Evaluation of chemical representations in physical chemistry textbooks
James M.
Nyachwaya
* and
Nathan B.
Wood
Department of Chemistry and Biochemistry, and School of Education, North Dakota State University, P.O. Box 6050, Fargo, ND 58108, USA. E-mail: James.nyachwaya@ndsu.edu
First published on 14th July 2014
Abstract
That different levels of representation are important for complete understanding of chemistry is an accepted fact in the chemistry education community. This study sought to uncover types of representations used in given physical chemistry textbooks. Textbooks play a central role in the teaching and learning of science (chemistry), and in some cases textbooks are the curriculum (Chiappetta and Fillman, 2007; Gkitzia et al., 2011). The books are not only central to instructors' curriculum; they are also a major resource for students' reference especially outside of class. Using a coding rubric developed by Gkitzia et al. (2011), the physical chemistry textbooks were analyzed to determine at what level(s) the included representations conveyed chemistry content. Representations were also analyzed for their characteristics or features. Results indicate that the analyzed texts contain at least one representation on 95% of the sampled pages and, on average each page contains about 1.4 representations. The vast majority of the representations are symbolic level representations, accounting for about 85% of representations. Particulate or submicroscopic representations were in a slightly higher proportion than macroscopic and multiple representations, but these collectively only accounted for about 15% of the representations in the textbooks. Our results indicate no significant difference in the types of representations used in textbooks for chemistry and life science majors. Across editions of a number of textbooks, there does not seem to be a difference in the type and proportion of representations used. An analysis of the representations showed that virtually all were completely related to the accompanying text, had surface features that were clear and explicit, and captions were concise, explicit and completely described associated representations. Implications of our findings to the chemistry education community are described.
Introduction
One of the most influential ideas in chemistry education today was proposed by Alex Johnstone in 1982 (Gabel, 1993; Gilbert and Treagust, 2009; Talanquer, 2011; Taber, 2013). Johnstone noted that chemistry can be represented at three levels: the macroscopic, submicroscopic, and symbolic levels (1991). He defined the macroscopic level as involving observable phenomena that could be experienced through touch, smell and sight; the symbolic level as involving symbols, chemical formulas, and graphs; and the submicroscopic level as involving particles such as atoms, molecules and ions. The different levels of representation are integral to understanding chemical phenomena (Treagust et al., 2003), a fact that makes learning in chemistry challenging (Johnstone, 1991). To understand chemistry in a meaningful way, students need to be exposed to the three levels of representation in chemistry (Johnstone, 1991; Treagust et al., 2003; Chandrasegaran et al., 2008). Depending on the nature of phenomena to be understood and/or explained, it sometimes becomes necessary to integrate and navigate between all the three levels (Johnstone, 2000).Despite the importance of the different levels of representation to the learning of chemistry, research in chemistry education has consistently revealed that students struggle with the levels of representation—they struggle with both understanding the chemistry at each level, and translating between the levels. Research studies have documented struggles within individual levels, e.g. with interpreting chemical formulas (Ben-Zvi et al., 1987; Kern et al., 2010; Nyachwaya et al., 2011); chemical equations (Kozma and Russell, 1997; Nyachwaya et al., 2011; Naah and Sanger, 2012), translating between formulas, electron configurations and models (Keig and Rubba, 1993). Students also struggle translating between the levels (Chittleborough and Treagust, 2008; Davidowitz and Chittleborough, 2009; Kern et al., 2010; Nyachwaya et al., 2011; Naah and Sanger, 2012).
An important question derived from the documented students' struggles with representations in chemistry pertains to the exposure that students get to the different representations as they go through the ‘chemistry pipeline’. Textbooks, as an important resource, should present content, and therefore expose students to different levels of representation in chemistry. This study looks at representations in physical chemistry textbooks. Given that physical chemistry is traditionally offered as an upper level course in the chemistry track in most departments in the United States, (and we assume other places as well) this study will shed some light into the kinds of exposure students get in upper level courses.
Theoretical background
Representations in chemistry
About three decades ago, Alex Johnstone noted that Chemistry could be represented at three levels: the descriptive and functional, the representational, and the explanatory (1982). Johnstone later noted that chemistry can be represented at three levels: the macroscopic, the submicroscopic and symbolic levels (1991). He defined the macroscopic level as involving observable phenomena that could be experienced through touch, smell and sight; the representational level as involving symbols, chemical formulas, graphs, and symbols; and the submicroscopic form as involving particles such as atoms, molecules and ions (1982; 1991; 2000). These levels of representation (Gilbert and Treagust, 2009), also referred to as Johnstone's triangle (Lorenzo et al., 2010; Jaber and BouJaoude, 2011), have been very influential in the field of chemical education, informing both curriculum and research (Gilbert and Treagust, 2009; Talanquer, 2011), to the extent that researchers and educators may take Johnstone's ideas for granted (Taber, 2013). According to Johnstone (1991), the existence of these three levels, which requires students to engage in multilevel thinking (Taber, 2013), makes science difficult for students.One of the outcomes of interest in and use of Johnstone's ‘triangle’ (Lorenzo et al., 2010; Jaber and BouJaoude, 2011) is the different ‘faces’, interpretations and modifications of the levels of representation by the chemistry education community (Talanquer, 2011). This is also problematic because the chemical education community does not seem to have a common view of the ‘triplet’ relationship (Taber, 2013). Referring to the same ideas, researchers in the chemistry education community have used different terms. For example, Talanquer (2011) talked about ‘three main ways’, while Gilbert and Treagust (2009) used the terms ‘types of representation’. Even while referring to Johnstone's original levels, Gilbert and Treagust (2009) used the terms phenomenological, model and symbolic as equivalents to the macro, submicro and representational levels. Over the years, a similar trend is seen as researchers use different terms. For example, Gabel et al. (1987) used levels of description, while Gabel (1993, 1999) used levels of instruction and levels of representation respectively. As Talanquer (2011) noted, the different interpretations can be a source of confusion, especially if they are all meant to refer to the same ‘objects’ or components of the triplet.
Another area that is problematic in the way the idea of the three levels of representation has been used is the fact that the three levels of representations occupy three apices of ‘Johnstone's triangle’. Specifically, representations that have been traditionally ascribed to the apices of the triangle may transcend the one apex of the ‘triangle’ they are assigned to. For example, as Taber (2013) noted, an equation of a reaction showing formulas of reactants and products, which is a symbolic representation according to Johnstone's definition (2000), can be ascribed to the macroscopic and symbolic levels simultaneously. A graph in a physical chemistry textbook could represent a macroscopic level observation. Designating the graph as only a symbolic level representation is therefore problematic. In our past research (Nyachwaya et al., 2011), as well as others, (e.g.Naah and Sanger, 2012) and in accordance with Johnstone's original work, formulas and chemical symbols have been classified as part of the symbolic language. Unless care is taken during instruction, while referring to a representation, students will not necessarily know what level a teacher is referring to (Taber, 2013).
In addition to Johnstone's three levels, Dori and Hameiri (2003) proposed a fourth level, the process level, which they defined as encompassing the process of chemical reactions and how it pertains to the macroscopic, symbolic and particulate levels. Gkitzia et al. (2011) noted that there are representations that combine elements from the different levels, such as hybrid, multiple and mixed representations. According to the authors, a hybrid representation is one that combines characteristics of two levels of representation, while a multiple representation depicts a phenomenon at more than one level of representation simultaneously. A mixed representation on the other hand has one of the three levels of representation and another kind of representation such as an analogy (Gkitzia et al., 2011). More recently, Dangur et al. (2014) suggested the addition of a fifth level, which they called the quantum level, which involves understanding of the electronic structure of atoms, molecules and the solid state, and the relationship to quantum mechanics.
In response to the existence of different (and problematic) interpretations and use of the levels of representation, Talanquer (2011) proposed a ‘multi-dimensional knowledge space’ (p. 186), which he suggested could better capture chemistry knowledge than ‘Johnstone's triangle’. According to Talanquer (2011), the knowledge space characterizes chemistry knowledge according to different approaches of teaching (mathematical, conceptual, historical and contextual), types of knowledge (experiences, models and visualizations), dimensions (structure/composition, energy and time), and levels or scales (subatomic, molecular, supramolecular, multi-particle, mesoscopic and macroscopic).
Representations in chemistry textbooks
Textbooks play a central role in the teaching and learning of science (chemistry), and in some cases textbooks are the curriculum (Chiappetta and Fillman, 2007; Gkitzia et al., 2011). The books are not only central to instructors' curriculum; they are also a major resource for students' reference especially outside of class. For instructors and teachers, textbooks guide the organization of curriculum materials (Justi and Gilbert, 2002; Koppal and Caldwell, 2004). If used well, textbooks can be an effective companion in fostering effective teaching and learning. Given their central role, textbooks should help facilitate learning of science (and chemistry in particular). Representations play a central role in the teaching and learning of chemistry. Representations used in the books should therefore facilitate students' understanding of chemistry. However, the mere presence of a representation in a textbook does not mean that it will necessarily communicate and facilitate the intended content understanding (Harrison, 2001; Furío-Más et al., 2005; Gkitzia et al., 2011).For a representation in a textbook to be useful, it has to facilitate understanding of target chemistry concepts. According to Gkitzia et al. (2011), the surface features of the representation have to be explicitly communicated to the reader (students). Indeed, representations will not be useful if students cannot infer the right and intended meaning from them. According to the authors, representations have to be also completely related to the associated content in a text. In case a representation has an accompanying caption, the caption has to be clear, completely define the representation and enable a reader to understand the representation (Gkitzia et al., 2011). Equally important is the need for a representation to be presented in a form that students can understand in textbooks (Giordan, 1991).
Why physical chemistry textbooks?
The preceding section talked about the central nature of textbooks to teaching and learning of chemistry. Dangur et al. (2014) noted that instruction in physical chemistry has traditionally taken a quantitative approach, with little emphasis on qualitative aspects of understanding in the discipline. Based on the different levels of representation, physical chemistry teaching, according to the authors therefore focusses heavily on the symbolic level (mainly mathematical derivations). The danger of using or relying on one level of representation is that it denies students a chance to see phenomena presented at different levels. This also has implications on students' knowledge and ability to translate between the levels of representation (Chandrasegaran et al., 2007). Chemistry students have also been known to memorize, repeat mathematical equations and even solve problems algorithmically without having a conceptual understanding of underlying chemical concepts (Bunce et al., 1991; Nakhleh, 1993; Bodner, 2003; Papaphotis and Tsaparlis, 2008). This tendency has been linked to the way textbooks have been written and the instructional strategies they engender (Gabel, 2003). Rote memorization interferes with conceptual understanding (Nakhleh, 1993).Could the dominance of the quantitative approach to teaching physical chemistry (Dangur et al., 2014) be a function of types of representation predominantly used in physical chemistry textbooks? An instructor's curriculum is derived from resources such as textbooks (Chiappetta and Fillman, 2007; Gkitzia et al., 2011). Ideally, physical chemistry textbooks should present content at and using different levels of representation. Integrating different levels of representation is known to enhance the understanding of chemistry (Johnstone, 1991; Treagust et al., 2003; Chandrasegaran et al., 2008).
With increased interest in chemistry students' representational fluency (Kozma, 2000), textbooks have been analyzed to catalog the nature and types of representations therein. Most of the textbook analyses looking at representations have involved general chemistry textbooks, mostly used at the high school and college freshman levels (e.g.Gkitzia, et al., 2011). Physical chemistry is usually offered as an upper level course in most schools in the United States. Students enrolling in physical chemistry would have normally taken general chemistry, organic chemistry and biochemistry, where they get exposure to different levels of representation (e.g.Gkitzia et al., 2011). In analyzing representations in physical chemistry textbooks, our goal was to find out what opportunities or exposure if any, students get to learn about and use the different levels of representations in an upper level course such as physical chemistry. To the best of our knowledge, no such analyses exist for physical chemistry textbooks. Also, not much work has been done on upper level college textbooks. This study involves analyzing physical chemistry textbooks for the nature and types of representations used. This analysis involves textbooks spanning the undergraduate to graduate level continuum.
This study is guided by the following research question(s):
(i) What are the different types of representations used in physical chemistry textbooks?
(ii) How do the types of representations compare across texts for different audiences (chemistry majors and life science majors)?
(iii) How do representations compare across different editions of the same textbook?
(iv) What are the characteristics of representations in the physical chemistry textbooks?
These research questions are important for three reasons. First, it is now accepted in the chemistry education community that multiple representations aid in the understanding of chemistry (Johnstone, 1991; Treagust et al., 2003; Chandrasegaran et al., 2008). Where necessary, multiple representations are combined to help describe and explain phenomena. For physical chemistry, it is important to find out what types of representations are used in textbooks. Second, given the importance of representations, partially explained by a lot of research done on the topic, curriculum materials have been developed in response to the research. One question we sought to understand is whether and how physical chemistry, as a branch of chemistry, has responded to the research. Finally, as Gkitzia et al. (2011) noted, there are certain features of a representation that make it useful in facilitating the understanding of a concept. Characterizing representations will help us understand their features, and how likely they are to facilitate the learning of associated chemistry content.
Methodology
Textbook selection and sampling
The amazon.com website (http://www.amazon.com) was searched to identify several of the most commonly-ordered and used physical chemistry textbooks in the United States. The site was chosen because it is a common source of textbooks for students, particularly in the United States. A ‘query’ was run on the site for ‘physical chemistry’. Copies of the top 12 textbooks from the list that came up were obtained for analysis. Copies of the textbooks were then obtained from colleagues, and our campus library. Given that physical chemistry textbooks are typically very lengthy, representative samples of pages were selected from each book. From each textbook, specific pages were identified for analysis by using a random-number generator (http://www.random.org). A sufficient total number of pages were analyzed from each text to provide a sample with 95% confidence level (5% confidence interval, α = 0.05) given the total population of pages in the given books (Cohen et al., 2011).Coding and analysis
To code and analyze representations in the physical chemistry textbooks used in this study, we used a rubric developed by Gkitzia et al. (2011). This rubric is based on Johnston's three levels of representation (macroscopic, symbolic and submicroscopic/particulate). In using this rubric, we acknowledge that there are problems with how the three levels are understood and used in the chemistry education community as described in the theoretical background. The rubric has the following criteria:(1) Types of representation – a representation could fall into six categories: macroscopic, submicroscopic, symbolic, hybrid, multiple and mixed (Gkitzia et al., 2011). The macroscopic, submicroscopic and symbolic are based on Johnstone's original definitions (1991). A hybrid representation is one that combines characteristics of two levels of representation, while a multiple representation depicts a phenomenon at more than one level of representation simultaneously. A mixed representation on the other hand has one of the three levels of representation and another kind of representation such as an analogy (Gkitzia et al., 2011). It is worth noting that multiple, hybrid, and mixed representations are not levels of representation on their own.
(2) Surface features of a representation – they could either be explicit, implicit or ambiguous.
(3) Relationship of representations to the text refers to whether there is an explicit reference to the representation within the text.
(4) Existence and properties of a caption – for representations that have a caption, the caption should ideally be brief, explicit and comprehensive.
(5) Degree of correlation between components comprising a multiple representation, the relatedness between the components or elements of the representations.
Using the above criteria (Gkitzia et al., 2011), one researcher coded representations in two of the twelve textbooks. The same textbooks were coded by the same researcher after two weeks to determine intra-rater reliability, which came out to be 99%. The second researcher coded one of the two textbooks for comparison. The inter-rater reliability was established at 97%. Differences were resolved through discussion. In each text's sampled pages, each page was analyzed for the type of representation present if any. We should stress here that our coding is guided by the Gkitzia et al. (2011) rubric. In adopting this rubric, we are aware as discussed above that there are inherent problems in simply classifying representations into the three levels proposed by Johnstone, and in light of Talanquer's (2011) proposed complex knowledge space.
In order to answer the research questions posed in this study, different kinds of analyses were done. First, all twelve textbooks were analyzed for the different types of representations. Upon examination, we found that the physical chemistry textbooks were written for different audiences – students in the ‘chemistry majors’ track and others, such as life sciences. Texts written for these audiences (based on their titles, such as physical chemistry for life sciences) were analyzed to see the similarities and differences between representations in these texts if any. We were also interested in looking at any changes in the type and number of representations in different editions of the same book (by the same author). Four books were chosen for this analysis. Two editions of each textbook were analyzed. Four (same) chapters from each text across two editions were analyzed.
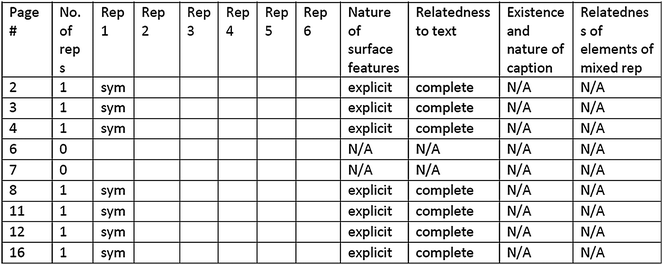
Each representation was further analyzed for surface features, relatedness to the accompanying text, and for representations with a caption, the nature of a caption. These attributes were adopted from Gkitzia et al. (2011). Each representation was classified and characterized at the same time. Excel was used during the coding and analysis processes. Fig. 1 shows a snapshot of an Excel page of one of the textbooks analyzed.
Results
The results of the analysis done will be presented by research questions.Question 1: (i) What are the different types of representations used in physical chemistry textbooks?
Table 1 gives a summary of the number of pages sampled from each book, the total number of representations from the sampled pages, the average number of representations per page from those sampled, and the percentage of pages with at least one representation from the sample in each book.| Textbook | Number of pages sampled | Total number of representations | Average number of representations per page | % of pages with at least 1 representation |
|---|---|---|---|---|
| Atkins (1994) | 281 | 405 | 1.4 | 97 |
| McQuarrie and Simon (1997) | 279 | 358 | 1.3 | 99 |
| Laidler and Meiser (1999) | 266 | 330 | 1.2 | 99 |
| Levine 5th edn (2002) | 266 | 376 | 1.4 | 98 |
| Chang (2005) | 231 | 301 | 1.3 | 95 |
| Sibley et al. (2005) | 265 | 330 | 1.2 | 96 |
| Atkins and De Paula, (2006) | 278 | 432 | 1.6 | 94 |
| Engel and Reid, (2006) | 266 | 392 | 1.5 | 99 |
| Patterson (2007) | 85 | 92 | 1.1 | 96 |
| Atkins and De Paula, (2011) | 213 | 285 | 1.3 | 95 |
| Cooksy (2014) | 220 | 308 | 1.4 | 99 |
| Ball (2015) | 251 | 324 | 1.3 | 99 |
As can be seen from the table, on average, each of the sampled pages in each textbook had at least one representation. Even though not every page had a representation, a very high percentage of the sampled pages had a representation on them (94% to 99%). This is an encouraging trend in the context of having representations used alongside text to convey content in the textbooks. An important next question is: What are the different types of representations in the textbooks?
Our results showed that different types or levels of representation were used in the physical chemistry textbooks sampled. Table 2 gives a summary of the types and proportion of representations used in the analyzed physical chemistry textbooks. Note that Table 2 is divided into two sections. The top section is for physical chemistry textbooks for ‘chemistry majors’, while the bottom section has textbooks for ‘life science majors’.
| Book/representation | Macroscopic (%) | Submicroscopic (%) | Symbolic (%) | Multiple (%) |
|---|---|---|---|---|
| For chemistry majors | ||||
| Atkins (1994) | 4.90 | 6.20 | 88.90 | 0.00 |
| McQuarrie and Simon (1997) | 1.31 | 0.55 | 98.14 | 0.00 |
| Laidler and Meiser (1999) | 3.33 | 0.91 | 95.76 | 0.00 |
| Levine 5th edn (2002) | 4.26 | 2.13 | 92.56 | 1.05 |
| Sibley et al. (2005) | 0.90 | 1.50 | 97.50 | 0.00 |
| Atkins and De Paula (2006) | 7.90 | 10.2 | 82.20 | 0.50 |
| Engel and Reid, (2006) | 5.60 | 4.00 | 90.30 | 0.00 |
| Cooksy (2014) | 5.19 | 8.77 | 86.04 | 0.00 |
| Ball (2015) | 4.62 | 2.77 | 92.31 | 0.30 |
| Life science majors' | ||||
| Chang (2005) | 4.00 | 7.30 | 87.40 | 1.00 |
| Patterson (2007) | 0.00 | 0.00 | 100 | 0.00 |
| Atkins and De Paula, (2011) | 4.20 | 13.30 | 80.70 | 0.70 |
A number of observations can be made from Table 2. Of the twelve books analyzed, five had four types of representations; six had three types of representations while one had only one type of representation. Symbolic representations were the most common type of representation used, followed by sub-microscopic representations. Generally, of the three common representations used in the textbooks macroscopic representations were used least across the textbooks. None of the twelve textbooks contained mixed or hybrid representations in the sampled pages. Even though the range in years of publication was 1994–2011, it is interesting to note that the proportions in individual categories of representations are not very different over that time span. One wonders whether physical chemistry as a branch of chemistry has been slow to responding to ‘reform’ in terms of including multiple representations—especially higher proportions in the macroscopic, submicroscopic and multiple levels of representation.
(ii) How do the types of representations compare across texts for different audiences (chemistry majors and life sciences)?
One idea that was investigated in this study was whether there is a difference in the types and proportion of representations used in physical chemistry textbooks for audiences other than chemistry majors—specifically for life sciences, such as biochemistry. Our hypothesis before the analysis was that there would be more submicroscopic representations since these textbooks were written for life science majors. Worth noting from Table 2 is the fact that the symbolic level representations are most common in the three ‘life science’ textbooks. A majority of the symbolic representations were mathematical derivations. Overall, the proportions of macroscopic and sub-microscopic representations are very similar to those of ‘chemistry majors’ textbooks. This is an interesting finding, given the audience of the textbooks. In fact, it is interesting that one of the three textbooks has 100% symbolic representations.(iii) How do representations compare across different editions of the same textbook?
As newer editions of textbooks get published a number of things may change, such as the number and order of topics, number of pages, and the nature of representations. For this study, one idea we explored was to compare two editions of the same book, by the same author, to look at changes, if any, to the type of representations used. Two editions each of four different textbooks were analyzed. Table 3 gives a summary of the comparison of the type and proportion of representations in each edition of a given textbook.| Type of representation | Macro (%) | Micro (%) | Symbolic (%) | Multiple (%) |
|---|---|---|---|---|
| Atkins 2nd edn (1982) | 6.00 | 5.00 | 89.00 | 0.00 |
| Atkins 5th edn (1994) | 5.70 | 4.60 | 89.70 | 0.00 |
| Levine 5th edn (2002) | 0.45 | 2.60 | 96.95 | 0.00 |
| Levine 6th edn (2009) | 1.31 | 2.89 | 95.80 | 0.00 |
| Engel and Reid 1st edn (2006) | 2.01 | 8.06 | 87.92 | 2.01 |
| Engel and Reid 2nd edn (2010) | 4.45 | 10.19 | 81.53 | 3.82 |
| Atkins and De Paula 1st edn (2006) | 4.00 | 1.50 | 93.80 | 0.70 |
| Atkins and De Paula 2nd edn (2011) | 7.10 | 7.10 | 84.90 | 0.90 |
Worth noting from Table 3 is a very similar trend both in proportion and type of representations used in the two editions of each textbook analyzed. In general, it appears that of the changes made across the two editions of each textbook, specifically to the same topics, not much changed in terms of the type and proportions of representations and the number of representations used. For the textbooks analyzed here, across the editions, there does not seem to be an appreciable change in the proportion of the three types of representations used, as the symbolic representation is the most commonly used. In particular, if an earlier edition of a textbook lacked a type of representation (such as multiple representations), a newer edition did not have or include that representation.
Question 2: What are the characteristics of the representations found in the physical chemistry textbooks?
Representations were analyzed for their surface features, whether individual representations were related to the text, and for representations that had a caption, the properties of the caption (Gkitzia et al., 2011). In the textbooks analyzed for this study, 99.2% of all representations had explicit surface features. This means that it was not left to the reader to figure out what a representation contained. As an example, graphs were clearly labelled, and axes were labelled with corresponding units among other features. This as characteristic of the representations, makes it easy for users, especially students, to make sense of a representation. In 0.8% of the representations features of the representations were implicit, a fact that may make the representations inaccessible to users. As Gkitzia et al. (2011) noted students are especially likely to end up with misconceptions when aspects of representations are left to interpretation.All of the representations identified in the analyzed textbooks were completely related to the accompanying text. In all the cases, as the phenomenon was described in the text as part of the content, reference was made to the representations, which were in most cases referred to as a figure, assigned a number. This explicit relationship enables users, more importantly students, to link representations to phenomena and content describing it.
Of the representations that had captions, 100% of the captions were brief and explicit, and completely described the accompanying representations. This makes the representations described by the caption clear and understandable to users. Representations that did not have captions were mainly mathematical equations which were derivations, which are not expected to have captions. Gkitzia et al. (2011) describe such representations as being incorporated, meaning that they form part of the text. Looking at the characteristics of the representations presented here shows an encouraging trend, especially in facilitating student understanding of the representations. That said, studies should be made to determine the utility of these characteristics to students, or if students can recognize their value.
Discussion and implications
This study adds to current literature in the field, particularly on representations in textbooks. This study specifically adds findings in representations in physical chemistry textbooks. Since some of the textbooks we analyzed cover the content of undergraduate and graduate levels, this study gives the chemistry education community a glimpse into the exposure to various representations that students get as they move through the chemistry ‘pipeline’.One would ideally expect to see attempts in textbooks to not only use different representations, but to also integrate the representations as well. The closest such integration was seen in the very few multiple representations, as evidenced in Table 1. An advantage of such a presentation is that it enables learners to see phenomena at multiple levels (e.g. the same phenomena at symbolic and submicroscopic levels), which could help students transfer between the different levels (Chandrasegaran et al., 2007). In contrast, students learning and experiencing phenomena at one level are likely to end up with fragmented knowledge (Treagust et al., 2003), not knowing how different levels or representation of the same phenomena are related. Saying this does not downplay the important fact that representations are used in the textbooks.
Given the central role that textbooks play in science (chemistry), there is a need for increasing the use of other representations. It is true that physical chemistry has long been characterized by mathematical derivations involving symbols, tables and graphs. Is there room for other representations beside the symbolic level? While it is encouraging that other levels of representation are used, their proportions are very low. As noted above, representations in one of the textbooks analyzed were 100% symbolic. How and where will students get exposure to other representations if textbooks don't use them?
The results of this study communicate an important message about the nature of education in physical chemistry; specifically as a sub-discipline of chemistry that is heavy in symbolic level representations. The results analyzed here show that mathematical derivations are the most common symbolic level representations. Research has shown that students struggle with symbolic level representations (Kozma and Russell, 1997). If representations in textbooks are predominantly symbolic, we expect students to struggle with making sense of them. There is a real opportunity here for other representations to be brought in. As part of reform efforts in chemistry, it is important for this image of the nature of chemistry to be addressed.
Dangur et al. (2014) noted that instruction in physical chemistry tends to be primarily quantitative. We speculate that much of this state can be attributed to the fact that representations in physical chemistry textbooks are mostly symbolic, and the symbolic representations are mostly mathematical derivations. Any teaching approach that focuses on some of the levels results in confusion, information overload, decreased student motivation and ultimately less student achievement (Talanquer, 2011). Studies in other areas of chemical education, such as problem solving, have shown that instruction that focuses at the symbolic level, particularly equations and formulas, encourages rote memorization in students, where they can go through a derivation, for example, without necessarily understanding the underlying chemistry (e.g.Nakhleh, 1993). The fact that symbolic representations, specifically mathematical derivations, are the most common types of representation found in the textbooks analyzed should be of concern to the chemistry education community. As noted above, textbooks are central to instruction and learning of chemistry. If instructors then rely on the textbooks as written, and students use the textbooks as resources, what is presented in the textbooks is what is learned.
The findings presented here have implications for physical chemistry textbook authors, instructors and other curriculum developers. This study analyzed textbooks published between 1994 and 2011. As the results have shown, the proportions of different types of representations across the textbooks are very similar. This suggests over the years that, as newer books get published, there has not been much change in the diversity of representations used. The results of this study have also shown that even across editions of the same textbook, by the same author, there is virtually no difference in the proportion and type of representations used. The newer editions of textbooks are not being informed by a need to include multiple representations. While most of the textbooks from this analysis have more than one representation used, it is important to increase the use (proportion) of all representations, as much as the symbolic level is used.
For classroom instructors, using different levels of representation during instruction enhances the understanding of chemistry (Cheng and Gilbert, 2009). Given the influence of textbooks in the teaching of chemistry, it is incumbent upon teachers to intentionally look for other resources, especially representations to augment their teaching. Knowing the importance of using representations in teaching chemistry, teachers have to use resources that include representations as part of their content. For a teacher, this may mean combining a number of textbooks while developing curriculum to ensure that students get the necessary exposure and learning. Also, a careful choice of textbooks is necessary in this context to ensure that they include representations, and the useful features that will enhance learning.
Future studies
We are interested in pursuing a number of research questions that build off of this study. First, in recent years, most textbooks are accompanied by either a CD or web-based resources as supplemental to the textbooks. These supplemental resources have different representations associated with them. It will be interesting to study the nature of the representations contained therein. This is especially important since these are resources that students use outside of class. Second, our results indicate that most if not all of the sampled pages contained at least one representation. We are not sure whether all representations on a given page are necessary to convey intended meaning, or if they could lead to information overload, thereby being a distraction instead. Related to this would be a question of how useful students find the given representations. What features of a representation in a physical chemistry textbook do students pay attention to? Third, given that textbooks analyzed here have highest proportions of symbolic representations, it will be interesting to find out whether physical chemistry instructors make a conscious choice to seek other resources so as to supplement the symbolic level content during instruction. An important idea therefore would be to investigate whether and the extent to which physical chemistry instructors seek and integrate various resources when planning instruction to ensure that their students get exposure to different representations. It will also be interesting to find out if instructors only present what is in the textbooks they use.Limitations of this study
One possible limitation of this study stems from the fact that the data reported come from twelve physical chemistry textbooks. These textbooks are commonly used in the United States. Maybe in other countries, the commonly used textbooks are different from those analyzed here. Given the consistency of results, in terms of proportions of the different representations in books, chances are that this textbook sample is representative. Another possible limitation could be due to the fact that the results come from sampled pages, meaning that not all representations in the analyzed textbooks were captured. However, given that the sample size is selected, it is very likely that the sampled pages were representative of the whole textbook. The conclusions on whether textbooks for life science majors and newer editions of textbooks should have higher portions of other representations beside the symbolic level is based on the assumption that textbook authors should be paying attention to the need for and importance of representations when writing books. This for example may not necessarily be the intention of the authors and publishers as newer editions of textbooks come out. It is however not an unreasonable expectation.References
- Atkins P. W., (1994), Physical Chemistry, 5th edn, NY: Freeman and Company.
- Atkins P. W., (1982), Physical Chemistry, 2nd edn, NY: Freeman and Company.
- Atkins P. and De Paula J., (2011), Physical Chemistry for the life sciences, 2nd edn, NY: W. H. Freeman and company.
- Atkins P. and De Paula J., (2006), Atkins' physical chemistry, 8th edn, NY: W. H. Freeman and company.
- Ball D. W., (2015), Physical Chemsitry, 2nd edn, Connecticut: Cengage Learning.
- Ben-Zvi R., Eylon B. and Silberstein J., (1987), Students' visualization of a chemical reaction, Educ. Chem., 24, 117–120.
- Bodner G. M., (2003), Problem solving: the difference between what we do and what we tell students to do, Univ. Chem. Educ., 7, 37–45.
- Bunce D. M., Gabel D. and Samuel J., (1991), Enhancing chemistry problem solving achievement using problem categorization, J. Res. Sci. Teach., 28(6), 505–521.
- Chandrasegaran A. L., Treagust D. F. and Mocerino M., (2008), An evaluation of a teaching intervention to promote students' ability to use multiple levels of representation when describing and explaining chemical reactions, Res. Sci. Educ., 38, 237–248.
- Chandrasegaran A. L., Treagust D. F. and Mocerino M., (2007), The development of a two-tier multiple-choice diagnostic instrument for evaluating secondary school students' ability to describe and explain chemical reactions using multiple levels of representation, Chem. Educ. Res. Pract., 8(3), 293–307.
- Chang R., (2005), Physical Chemistry for the Biosciences, Sausalito, Ca: University science books.
- Cheng M. and Gilbert J. K., (2009), Towards a better utilization of diagrams in research, in Gilbert J. K. and Treagust D. (ed.) Multiple representations in chemical education, New York: Springer, pp. 55–73.
- Chiappetta E. L. and Fillman D. A., (2007), Analysis of five high school biology textbooks used in the United States for inclusion of the nature of science, Int. J. Sci. Educ., 29(15), 1847–1868.
- Chittleborough G. and Treagust D., (2008), Correct interpretation of chemical diagrams requires transforming from one level of representation to another, Res. Sci. Educ., 38, 463–482.
- Cohen L., Manion L. and Morrison L., (2011), Research methods in education, 7th edn, London: Taylor & Francis, Inc.
- Cooksy A., (2014), Physical Chemistry: Quantum Chemistry and Molecular Interactions, New Jersey: Pearson.
- Dangur V., Avargil S., Peskin U. and Dori J. Y., (2014), Learning quantum chemistry via visual-conceptual approach: students' bidirectional textual and visual understanding, Chem. Educ. Res. Pract., 2014, 15, 297–310.
- Davidowitz B. and Chittleborough G., (2009). Linking the macroscopic and sub-microscopic levels: Diagrams, in Gilbert J. K. and Treagust D. (ed.) Multiple representations in chemical education, the Netherlands: Springer, pp. 169–191.
- Dori Y. J. and Hameiri M., (2003). Multidimensional analysis system for quantitative chemistry problems: symbol, macro, micro, and process aspects, J. Res. Sci. Teach., 40(3), 278–302.
- Engel T. and Reid P., (2006), Physical Chemistry, New Jersey: Pearson.
- Engel T. and Reid P., (2010), Physical Chemistry, 2nd edn, New Jersey: Pearson.
- Furío-Más C., Calatayud M. L., Guisasola J. and Furío-Gómez C., (2005), How are the concepts and theories of acid–base reactions presented? Chemistry in textbooks and as presented by teachers, Int. J. Sci. Educ., 27, 1337–1358.
- Gabel D. L., Samuel K. V. and Hunn D., (1987), Understanding the particulate nature of matter, J. Chem. Educ., 64(8), 695–697.
- Gabel D. L., (1993), Use of the particle nature of matter in developing conceptual understanding, J. Chem. Educ., 70(3), 193.
- Gabel D., (1999), Improving teaching and learning through chemistry education research: a look to the future, J. Chem. Educ., 76(4), 548–554.
- Gabel D. L., (2003), Enhancing conceptual understanding of Science, Educ. Horizons, 81, 70–76.
- Gilbert J. K. and Treagust D., (2009), Introduction: macro, submicro and symbolic representations and the relationship between them: key models in chemical education, in Gilbert J. K., Treagust D. (ed.) Multiple representations in chemical education, Dordrecht: Springer, pp. 1–8.
- Giordan A., (1991), The importance of modelling in the teaching and popularisation of science, Impact Sci. Soc., 164, 321–338.
- Gkitzia V., Salta K. and Tzougraki C., (2011), Development and application of suitable criteria for the evaluation of chemical representations in school textbooks, Chem. Educ. Res. Pract., 12(1), 5–14.
- Harrison A. G., (2001), How do teachers and textbook writers model scientific ideas for students? Res. Sci. Educ., 31, 401–435.
- Jaber L. Z. and BouJaoude S., (2011), A Macro-Micro-Symbolic Teaching to Promote Relational Understanding of Chemical Reactions, Int. J. Sci. Educ., 1–26.
- Johnstone A. H., (1982), Macro- and micro- chemistry, Sch. Sci. Rev., 64(227), 377–379.
- Johnstone A. H., (1991), Why is science difficult to learn? Things are seldom what they seem, J. Comput. Assist. Lear., 7, 75–83.
- Johnstone A. H., (2000), Teaching of chemistry: Logical or psychological? Chem. Educ.: Res. Pract. Eur., 1(1), 9–15.
- Justi R. S. and Gilbert J. K., (2002), Models and modelling in chemical education, in Gilbert J., De Jong O., Justi R., Treagust D. and Van Driel J. (ed.) Chemical education: towards research-based practice, Dordrecht: Kluwer, pp. 213–234.
- Keig P. F. and Rubba P. A., (1993), Translation of representations of the structure of matter and its relationship to reasoning, gender, spatial reasoning and specific prior knowledge, J. Res. Sci. Teach., 30, 883–903.
- Kern A. L., Wood N. B., Roehrig G. H. and Nyachwaya J. M., (2010), A qualitative report of the ways high school chemistry students attempt to represent a chemical reaction at the atomic/molecular level, Chem. Educ. Res. Pract., 11, 165–172.
- Koppal M. and Caldwell A., (2004), Meeting the challenge of science literacy: project 2061 efforts to improve science education, Cell Biol. Educ., 3, 28–30.
- Kozma R., (2000), The use of multiple representations and the social construction of understanding in chemistry, in Jacobson M. and Kozma R. (ed.) Innovations in science and mathematics education: Advanced designs for technologies of learning, Mahwah, NJ: Erlbaum, pp. 11–46.
- Kozma R. B. and Russell J., (1997), Multimedia and understanding: expert and novice responses to different representations of chemical phenomena, J. Res. Sci. Teach., 34, 949–968.
- Laidler M. J and Meiser J. H., (1999), Physical Chemistry, 3rd edn, New York: Houghton Mifflin.
- Levine I. N., (2002), Physical Chemistry, 5th edn, Boston: McGrawHill.
- Levine I. N., (2009), Physical Chemistry, 6th edn, Boston: McGrawHill.
- Lorenzo M. G., Farre A. S. and Rossi A. M., (2010), Teachers' discursive practices in a first organic chemistry course, in Çakmakci G. and Tas-ar M. F. (ed.) Contemporary Science Education Research: Scientific Literacy and Social Aspects of Science, Ankara, Turkey: Pegem Akademi, pp. 13–22.
- McQuarrie D. A and Simon J. D., (1997), Physical Chemistry: A molecular Approach, Sausalito: University Science Books.
- Naah B. M. and Sanger M. J., (2012), Student misconceptions in writing balanced equations for dissolving ionic compounds in water, Chem. Educ. Res. Pract., 13, 186–194.
- Nakhleh M. B., (1993), Are our students conceptual thinkers or algorithmic problem solvers? J. Chem. Educ., 70, 52–55.
- Nyachwaya J., Mohamed A., Roehrig G. H., Wood N. B., Kern A. L. and Schneider J. L., (2011). The development of an open-ended drawing tool: an alternative diagnostic tool for assessing students' understanding of the particulate nature of matter, Chem. Educ. Res. Pract., 12, 121–132.
- Papaphotis G. and Tsaparlis G., (2008), Conceptual versus algorithmic learning in high school chemistry: the case of basic quantum chemical concepts, Part 2. Students' common errors, misconceptions and difficulties in understanding, Chem. Educ. Res. Pract., 9, 332–340.
- Patterson G., (2007), Physical Chemistry of Macromolecules, 2nd edn, Taylor and Francis.
- Sibley R. J., Alberty R. A. and Bawendi M. G., (2005), Physical Chemistry, 4th edn, New Jersey: John Willey and Sons.
- Taber K. S., (2013), Revisiting the chemistry triplet: drawing upon the nature of chemical knowledge and the psychology of learning to inform chemistry education, Chem. Educ. Res. Pract., 14(2), 156–168.
- Talanquer V., (2011), Macro, Submicro, and Symbolic: the many faces of the chemistry “triplet”, Int. J. Sci. Educ., 33(2), 179–195.
- Treagust D. F., Chittleborough G. and Mamiala T. L., (2003), The role of submicroscopic and symbolic representations in chemical explanations, Int. J. Sci. Educ., 25, 1353–1368.
| This journal is © The Royal Society of Chemistry 2014 |