The significance of implicit knowledge for learning and teaching chemistry
Keith S.
Taber

Faculty of Education, University of Cambridge, UK. E-mail: kst24@cam.ac.uk
First published on 6th August 2014
Abstract
This article discusses the nature of implicit knowledge, something which is considered to be highly influential in learning. The notion of implicit knowledge is important in conceptualising studies exploring student thinking and learning in chemistry, and in considering how the results of such studies should be interpreted to inform teaching. Research on cognition suggests that a good deal of the knowledge that people call upon in interpreting their world and making decisions is not accessible to conscious introspection. This has consequences in chemistry education research as individuals are not able to directly report implicit knowledge – so it can only be elicited indirectly. A corollary is that the results of many research studies reporting student conceptions in chemistry need to be understood as reflecting – at least in part – cognition drawing upon implicit knowledge. The distinction between explicit and implicit knowledge is an important one in understanding chemistry learning given that implicit knowledge operates automatically in cognition without deliberation. This suggests that strategies designed to counter students' alternative conceptions may need to be quite different when such ideas derive from the operation of implicit knowledge rather than students' explicit knowledge. The importance of implicit knowledge elements sometimes labelled as p-prims has been widely recognised in physics education research, and it is argued here that research into student thinking and learning in chemistry needs to take more account of the distinction between explicit and implicit knowledge elements if it is to better inform teaching. Research is needed to understand the repertoire and action of implicit knowledge elements active in chemistry learning. This will then facilitate the design of studies to test out teaching approaches that can recruit the most suitable implicit knowledge elements to support learning of canonical chemical ideas.
Introduction
This article explores the theoretical perspective of the role of implicit knowledge in learning chemistry and considers its significance in the research programme to inform chemistry teaching through exploring the contingent nature of student learning. In this programme it is considered that learning is contingent on, and channelled by, existing knowledge and understanding. The assumption is that teachers should be able to teach better if they have a good understanding of what students understand, and how they think about the topics to be studied – including whether they hold any alternative conceptions. The article begins by considering the notion of implicit knowledge and how it relates to how knowledge is more generally understood in studies of learners' ideas. Having set out what is meant by implicit knowledge, the article then discusses how implicit knowledge acts in cognition and how it comes to be acquired. Key work which has considered the role of implicit knowledge elements in learning science is then reviewed, before considering how these ideas have been adopted in studies exploring student thinking in chemistry. Given the limited amount of work in this area within chemistry education research (CER), and the potential value of this perspective, the challenges of developing the research programme in this direction are considered.The nature of a learner's knowledge
A central concern in chemistry education is learners' knowledge and understanding. Students are assessed largely in terms of their knowledge and understanding of the subject. Chemistry teaching is – not exclusively, but to a large extent – concerned with developing learners' knowledge and understanding of the subject. Those teaching the subject are advised that teaching needs to take into account the learners' prior knowledge and understanding (Driver and Easley, 1978; Gilbert et al., 1982; Taber, 2014a), and are encouraged to undertake diagnostic assessment to inform their teaching (Treagust, 1995, 2006; Taber, 2002, 2014b). Understandably, key books reporting CER give prominence to studies of student knowledge and understanding of the subject (Gilbert et al., 2002; Gilbert and Treagust, 2009; Tsaparlis and Sevian, 2013).Terms such as ‘knowledge’ and ‘understanding’ are ubiquitous in the discourse of education, although they are seldom operationally defined in reports of CER. Such widely used (and, one would expect, understood) terms are commonly taken-for-granted in educational writing, yet it seems less clear that the ‘implicit knowledge’ discussed in this article (i.e. knowledge a person applies that they are not able to deliberately access) would be accepted as ‘knowledge’ in the everyday sense of the word. It is therefore important to clarify the use of the terms ‘knowledge’ and ‘implicit knowledge’ when used as technical terms (as in CER research).
Explicit and implicit knowledge
A distinction that is important for understanding learning is between knowledge that can be classed as explicit or as implicit. Implicit knowledge could be understood as the things we know (in the sense of being able to take them into account in making decisions for example) but are not aware that we know. The phenomenon of implicit knowledge may be illustrated by a medical condition known as blindsight (Gazzaniga et al., 1994). This is a condition where a person claims they cannot see, and indeed genuinely seems to not have ‘normal’ visual experience because of some form of damage in the brain, but nonetheless retains the ability to report some information perceived visually. If a doctor asks a patent with this condition to identify an object being held in front of them, they will not be able to do so, as they cannot see the object. That is, they have no conscious experience of vision. Yet if they are instead persuaded to guess what the object is, then they can often give accurate reports. In some sense they can apply information from the visual field, but they are not aware of seeing an object. Although useful knowledge is represented in the brain the person with blindsight is not aware of this and experiences the activation of this knowledge as no more than having a hunch.This condition seems quite bizarre. It seems to occur when the main neural pathways concerned with vision become damaged. However, the brain has auxiliary pathways for processing sensory information from the retina although these do not lead to the usual visual experience of seeing the world. A person with normal vision will see the doctor holding a pen or some other object and will be aware of this. The person with blindsight does not ‘see’ in this usual sense, and has no conscious awareness that they are processing visual information, yet the processing has gone on within the ‘cognitive system’ (i.e. the processing system within the central nervous system that supports our cognition). At some level the person with blindsight who is able to identify objects being ‘shown’ to them has ‘knowledge’ of objects in the visual field (as shown by the successful ‘guessing’), but they are not aware of that knowledge and would deny having it.
Although blindsight is very rare, it reflects a general principle that the thinking we are consciously aware of reflects only a small part of the processing that occurs within our cognitive systems, and more significantly for the present article, that not all the knowledge applied by people is explicit knowledge: knowledge that they are aware they have and can deliberately access. Rather, much knowledge is implicit in the sense that we may draw upon it without being aware of doing so. Put simply, we would be unable to confidently answer the question ‘how did you know that?’
A relevant concept here is intuition, something which can play an important role in much informal decision-making. This contrasts with the form of decision-making that uses explicit criteria to evaluate options, and which allows a decision to be justified through a logical chain of argument. We might wish to label this as scientific decision-making (Newton et al., 1999), although it may be more generally thought of as deliberate decision making in the sense that it relies on conscious deliberation. This type of process is open to explanation, and re-checking. However, in everyday life many of our decisions are not subject to such a deliberate process, but rather we may just ‘go with our gut instinct’ (cf.Claxton, 1993). Sometimes such decision-making leads to us being confident that we have made the correct decision, even though we cannot explain the basis of the decision beyond reporting that it ‘felt right’.
Intuition is not necessarily a poor basis for decision-making. For one thing, it provides a much faster process than deliberate decision-making. We simply could not function effectively if we needed to approach every one of the myriad decisions we need to make in everyday life through a process of consciously setting out all the options, selecting criteria, mustering available evidence, considering alternative scenarios by running mental simulations…and so forth each time we needed to decide which jacket to wear or whether to have tea or coffee, or which writing implement to pick up to make a note. It is now widely accepted that many aspects of human cognition can be understood by considering that there are two complementary systems at work (Evans, 2008): system 1 (also referred to by different theorists using terms such as automatic, experiential, heuristic, implicit, associative, holistic, reflective and impulsive) and system 2 (also variously labelled with such terms as controlled, rational, systematic, explicit, analytic, reflective and higher order).
Intuition is certainly not a mystical process, even if it seems a mysterious one. Intuition can often be highly honed and is ‘educated’ in the sense of drawing upon considerable experience (diSessa, 1983). This is perhaps related to what Louis Pasteur meant by chance favouring a prepared mind. Our brains are able to use experience to develop cognitive resources that support us in our work even though we may not be aware of their existence. Experience in a field of activity enables us to see things others do not. So, for example, an experienced paleontologist can often spot small fossil fragments that do not stand out from their context to the casual observer, even though they may not be able to report that they are looking for any particular feature – rather they have developed expertise in visual recognition of fossil candidates through the trial and error of a great many hours of doing the work. Similar effects are found in learning to see like an expert through a microscope or telescope.
The chemist and philosopher Michael Polanyi (1962, 1970) argued that scientific work relies heavily on what he termed ‘tacit knowledge’ and this can be very clear in the laboratory where, for example, a technician with many hours of experience with a particular instrument can often use it effectively even when others working in the lab may consider it temperamental and unreliable. The technician has developed specific tacit knowledge of how to get the best out of that particular instrument. This can be quite a significant phenomenon as the sociologist of science Harry Collins (2010) reports that despite the scientific practice of detailing new techniques in scientific papers, knowledge-transfer between laboratories is sometimes only possible by scientists visiting and spending time in a lab where a new technique or instrument has been perfected: the kind of specialised (tacit) knowledge needed to master the technique is not consciously available so it cannot be specified in technical papers. Rather it is a matter of seeing, and copying, and ‘getting a feel’ for what is needed. Van Eijck and Roth discuss a parallel situation where a scientist working in a salmon hatchery was unable to replicate a technique that an experienced fish culturist of high technical expertise was able to recreate readily. Despite an experimental set up described as “simplicity itself” and the scientist being characterised as having an “abundance of scientific knowledge” there was a flaw in his approach where “he didn't have that insight to try to figure out” (van Eijck and Roth, 2007, p. 941).
Polanyi did not just see tacit knowledge as related to laboratory activity. He referred to what he called the “unaccountable element in science” (Polanyi, 1962/1969), that prevented scientific work being reduced to following learnt algorithms – akin to the more simplistic notions of a scientific method that have been criticised as misrepresenting scientific processes in some school science curriculum documents (Taber, 2008b; Hofstein and Kind, 2012). Scientific work has both divergent phases of exploration and convergent phases of logical justification through argumentation (Kuhn, 1959/1977). Scientific research reports occupy a genre that generally offers a tidy, idealistic account of what seems in retrospect (the context of justification) a linear process of research (Medawar, 1963/1990). Yet the logical argument in a research paper only reflects part of the scientific process: science also relies on creative, imaginative thinking that largely takes place away from consciousness (Taber, 2011) and draws upon our implicit knowledge or intuition.
Is ‘implicit knowledge’ really knowledge?
Before preceding it is useful to explore how the term knowledge is to be understood in this article. Van Eijck and Roth's fish culturist referred to the highly knowledgeable scientist as having a lack of ‘insight’. ‘Insight’, like ‘knowledge’, is one of a range of terms relating to mental properties and activities (‘learning’, ‘understanding’, and ‘thinking’ are other examples) which are ubiquitous in educational discourse. That is, teachers and others working in education regularly refer to ‘knowledge’ and seem to have a sufficiently shared understanding of the term for their communications to generally be unproblematic. However, such terms are seldom defined in precise ways because for most professional purposes a fairly vague meaning that is understood in general terms suffices. These widely used, but seldom defined, terms for discussing mental processes and attributes have been collectively labelled the mental register (Taber, 2013b).A core area of human cognition concerns what is known as ‘theory of mind’ which is acquired as part of normal development in childhood – such that we come to infer that others have minds like ourselves, and so are likely to have mental experiences like our own (Wellman, 2011). As this appreciation first develops when we are very young, our understanding of mental phenomena – though quite vague and indeed often technically suspect (Taber, 2013b) – tends to become a taken-for-granted part of our habitual ways of thinking and talking that we retain throughout life. Consequently people generally consider that they know what – for example – ‘knowledge’ is, and seldom feel a need to characterise it through a formal definition or set of criteria.
In everyday life, this is seldom a problem. However, within an area of scholarship where knowledge is an important focus (such as CER for example) there is a need to treat ‘knowledge’ as a technical term, so that we are able to decide when something we examine is, or is not, ‘knowledge’ – just as chemists expect to have means of knowing when they are dealing with an ‘acid’ or an ‘alloy’ for example. Philosophers have explored the nature of and criteria for knowledge over many centuries. A traditional notion of knowledge from philosophical traditions is that knowledge is true, reasoned, belief (Bhaskar, 1981; Matthews, 2002). So if a scientist makes a knowledge claim (in a scientific paper perhaps) for example that compound X forms rhombic crystals, then this would be considered knowledge providing (a) the scientist was strongly committed to the claim (believed it herself) AND (b) the compound did in fact form rhombic crystals AND (c) the scientist had good grounds for her beliefs (perhaps she had prepared the crystals herself and carefully examined them under the microscope). If all three of these conditions are met, then we can say the scientist has knowledge that the compound forms rhombic crystals. However, if she was just guessing, or if – despite the compound having rhombic crystals – her belief was based on mistakingly examining a sample of another compound in an adjacent petri dish so that she lacked sufficient grounds for the claim, then this would not count as knowledge (in this philosophical sense) despite the statement itself being correct.
This notion of true, reasoned, belief runs into problems in science where all knowledge claims are considered in principle provisional and so we can never know for certain that any knowledge claim is true (Osborne et al., 2003) – so a strict application of the philosophical notion cannot apply to scientific knowledge. In education (and educational research) we have the additional issue that often when we explore learners' ideas as the foci of our research, what we are interested in is not just what learners genuinely believe and can offer strong grounds for believing – we are also concerned with what they suspect is the case, or what they may believe despite having poor grounds. We are interested in knowing (for example) that a learner thinks that oxidation always involves an addition of oxygen – regardless of how the student came to that view. We are interested in knowing that a student thinks ionic bond formation in a salt he has prepared is due to electron transfers from metal atoms to nonmetal atoms – even though this is incorrect according to the canonical scientific account and is an illogical deduction when considering the neutralisation and evaporation procedure he has followed.
Moreover, we are interested in ‘knowing’ these things even if in CER the grounds for such ‘knowledge’ are necessarily our best interpretations of inferences drawn from messy and complex data sets that only offer indirect access to students' actual thinking processes (Taber, 2013b). It would be possible to limit our use of the term ‘knowledge’ to something akin to the ideal philosophical meaning, and to propose an alternative term to describe ‘what (we think that) learners think is the case, or is possibly the case’, however in practice the term knowledge is already used loosely in education discourse.
In educational work then it is useful to take a wider view of what we mean by knowledge to encompass those mental representations of aspects of the world relevant and important to the work of teaching subjects such as chemistry – some of which will be incorrect from the canonical perspective; some of which will be committed to less strongly by the learner; and some of which will be supported by grounds that would be judged weak or dubious from the scientific viewpoint. It has been suggested that a more useful understanding for supporting research in science education is that in practice the “learner's knowledge refers to what they believe to be the case or simply consider as a viable possibility…the range of notions under current consideration as possibly reflecting some aspect of how the world is” (Taber, 2013b, p. 179). Such a definition needs to be used with care as it admits both highly committed and much less certain beliefs, and indeed sometimes several alternative notions that are logically inconsistent. However, in practice, the large body of research into student ideas about chemical topics does indeed encompass such a catholic range of notions.
The role and development of implicit knowledge in cognition
The cognitive system
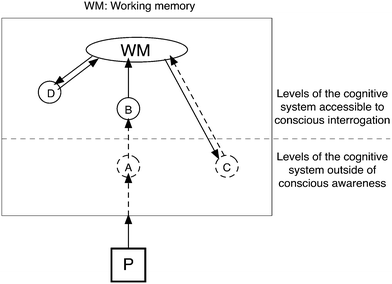
Potential knowledge elements were referred to above as mental representations, a term which acknowledges that a person's experiences lead to changes in the brain. In development during childhood and adolescence these changes can lead to new cognitive abilities (Piaget, 1970/1972). We can understand the human central nervous system as an apparatus that allows us to be responsive to our environment, and in part it does this by allowing us to interpret sensory data. The apparatus is in part a decision-making system – and in order to function effectively it is also a model building system. It is often useful in research to describe and analyse cognition at the system level, that is in terms of the information processing within the cognitive system (represented very simply in Fig. 1). This is seen as a more useful level of description for many purposes than talking in terms of the physical structure of the brain, although it is widely assumed that all the functionality than can be studied at the cognitive system level can in principle (if not always yet in practice) be explained in terms of anatomy/physiology/biochemistry of the tissues from which the system is realised (Taber, 2013b).The cognitive system level of description then acts as a useful bridge between the level of the register of terms commonly used for discussing mental activity (thinking, understanding, knowing, insight, etc. – generally understood, but often imprecise) and the physical level description of synapses and nervous impulses (underpinning cognition, but vastly complex and not generally helpful in considering educational questions). The cognitive system interprets incoming sensory data in terms of its evolving model of the external world (constructed from previous experience of sensing and acting in the world). What is being referred to here is in a broad sense memory, but not limited to those things we are aware we can remember (which are represented by D in Fig. 1).
Conscious experience may be understood as reflecting the higher level of the cognitive system as consciousness is only able to access resources above a certain threshold level within the system (see Fig. 1). The terms ‘higher’ and ‘above’ here are metaphorical (Lakoff and Johnson, 1980a), and are meant in the sense of being ‘further’ (sic) from raw sensual data in terms of processing. Sensory impulses (the representations of sensory information in the nervous system) are relayed through several stages of processing before being represented in the form of percepts that people are aware of. The designation of ‘higher’ as more processed is somewhat arbitrary, and for example ‘deeper’ might be an equally suitable alternative metaphor (i.e.Fig. 1 could equally well be drawn inverted).
A great deal of processing occurs at pre-conscious levels. For example, when we see a laboratory flask, we are conscious of seeing an object. Yet before that is possible a great deal of mental processing has gone on at a preconscious level that has discriminated the object from a complex visual field, regardless of the angle of sight, distance, lighting conditions, background etc. (a flask looks a different shape, size, colour etc. depending upon viewing conditions). Although we can appreciate the processing that must have occurred, we are not aware of it, just as when we hear talk it generally ‘arrives’ in consciousness as a stream of words distinguished from background, and just as people are able to coordinate complex patterns of muscle contractions to, for example, climb stairs, without generally having any conscious awareness of which muscles they are contracting and how they sequence the myriad individual actions. This is represented in Fig. 1 where sensory information about a phenomenon (P) in the outside world is initially processed at a preconscious level and only a preprocessed mental representation (B) is made accessible to consciousness. In vision, for example, the retina act as a kind of sensory interface that converts patterns of photonic stimulation into electrical impulses of the kind the nervous system can process (Taber, 2013b). Components of the cognitive system process these signals and recognise features that are used to generate representations of the outside world. In effect, there are cognitive resources that are able to interpret the sensory signals in terms of the internal model of the world. In a sense these cognitive resources ‘know’ how to interpret certain input patterns to generate mental representations that will make sense to the conscious mind. In effect then there are knowledge elements at work in the brain that are not directly accessible to consciousness (such as A in Fig. 1) but which generate representations (such as B in Fig. 1) that are accessible. Working memory, generally considered to be the focus of conscious thought (Baddeley, 2003), can only access the preprocessed representation (B) and not the raw sensory ‘data’ – the initial representation of the sensory stimulus in the system. As the implicit knowledge elements work below the level of conscious awareness they are normally relied upon without critique. We usually only question the work of these implicit knowledge elements when it is obvious they have got something wrong – such as when we recognise a friend in a public place, but then when they ignore our greetings realise it is not them at all. Fig. 1 is a gross simplification of these processes – as much perceptual data is processed through a number of stages before perception occurs.
As with perception and motor activity, much of what we might label our ‘thinking’ actually occurs out of the reach of conscious awareness. Consider for example creative problem-solving where it is very common for solutions to be experienced as ‘popping into the head’ when the person is actually otherwise engaged – going for a walk, playing sport, watching a movie. A common popular cultural representation is of a light turning on in the head at the moment of insight. Kekulé's report of how he solved the puzzle of the (cyclic) structure of the benzene molecule after a mental image appeared in a reverie and the story of how Archimedes had a moment of insight (leading to his eponymous principle) when taking a bath are perhaps among the most famous examples of such insight in scientific work – although there are many others such as Nobel laureate Barbara McClintock's description of relying on preconscious thinking to solve scientific puzzles (Taber, 2011). Of course a potential solution does not actually ‘pop into the head’, but is rather produced in the brain at a preconscious level, and then presented to (represented in) consciousness. Often the activity is deliberate in the sense that a problem was actively being sought – but the processing is being worked on ‘in the background’ whist we (consciously) think about something else. The thinking involved in finding a potential solution is not accessible to consciousness – only the outcome. This is represented simplistically in Fig. 1 by the activity of the cognitive resource labelled C (a somewhat more nuanced, but still simplified, representation of this process can be found in Taber, 2013b, p. 153, Fig. 7.5).
It might seem to be stretching our usual definition of knowledge to consider the kinds of processes involved in producing insight as drawing upon implicit knowledge – as it suggests we do not always know what we know. However preconscious processes rely upon features of the human cognitive apparatus that act as representations of the external world that we use to interpret experience and determine how to act in the world. These cognitive resources are parts of the way the brain models the world – and in that sense they should be considered implicit knowledge elements. Moreover, implicit does not imply instinctive, as this type of knowledge can be learnt just as explicit knowledge is learnt.
Developing implicit knowledge
The human brain can be understood to modify its structure to represent past experience and so to change its characteristics over time. Such changes do not happen in gross structure, but at the level of synaptic connections – how neurons are connected – which determines how signals are processed within the system. Although this is not understood in great detail, it is widely accepted that learning depends upon such synaptic changes. This same principle applies to both explicit declarative knowledge and implicit knowledge. So, for example, a student who had never heard of niobium might be told that niobium is a metallic element. This information might be learnt, so that after learning the student could respond to the question ‘what is niobium’ with the response ‘a metallic element’, when before learning they would not have not been able to offer any confident answer to the question.† Scientists think that this is facilitated by changes in neural connections (such as modifying connection strengths such that activation of one neuron is more or less likely to trigger activation in another). The extent to which this hypothetical student has simply rote learnt the phrase ‘niobium is a metallic element’ or has integrated it into a network of meanings for concepts such as chemical element and metal would reflect different degrees of synaptic restructuring associated with the learning process – and it is worth noting that this is not a discrete event, but initial learning may be followed by consolidation processes that bring about greater integration over longer time scales (Taber, 2013b).Similar processes seem to occur at the levels of the system that are preconscious as at the ‘higher’ levels where the individual has conscious access (for example remembering that they have been told niobium was a metallic element). Indeed starting very early in life the brain acts as a system capable of abstracting commonalities from experiences to model how the world works. A useful comparison here is with arrays of transistors acting as artificial neural nets which can be tuned (by modifying connection strengths) to ‘learn’. Such simple systems can use feedback to become better at various tasks (perhaps identifying objects from a camera feed) although there is no suggestion of any awareness in the system. The human brain seems to work in a similar way (and indeed is the model for the artificial neutral nets!) but does so without needing any external controller to give feedback on how successful it is being. Rather the brain comes to recognise regularities in experience of the world which are so common that they start to act as, in effect, expectations of future experience, and so are used as templates for organising experience. This takes place automatically from early in life and without any conscious control or awareness.
Approaches to describing implicit knowledge in science learning
The experiential gestalt of causation
In 1980 the cognitive linguist George Lakoff and the philosopher Mark Johnson discussed something they labelled the experiential gestalt of causation to describe how people perceive cause and effect in the world. We come to expect both that events have causes, and that those causes involve an active agent acting on a passive patient using some kind of instrument (Lakoff and Johnson, 1980b). We might imagine a golf ball moving off being understood as the action of a golfer using a golf club – but Lakoff and Johnson's point is that we come to intuitively understand the world working this way even when the three components of the gestalt (the agent, the patient and the instrument) are not clearly obvious. That is, when faced with an event we are likely to implicitly identify (assume) an apparent causative agent and instrument/mechanism, and – because this all occurs pre-consciously – what we become aware of is the gestalt presented to consciousness as an (in effect) explained event.The significance of this idea for learning in the sciences was developed by Björn Andersson (1986) who discussed how many “everyday physical and chemical conceptions” (p. 155) – conceptions that are often at odds with canonical scientific concepts – could be understood as the application of this basic expectation about how the world works. Andersson suggested a whole range of common ‘misconceptions’ in physics and chemistry derived from the action of this implicit knowledge element.
Knowledge-in-pieces and the challenge to the notion of alternative frameworks
The importance of such implicit knowledge elements was also highlighted by those who were critical of some treatments of learners' ideas in reports of alternative conceptions and frameworks (Claxton, 1993; Solomon, 1983, 1994). Descriptions of students' alternative conceptualisations of science concepts were considered by some to over-state the theory-like nature of those conceptions. Just because research elicited alternative ideas in place of the scientific principles and theoretical frameworks established in the science curriculum did not imply that these alternatives had a similar nature or status to the scientific notions they substituted for. That is, children's ideas in science were not just not identical to the scientific principles, but could indeed be of a very different nature: so they did not reflect well-integrated sets of abstract general ideas that would be applied consistently across a well established range of application. Claxton (1993) in particular argued that children's ideas about the natural world were not generally theoretical in nature and often comprised what he termed ‘gut science’. Like Claxton, Solomon (1983) focused on how student thinking was often distinct from mature scientific thinking, highlighting the way people in society commonly adopt a ‘natural attitude’ that lacks the logical and evidential norms of scientific dialogue.These critics were right to point out how learners' knowledge could not be assumed to be like scientific conceptual structures. However they perhaps underplayed the extent to which science learners' alternative conceptualisations could sometimes be complex, well-integrated, coherent and systematically applied. After all, even the most successful scientists began as novice learners, and the literature on learners' alternative ideas in science suggests it is possible to find examples of highly coherent and consistent thinking applied extensively over a domain as well as examples of isolated, incoherent, and unstable conceptions (Taber, 2009).
The lack of coherence and consistency in application of much learners' thinking as reported in studies led to one research team referring to learners as having ‘knowledge-in-pieces’ (diSessa, 1988; Smith et al., 1993). Smith and colleagues wrote of “systems of knowledge with numerous elements and complex substructure that may gradually change, in bits and pieces in different ways” (p. 148). These authors were interested in how the knowledge structure of a novice, characterised as fragmented, could evolve towards expertise, and they highlighted how such expert knowledge had to be constructed from the resources available – the learner's knowledge-in-pieces.
Phenomenological primitives and learning science
In particular Andrea diSessa (1983, 1993) developed an account of a kind of implicit knowledge element labelled as a phenomenological primitive, or a p-prim. diSessa undertook extensive interviews with college physics students and reported that a good deal of student thinking about physical systems and phenomena (that is, what the students reported as explicitly thinking) could be understood as deriving from implicit knowledge elements, p-prims. A few examples of diSessa's candidates for p-prims are shown in Table 1. The argument is that p-prims develop in response to the brain's inherent tendency to make sense of experience by finding patterns and so comprise representations of commonalities in experience.| P-prim | An implicit expectation that |
|---|---|
| Intrinsic or spontaneous resistance | “Especially heavy or large things resist motion” (p. 218) |
| Dying away | “All motion…gradually dies away” (p. 219) |
| Change takes time | “Changes take time to ‘blossom’.” (p. 219) |
As an example, we might consider early childhood experience that effects are often greater when we are close to the source. Of course if we are dealing with early life experiences, the child does not conceptualise in those terms, and does not have the language to do so, but perhaps many experiences of getting closer to a fire, to a loudspeaker, to a desk-lamp, to a spluttering garden hose, and so on lead to experiencing a greater intensity of heat, sound, brightness, splashing and so forth. The response to this could be the development of a p-prim that we might label in such terms as closer-stronger. However the p-prim does not operate verbally but rather acts as a pattern recognition ‘device’ within cognition. If the child plays with some strong magnets, and finds they repel more as they are pushed together this may be recognised as fitting with a good deal of previous experience – the p-prim is activated. The recognition is not a conscious act, but rather leads to experiencing the novel experience with magnets as being familiar (and so ‘natural’) rather than something at odds with expectations that needs an explanation.
It is important to note that p-prims are ontologically quite different from ‘alternative conceptions’ in a number of ways. As a learner is aware of their conceptions and can directly represent them to others (in talk, gestures, drawing, writing, etc.) they can in principle examine them critically and debate them. P-prims are however not consciously accessible but rather part of the process of interpreting our experience to present it to consciousness. This not only makes them harder for researchers to investigate, but also makes them more insidious as they act ‘out of mind’ and prior to us being aware of there being anything to interpret: they are acting in perception rather than conceptualisation. This is an important point which is not well reflected in (necessarily verbal) descriptions such as those in Table 1. diSessa reported his explicit conceptualisations of his own models of learners’ implicit knowledge based on his deliberate interpretation of their explanations. Inevitably, the formal representations of p-prims in academic reports are verbal and explicit – unlike p-prims themselves.
Related to this, p-prims should not be judged right or wrong, canonical or non-canonical in the way conceptions can be. A student who thinks that a sulphur atom can only form two bonds can be considered to have erred and (if this is a stable feature of their thinking) to have an alternative conception. By contrast, implicit knowledge elements are abstractions from patterns in past experience (and in that sense not meaningfully understood as wrong) which act as interpretive resources for making sense of current experience. A learner who has developed a p-prim that motion dies away has simply noticed a salient pattern in her experience that has come to be used as an ‘expectation’ for making sense of the world. It is a reasonable abstraction and will prove very useful in making sense of much further experience in life. However, the expectation may make it more difficult to learn Newton's first law of motion (the principle of inertia) as the idea that a moving object will continue to move at the same velocity unless acted upon by a force does not fit the intuition the learner has developed about the world. This makes it likely that an alternative conception will be developed along the lines that a moving object will come to a stop unless a continuous force is acting to keep it moving.
The alternative conception (which is explicit and can be readily verbalised, and may be used with deliberation, for example in working on school or college physics problems) is non-canonical and can be labelled wrong. The p-prim itself though is quite different in nature, even if this difference is obscured when we necessarily formulate p-prims verbally to discuss them. So p-prims are not wrong or right – but their activation can sometimes support the development of alternative conceptions. However, they can also sometimes support the development of canonical conceptualisations, and Clement et al. (1989, p. 555) recommended the identification of “intuitive knowledge structures” that can act as anchors for the learning of canonical science concepts. Students who are successful in academic studies of science sometimes claim that they find scientific concepts perfectly intuitive (Brock, 2006).
P-prims are important for learning in subjects such as chemistry as they relate to what is familiar and makes sense in our experience. One consequence is that phenomena that activate a well-established p-prim may seem to make sense without further explanation as they fit into the learners' expectations about how the world is. The scientific attitude is to question why things happen as they do and seek theoretical models and explicit fundamental principles. The ‘natural attitude’ (Schutz and Luckmann, 1973) taken in everyday life, however, is to largely accept what seems normal, and only to seek to explore what is novel – what does not fit and so seem to make sense. This may be considered reasonable from an evolutionary perspective, as what is novel in the environment is likely to be worth paying more attention to as a potential threat or opportunity. If students are asked to explain why familiar natural phenomena occur, they often do not have any ready answers as they have just come to take the phenomena for granted as something familiar. The familiar fits into our expectations and so seems to make sense, and so does not need further explanation. Students will often resort to ‘explaining’ the familiar as just being the natural way of things (Watts and Taber, 1996).
It has been noted by some commentators on research into student thinking and learning that one danger of research exploring people's conceptions is that the researcher asks questions about things that the informant actually has never had reason to develop any explicit ideas about. In his work with children Piaget (1929/1973) referred to how a child would often co-operate by ‘romancing’ an answer – making up something on the spur of the moment to meet the social need of responding to a question. It was important therefore to discriminate between children's responses which reflect their well-established thinking from those which are just constructed to meet the needs of an interviewer's strange question. This can apply with older informants as well. So a child who is asked why it is hotter in Summer than Winter and who has never had reason to think about that question may still suggest something that seems viable – perhaps that the earth is closer to the sun in Summer. Such a response will draw upon available cognitive resources (perhaps a p-prim such as the closer-stronger pattern), and because this makes sense this may then become an established idea in the student's thinking if it is not challenged (perhaps by asking about why it is Summer in Australia when it is Winter in Canada).
P-prims are therefore sometimes unhelpful to teachers as when learners (implicitly) recognise that something fits with an existing p-prim they can feel it does not require further explanation, and indeed it may be difficult to shift learners away from that way of thinking when their intuitive notion does not match the scientific account. Conversely, p-prims are resources that can be called upon when learning. So, for example, if teaching about the periodic relationship between ionisation energies and atomic structure, the intuition that being closer means a stronger effect can potentially support our teaching objectives.
Alternative perspectives on implicit knowledge
Although the p-prims theory has become well known (especially in physics education research, PER), there are alternative theories of how learners draw upon implicit knowledge of the world in learning science. Some commentators such as Stella Vosniadou and Michelene Chi do not accept that implicit knowledge is as fragmentary as diSessa's (1993) p-prims, and posit greater coherence and/or structure to at least some of learners' intuitive understandings.Vosniadou (2002) considers that children develop an explanatory system with some coherence to make sense of the physical world, that includes particular ontological and epistemological presuppositions (for example about the nature of objects, and what kind of motion requires a cause), and she refers to this as a framework theory. Vosniadou argues that children interpret new information (whether from their direct experience, or in terms of what they are told) through this initial framework theory – constraining their ability to appreciate scientific models and concepts. Vosniadou considers that by the time students are formally taught physics they will have already organised p-prims into something like a system of informal theories (Vosniadou et al., 2008), so their naive physics will be less piecemeal that the ‘knowledge-in-pieces’ perspective suggests.
Although Vosniadou refers to these naive ideas as ‘theories’ she suggests (Vosniadou, 2014) they may differ from learners' scientific knowledge in terms of being processed through system 1 rather than system 2 (see above). Vosniadou and colleagues (Vosniadou et al., 2008) suggest that children develop framework theories in domains other than physics such as psychology, mathematics and language. Chemistry is not usually considered a discipline where children will spontaneously develop a domain-related framework theory, and perhaps for this reason has not been a strong focus of research in developmental psychology.‡
Chi and her coworkers (e.g.Chi and Slotta, 1993) accept much of the argument diSessa (1993) makes about people developing a robust intuitive physics, which is phenomenological (in the a sense of representing abstraction of experience) and primitive (in that it is not seen to need to be explained), but consider that there is more structure or coherence to this intuitive knowledge than diSessa suggests. Rather Chi argues that people understand the world in terms of an implicit ontology with distinct ‘ontological trees’ (Chi, 2008), relating to such categories as matter, process and mental states. She considers that much of the research into learners' alternative ideas in science can be explained in terms of their implicit ontologies of the world, as new concepts may be developed on the wrong ontological tree. Physical concepts such as light, heat and current may be understood as forms of material substance, rather than as instances of a kind of process – examples of what are understood in science as constraint-based interactions (Chi et al., 1994). Chi argues that miscategorisations of this kind – understanding heat as a material substance for example – act as major impediments to learning the scientific concepts and so tend to be associated with robust alternative conceptions.
Whilst, like diSessa, much of Chi's work has had a particular focus on physical concepts, she has developed her ideas in a ‘domain-general’ direction with a focus on the distinction between direct and emergent processes (Chi, 2005). She argues that emergent processes are commonly mis-categorised by learners as direct processes. A direct process can be analysed as having a linear cause and effect (Chi uses the example of the heart pumping blood) where an emergent process arises from the interaction of several components. Chi uses the example of diffusion where “the mechanism … must be explained in terms of the collective interactive outcomes of all the constituent components” (p. 174). Chi considers that students tend to intuitively interpret processes as being direct (with a straightforward and one-way cause and effect relationship) and so miscategorise and therefore misunderstand emergent processes. This proposal has resonances with Andersson's (1986) argument, referred to earlier, that learners' commonly understand cause in science in terms of an active agent acting on a passive patient.
Wilensky and Resnick (1999) have argued that many problems in learning science relate to learners not appreciating the nature of emergent systems. This is particularly relevant in chemistry where the macroscopic phenomena to be explained often need to be understood as emerging from interacting systems of submicroscopic particles (Stieff and Wilensky, 2003; Rappoport and Ashkenazi, 2008).
A more nuanced understanding of the learners' implicit knowledge
diSessa's (1993) characterisation of p-prims populates the implicit levels of the learners' cognitive system with discrete knowledge elements that act independently in perception and cognition. Chi and Vosniadou and their colleagues claim that not all implicit knowledge is as fragmentary as the p-prims model suggests. Vygotsky (1934/1994) discussed how the learner's spontaneous concepts interacted with verbally taught academic concepts during a process of mutual development. Learners' explicit conceptions are then often best understood as melded concepts that result from – sometimes extensive – interaction between intuition and interpretations of taught concepts (Taber, 2013b).Knowledge may begin ‘in-pieces’, but clearly can be integrated into complexes and systems. An important capability of the cognitive system is to be able to ‘re-represent’ existing elements within new, more synthetic, structures (Karmiloff-Smith, 1996). The work of researchers such as Chi and Vosniadou indicates that such synthetic processes often produce systematic knowledge structures that remain at the implicit level.
Although these authors contrast their accounts with diSessa's (1993) p-prims, it should be noted that diSessa's work was part of a programme of research (Smith et al., 1993) that sought to recognise a greater diversity of types of knowledge element than had previously been the focus of research on science conceptions. That is, p-prims were seen as important elements of cognition, but elements that would be drawn upon in developing other more organised and systemic knowledge components. Chi and Vosniadou make the case that these (relatively) higher level knowledge structures may still often operate pre-consciously. It seems then that the learner's implicit knowledge consists of components at a range of grain sizes – some primitive and discrete, some more complex and organised, yet still operating below conscious awareness.
Applying the implicit knowledge perspective in chemistry education
An important direction for the research programme exploring student thinking in chemistry
Research that explores student thinking is justified in terms of its potential for informing teaching (Taber, 2006, 2009). Among the central assumptions§ of the research programme into the contingent nature of learning in science are that (Taber, 2006: p. 139):• Learners come to science learning with existing ideas about many natural phenomena
• Learners' existing ideas have consequences for the learning of science
•It is possible to teach science more effectively if account is taken of the learners' existing ideas
Alternative conceptions are understood to have various origins (Taber, 2014b) – such as intuitive ideas, false ideas with social currency, inferences from linguistic cues and so forth. Sometimes it may be clear where elicited ideas originate. Common scientifically incorrect thinking about force and motion – that a force is needed to maintain motion – can be understood to be based on interpretations of common experience (Gilbert and Zylbersztajn, 1985). The notion that acids are necessarily hazardous derives from everyday ideas and ways of classifying acids (so orange juice is not considered an acid in everyday discourse). The notion that neutralisation necessarily gives a neutral product seems to be based on a linguistic cue (Schmidt, 1991).
However, in chemistry we find a good many common alternative ideas that do not seem to be based on direct experience of the phenomena, linguistic cues, or common everyday ideas. In particular, when students learn about models of the material world at sub-microscopic scale, they often develop alternative conceptions, and some of these seem very common (see contributions in Gilbert and Treagust, 2009; Tsaparlis and Sevian, 2013). In part this could be explained in terms of the same incorrect or anachronistic ideas being taught by generations of teachers to their students – some of whom then go on to teach the same ideas (Taber and Tan, 2011). This however can at best only be a partial explanation – even if this kind of pedagogic reproduction occurs there has to be a basis for why some technically incorrect ideas are able to be retained (during university studies and teacher preparation courses) and replicated despite being inconsistent with canonical chemistry (cf.Blackmore, 2000). For example, it is very common for students to develop conceptions around the core idea that chemical reactions occur so that atoms can obtain full electron shells/octets (Taber, 2013a) despite this being both inconsistent with canonical chemistry and internally inconsistent (as in many examples where students apply this idea the atoms or ions of the reactants already have the target electronic configurations). There seems to be something inherently attractive about this idea such that it is regularly developed by students, and retained despite subsequent learning about alternative principles more in keeping with scientific accounts. What seems quite likely is that the ‘octet rule principle’ (that chemical processes occur to bring about ‘full’ electron shells) is the result of students interpreting teaching in terms of implicit knowledge elements. These operate at a preconscious level in cognition to match sensory information (including from teaching) to existing well-established patterns such that when the sensory information becomes represented in consciousness it is already perceived in terms of a familiar pattern for how the world is. Although implicit knowledge elements are by nature operating pre-consciously, they can lead to more explicit formal knowledge elements becoming established (Karmiloff-Smith, 1996). That is, explicit conceptions (that can be readily accessed and formulated verbally) are constructed upon a foundation of implicit knowledge elements operating out of the purview of conscious awareness.
The argument from the knowledge-in-pieces perspective, then, is that by the time learners have well established explicit conceptions it becomes more difficult to redirect their thinking. As suggested earlier, this perspective does not see implicit knowledge elements such as p-prims as inherently right or wrong – rather they are understood to be a set of resources that are available to make sense of the world, that the learner is not aware of, but which influence thinking. The p-prims we develop reflect patterns in our experience. Sometimes in academic learning we understand teaching in terms of p-prims that are helpful (so learners readily accept that the closer the electron to the nucleus, the stronger the force to be overcome to remove it) and sometimes they are less helpful (as when a student thinks the earth is nearer the sun in Summer or that chemical change occurs to bring about full electron shells). Given that people develop a repertoire of such implicit knowledge elements, teaching will be more effective when it encourages students to perceive new concepts and principles in terms of those implicit knowledge elements available to the learner that best fit with the canonical scientific model. That is, teachers should seek to teach in ways that lead to learners operating with more productive p-prims in terms of the fit with scientific principles.¶
The challenge for the research programme
Of course whilst this makes good sense in principle, it is not immediately clear how teachers are to do this. To support teachers researchers need to identify the repertoire of p-prims operating in the population and find out much more about how to channel learning in terms of specific p-prims (and avoid the activation of others). The potential here is great – research makes it clear that some alternative conceptions are readily acquired, and very resilient to teaching, so that an approach which offers the potential to avoid such ideas taking hold initially would be a significant contribution to chemistry education. Students are not aware of their p-prims, but they would not need to be – rather research would seek to support teachers by finding ways to present particular curricular material such that it was likely to activate the most productive p-prims.A major problem is that we do not know to what extent different people develop the same set of p-prims. Indeed, identifying p-prims is necessarily difficult because learners cannot report them, and rather they have to be inferred from indirect evidence. diSessa's (1993) seminal work offers a catalogue of candidate p-prims important for physics education based on interviews with physics students. P-prims are not themselves subject-specific – they are rather general purpose elements for making sense of any kind of sensory input. This suggests that diSessa's p-prims could well be operating in the learning of chemistry as well as physics. However, studies undertaken with informants at one educational level and in one area of science cannot be assumed to apply to learners elsewhere. Even if diSessa's suggestions (which were not presented as a final and fully verified list) includes good candidates for p-prims active in science learning, it seems likely that studies in other areas of science would refine the characterisation of some of diSessa's candidates and extend the catalogue with new examples (Taber and García Franco, 2010). Taking this area of work forward in CER is likely to be challenging, but given its potential importance for teaching and learning chemistry there is a strong case for encouraging the CER community to explore this perspective further.
A new direction for CER?
Little work seems to have been done explicitly from this perspective to date in chemistry education. Earlier in this article I used an example from explanations about ionisation energies. Work undertaken with Daniel Tan in Singapore and other colleagues suggests there are common patterns of alternative conceptions found in different student populations in this topic (Tan et al., 2008). We have suggested that the popularity of ideas about the stability of ‘full’ shells and sub-shells may well have an origin in the activation of an implicit knowledge element (Tan and Taber, 2005). Tan undertook studies with pre-university students and graduate trainee chemistry students in an educational context where it was reasonable to consider the different samples as broadly comparable. His research suggests that some alternative conceptions that seem to develop under the influence of p-prim like implicit knowledge elements actually seem to become more common at higher educational levels (Taber and Tan, 2011). However, these studies did not specifically set out to investigate the role of p-prims.Karina Adbo undertook a case study of the developing thinking about key chemical concepts of a 16 year old Swedish student, referred to as Jesper, who presented a rich and somewhat idiosyncratic conceptualisation of the nature of matter at the scale of atoms and molecules (Adbo and Taber, 2014). This work derived from a wider project exploring how Swedish high school students learnt about core chemical concepts (Adbo and Taber, 2009). The project was not specifically focused on the role of implicit knowledge elements, but in analysing Jesper's case there were indications “that Jesper was influenced by strong intuitions about the way the world is: that is, he was unknowingly drawing upon implicit knowledge elements perhaps something like diSessa's (1993) p-prims” (Adbo and Taber, 2014, p. 1124).
One CER researcher who has given serious attention to the role of implicit knowledge in student learning in chemistry is Vicente Talanquer (2006, 2007, 2013). Although Talanquer does not tend to use the label p-prims, his research is informed by a theoretical framework that recognises the role of implicit knowledge elements in student thinking and learning. Talanquer (2006, p. 811) refers to ‘common sense chemistry’ that is “characterized by the use of patterns of reasoning that people unconsciously follow and apply without hesitating or considering other alternatives”. Talanquer frames his work in terms of ‘empirical assumptions’ (or later ‘implicit assumptions’) and ‘reasoning heuristics’ that operate in learners' thinking. Akin to the argument Andersson (1986) made about the experiential gestalt of causation, and diSessa (1993) proposed regarding his p-prims, Talanquer argues that these assumptions and heuristics act at a preconscious level and lead to students' presenting a wide range of distinct alternative conceptions.
Talanquer (2006, p. 813) identified “a set of assumptions about the characteristics of things in the natural world, their behavior and relationships” (see Table 2) as well as a set of commonly used heuristics which seem to operate as habits of mind. Talanquer (2013), working in the United States, found that substantive proportions of a sample of university level students demonstrated essentialist thinking (cf.Table 2) and that in some contexts this did not seem to be reduced with increasing level of study – reflecting what was found in Daniel Tan's studies in Singapore (Taber and Tan, 2011). Whilst Talanquer did not use the terminology of p-prims, his implicit assumptions seem to be quite similar in nature to the kind of implicit knowledge elements diSessa (1993) discusses.
| Implicit assumption | Relevance to understanding chemistry |
|---|---|
| Continuity | Assumes that matter is continuous and similar at different scales – impedes learning about the canonical submicroscopic structural models that are ubiquitous in chemistry |
| Substantialism | Process and interactions are seen as material – so heat may be understood as a kind of fluid, and bonds are assumed to be material links |
| Essentialism | “Objects and materials in the world have an underlying quality or inherent essence” (p. 812) – so properties are assumed to be retained in some form through chemical transformations (e.g. sodium chloride should retain properties that are inherent qualities of sodium and chlorine) |
| Mechanical causality | An active agent is needed to bring about a change in a system – so in a reaction between acid and metal the acid is attacking the metal |
| Teleology | Processes occur to meet some purpose or need – so systems shift because they need to minimise energy |
Perhaps one reason why diSessa's ideas have been influential, whilst not being adopted widely in empirical studies, is the difficulty that diSessa (1993) acknowledged in identifying implicit knowledge elements that learners are not aware of applying. diSessa himself presented and explained a set of 17 heuristics that he felt were useful in identifying p-prims. Researchers wishing to build on diSessa's work need to consider carefully his set of heuristics in the context of both his examples of candidate p-prims, and his detailed accounts of analysing examples of interview transcripts.
One study that did adopt the idea of p-prims in relation to students' ideas in chemistry as part of its conceptual framework was carried out by Alejandra García Franco who interviewed secondary age English students using an interview-about-events (White and Gunstone, 1992) based approach to find out how students explained their observations of a range of phenomena that chemists would explain in terms of reaction, mixing, diffusion etc. The interviews from this study were analysed in two quite distinct ways. This is seen as appropriate when different aspects of the same data may be illuminated by different (but complementary) analytical frameworks (Taber, 2008a).
García Franco's data were coded according to the extent to which learners drew upon particle ideas, and whether such ideas used were canonical or included alternative conceptions. This offered indications of the extent to which students in the sample spontaneously used particle ideas in their explanations, and used them in scientifically acceptable ways (García Franco and Taber, 2009). However the data were also read in the light of diSessa's (1993) heuristics for identifying p-prims with a view to identifying where students appeared to be applying something like p-prims:
So in identifying intuitive knowledge elements that students drew upon in their explanations, we looked for instances in which respondents were able to report features that they found obvious (e.g., rather than inferred from learned theoretical principles) and natural (so self-evident as not to need further explanation) in describing the mechanisms or causes accounting for the phenomena discussed but for which they were not able to spontaneously offer any deeper justification (Taber and García Franco, 2010, p. 110).
The outcome of this analysis was the identification of five themes (see Table 3) which appeared to be good candidates for implicit knowledge elements of the kind diSessa described as p-prims. Taber and García Franco discuss how some of their themes seem to fit (or extend) p-prims suggested by diSessa, whilst some themes seem new. There are some clear links between the themes that derived from the analysis of García Franco's interview data (Table 3) and the assumptions identified by Talanquer from his analysis of literature (Table 2): so ‘component gives property’ appears to fit under ‘essentialism’, and ‘changes require active agents’ and ‘there is one active partner’ both link to Talanquer's ‘mechanical causality’. This type of convergence between different studies is promising, although the different ways of describing and characterising implicit knowledge elements indicates how this area of work requires further development.
| Theme identified | Relevance to understanding chemistry |
|---|---|
| Component gives property | Seeing a substance as being a complex of discrete different qualities (such as colour) acting in isolation |
| Changes require active agents | So mixing only occurs if an agent such as stirring or heating is at work. |
| There is one active partner | Reactions generally involve a more active substance acting on a more passive substance |
| Substances (naturally) react | It is in the nature of some substances to react (so no further level of explanation is needed) |
| Things have a (natural) predetermined configuration | Spreading-out will occur to achieve a kind of balance or other preferred configuration |
Conclusion: progressing the research programme
Work in the learning sciences suggests that implicit knowledge has an important role in learning and other cognitive processes (Karmiloff-Smith, 1996), something that reflects Polanyi's (1962) philosophy with its recognition of the importance of tacit knowledge in science. Work in PER (diSessa, 1988, 1993; Smith et al., 1993; Hammer, 1996) has stressed the importance of reformulating the constructivist programme into learning in science (Taber, 2006, 2009) in terms of a knowledge-in-pieces model that recognises how student thinking commonly draws upon implicit cognitive resources and is not based purely on explicit conceptions.In CER there is extensive work on students' conceptions in chemistry (Kind, 2004; Duit, 2009), but only a little of this work (e.g., Tan and Taber, 2005; Talanquer, 2013; Adbo and Taber, 2014) has to date been strongly influenced by arguments about the importance to the research programme of identifying implicit knowledge elements, such as p-prims, that are active during chemistry learning. One study has sought to identify a range of candidate p-prims operating in student intuitions about chemical phenomena (Taber and García Franco, 2010), but this work was limited to students of secondary age range in a single educational context.
The importance of the arguments about the role of implicit knowledge in learning, and the potential of a better understanding of the action of implicit knowledge for informing chemistry teaching, suggests that this is an area that deserves much more attention. One major criticism of work in the constructivist programme (for a review, see Taber, 2009) has been that the extensive work identifying aspects of student thinking has often not been followed up by effective research into how to use this information in teaching. Challenging well-established alternative conceptions through teaching is often problematic – suggesting that it may be more effective to support teachers in finding ways to teach that recruit the most productive existing intuitions to support the formation of more canonical initial conceptions.
A complication here is that even if alternative conceptions commonly reflect the action of implicit knowledge, not all alternative conceptions reported in chemistry learning seem to be resistant to instruction (Taber, 1995) and this needs to be better understood. Research is needed to characterise those implicit knowledge elements that seem to act as ‘hard core’ assumptions (Lakatos, 1970; cf.Talanquer, 2006) that are taken for granted and act insidiously within the conceptual ecologies of learners and so lead to those tenacious alternative conceptions that largely survive the teaching intended to challenge them (Taber and Tan, 2011). For example, the literature reviewed here motivates the question of whether there is a difference between the more tenacious alternative conceptions operating in chemistry learning and the more labile alternative conceptions in terms of either being linked to a ‘framework theory’ (Vosniadou et al., 2008) or reflecting misclassification in terms of learners’ ontological trees (Chi, 2008).
The main focus in this article has been on how implicit knowledge may support or interfere with conceptual learning. However, as suggested earlier, Polanyi (1962) emphasised how tacit knowledge was important in laboratory work, as scientists build up a store of procedural knowledge that allows them to become fast and efficient in practical operations. This could also be a useful focus of CER to find out how learners come to automate complex procedures, and whether modifications of learning environments or practical instruction can support this process. There is a link here with studies on expertise, as experts come to quickly and effectively make decisions and spot solutions to problems, which may involve the coordination of both ‘system 1’ and ‘system 2’ processing (van Merriënboer, 2013), and there may well be scope here for research informing teaching in areas such as interpreting spectra and suggesting synthetic routes.
Of course this will be a highly ambitious direction for the research programme. Eliciting knowledge that learners are not aware they have, and which is not represented verbally, is highly problematic (Taber, 2013b). Furthermore, there are no assurances that different learners will develop sufficiently similar sets of p-prims (or other implicit knowledge elements) to allow researchers to suggest general classroom strategies. Even if this is the case, the argument that it will be possible to develop viable classroom strategies for teaching chemistry which can effectively recruit students' implicit knowledge productively is yet to be tested in practice. Despite these challenges, the current state of our understanding of how learning occurs strongly suggests that this will be an important area of work in responding to demands for more evidence-based chemistry teaching (Gilbert et al., 2004).
References
- Adbo K. and Taber K. S., (2009), Learners' mental models of the particle nature of matter: a study of 16 year-old Swedish science students, Int. J. Sci. Educ., 31(6), 757–786, DOI: http://10.1080/09500690701799383.
- Adbo K. and Taber K. S., (2014), Developing an Understanding of Chemistry: a case study of one Swedish student's rich conceptualisation for making sense of upper secondary school chemistry, Int. J. Sci. Educ., 36(7), 1107–1136, DOI: http://10.1080/09500693.2013.844869.
- Andersson B., (1986), The experiential gestalt of causation: a common core to pupils' preconceptions in science, Eur. J. Sci. Educ., 8(2), 155–171.
- Baddeley A. D., (2003), Working memory: looking back and looking forward, Nat. Rev. Neurosci., 4(10), 829–839.
- Bhaskar R., (1981), Epistemology, in Bynum W. F., Browne E. J. and Porter R. (ed.), Macmillan Dictionary of the History of Science, London: The Macmillan Press, p. 128.
- Blackmore S., (2000), The power of memes, Sci. Am., 52–61.
- Brock R., (2006), Intuition and Integration: Insights from intuitive students, MPhil thesis, Cambridge: University of Cambridge.
- Chi M. T. H., (2005), Commonsense Conceptions of Emergent Processes: Why Some Misconceptions Are Robust, J. Learn. Sci., 14(2), 161–199, DOI: http://10.1207/s15327809jls1402_1.
- Chi M. T. H., (2008), Three types of conceptual change: belief revision, mental model transformation, and categorical shift, in Vosniadou S. (ed.), International Handbook of Research on Conceptual Change. New York: Routledge, pp. 61–82.
- Chi M. T. H. and Slotta J. D., (1993), The Ontological Coherence of Intuitive Physics, Cognition instruct., 10(2&3), 249–260.
- Chi M. T. H., Slotta J. D. and de Leeuw N., (1994), From things to processes; a theory of conceptual change for learning science concepts, Learn. Instr., 4, 27–43.
- Claxton G., (1993), Minitheories: a preliminary model for learning science, in P. J. Black and Lucas A. M. (ed.), Children's Informal Ideas in Science, London: Routledge, pp. 45–61.
- Clement J., Brown D. E. and Zietsman A., (1989), Not all preconceptions are misconceptions: finding ‘anchoring conceptions’ for grounding instruction on students' intuitions, Int. J. Sci. Educ., 11(special issue), 554–565.
- Collins H., (2010), Tacit and Explicit Knowledge, Chicago: The University of Chicago Press.
- diSessa A. A., (1983), Phenomenology and the evolution of intuition, in Gentner D. and Stevens A. L. (ed.), Mental Models, Hillsdale, New Jersey: Lawrence Erlbaum Associates, pp. 15–33.
- diSessa A. A., (1988), Knowledge in peices, in Forman G. and Pufall P. (ed.), Constructivism in the Computer Age, New Jersey: Lawrence Erlbaum Publishers.
- diSessa A. A., (1993), Towards an epistemology of physics, Cognition instruct., 10(2&3), 105–225.
- Driver R. and Easley J., (1978), Pupils and paradigms: a review of literature related to concept development in adolescent science students, Studies in Science Education, 5, 61–84.
- Duit R., (2009), Bibliography – Students' and Teachers' Conceptions and Science Education, Kiel, Germany: IPN – Leibniz Institute for Science and Mathematics Education.
- Evans J. S. B. T., (2008), Dual-Processing Accounts of Reasoning, Judgment, and Social Cognition, Annu. Rev. Psychol., 59(1), 255–278, DOI: http://10.1146/annurev.psych.59.103006.093629.
- García Franco A. and Taber K. S., (2009), Secondary students' thinking about familiar phenomena: learners' explanations from a curriculum context where ‘particles’ is a key idea for organising teaching and learning, Int. J. Sci. Educ., 31(14), 1917–1952, DOI: http://10.1080/09500690802307730.
- Gazzaniga M. S., Fendrich R. and Wessinger C. M., (1994), Blindsight Reconsidered, Curr. Dir. Psychol. Sci., 3(3), 93–96.
- Gilbert J. K. and Treagust D. F. (ed.), (2009), Multiple Representations in Chemical Education, Dordrecht: Springer.
- Gilbert J. K. and Zylbersztajn A., (1985), A conceptual framework for science education: the case study of force and movement, Eur. J. Sci. Educ., 7(2), 107–120.
- Gilbert J. K., de Jong O., Justi R., Treagust D. F. and Van Driel J. H. (ed.), (2002), Chemical Education: Research-based Practice, Dordrecht: Kluwer Academic Publishers BV.
- Gilbert J. K., Justi R., Van Driel J. H., de Jong O. and Treagust D. F., (2004), Securing a future for chemical education, Chem. Educ. Res. Pract., 5(1), 5–14.
- Gilbert J. K., Osborne R. J. and Fensham P. J., (1982), Children's science and its consequences for teaching, Sci. Educ., 66(4), 623–633.
- Hammer D., (1996), Misconceptions or p-prims: How may alternative perspectives of cognitive structure influence instructional perceptions and intentions? J. Learn. Sci., 5(2), 97–127.
- Hofstein A. and Kind P., (2012), Learning in and from science laboratories, in Fraser B. J., Tobin K. and McRobbie C. J. (ed.), Second International Handbook of Science Education, Netherlands: Springer, vol. 24, pp. 189–207.
- Karmiloff-Smith A., (1996), Beyond Modularity: A developmental perspective on cognitive science, Cambridge, Massachusetts: MIT Press.
- Kind V., (2004), Beyond Appearances: Students' misconceptions about basic chemical ideas, 2nd edn, London: Royal Society of Chemistry.
- Kuhn T. S., (1959/1977), The essential tension: tradition and innovation in scientific research, in Kuhn T. S. (ed.), The Essential Tension: Selected studies in scientific tradition and change, Chicago: University of Chicago Press, pp. 225–239.
- Lakatos I., (1970), Falsification and the methodology of scientific research programmes, in Lakatos I. and Musgrove A. (ed.), Criticism and the Growth of Knowledge, Cambridge: Cambridge University Press, pp. 91–196.
- Lakoff G. and Johnson M., (1980a), The metaphorical structure of the human conceptual system, Cognitive Sci., 4(2), 195–208.
- Lakoff G. and Johnson M., (1980b), Metaphors we live by, Chicago: University of Chicago Press.
- Matthews M. R., (2002), Constructivism and Science Education: A Further Appraisal, J. Sci. Educ. Technol., 11(2), 121–134.
- Medawar P. B., (1963/1990), Is the scientific paper a fraud? in Medawar P. B. (ed.), The Threat and the Glory, New York: Harper Collins, pp. 228–233; (Reprinted from: The Listener, vol. 70: 12th September, 1963).
- Newton P., Driver R. and Osborne J., (1999), The place of argumentation in the pedagogy of school science, Int. J. Sci. Educ., 21(5), 553–576.
- Osborne J., Collins S., Ratcliffe M., Millar R. and Duschl R., (2003), What “ideas-about-science” should be taught in school science? A Delphi study of the expert community, J. Res. Sci. Teach., 40(7), 692–720, DOI: http://10.1002/tea.10105.
- Piaget J., (1929/1973), The Child's Conception of The World (J. Tomlinson & A. Tomlinson, Trans.), Granada: St. Albans.
- Piaget J., (1970/1972), The Principles of Genetic Epistemology (W. Mays, Trans.), London: Routledge & Kegan Paul.
- Polanyi M., (1962), Personal Knowledge: Towards a post-critical philosophy (Corrected version ed.), Chicago: University of Chicago Press.
- Polanyi M., (1962/1969), The unaccountable element in science, in Greene M. (ed.), Knowing and Being: Essays by Michael Polanyi, Chicago: University of Chicago, pp. 105–120.
- Polanyi M., (1970), The logic of tacit inference, in Crosson F. J. (ed.), Human and Artificial Intelligence, New York: Appleton-Century-Crofts, pp. 219–240.
- Rappoport L. T. and Ashkenazi G., (2008), Connecting Levels of Representation: emergent versus submergent perspective, Int. J. Sci. Educ., 30(12), 1585–1603, DOI: http://10.1080/09500690701447405.
- Schmidt H.-J., (1991), A label as a hidden persuader: chemists' neutralization concept, Int. J. Sci. Educ., 13(4), 459–471.
- Schutz A. and Luckmann T., (1973), The Structures of the Life-World (R. M. Zaner & H. T. Engelhardt, Trans.), Evanston, Illinois: Northwest University Press.
- Smith J. P., diSessa A. A. and Roschelle J., (1993), Misconceptions reconceived: a constructivist analysis of knowledge in transition, J. Learn. Sci., 3(2), 115–163.
- Solomon J., (1983), Learning about energy: how pupils think in two domains, Eur. J. Sci. Educ., 5(1), 49–59, DOI: http://10.1080/0140528830050105.
- Solomon J., (1994), The rise and fall of constructivism, Studies in Science Education, 23, 1–19.
- Stieff M. and Wilensky U., (2003), Connected Chemistry—Incorporating Interactive Simulations into the Chemistry Classroom, J. Sci. Educ. Technol., 12(3), 285–302.
- Taber K. S., (1995), Development of Student Understanding: A Case Study of Stability and Lability in Cognitive Structure, Research in Science & Technological Education, 13(1), 87–97.
- Taber K. S., (2002), Chemical Misconceptions – Prevention, Diagnosis and Cure: Theoretical background, vol. 1, London: Royal Society of Chemistry.
- Taber K. S., (2006), Beyond Constructivism: the Progressive Research Programme into Learning Science, Studies in Science Education, 42, 125–184.
- Taber K. S., (2008a), Of Models, Mermaids and Methods: The role of analytical pluralism in understanding student learning in science, in Eriksson I. V. (ed.), Science Education in the 21st Century, Hauppauge, New York: Nova Science Publishers, pp. 69–106.
- Taber K. S., (2008b), Towards a curricular model of the nature of science, Sci. Educ., 17(2–3), 179–218, DOI: http://10.1007/s11191-006-9056-4.
- Taber K. S., (2009), Progressing Science Education: Constructing the scientific research programme into the contingent nature of learning science, Dordrecht: Springer.
- Taber K. S., (2011), The natures of scientific thinking: creativity as the handmaiden to logic in the development of public and personal knowledge, in Khine M. S. (ed.), Advances in the Nature of Science Research – Concepts and Methodologies, Dordrecht: Springer, pp. 51–74.
- Taber K. S., (2013a), A common core to chemical conceptions: learners' conceptions of chemical stability, change and bonding, in Tsaparlis G. and Sevian H. (ed.), Concepts of Matter in Science Education, Dordrecht: Springer, pp. 391–418.
- Taber K. S., (2013b), Modelling Learners and Learning in Science Education: Developing representations of concepts, conceptual structure and conceptual change to inform teaching and research, Dordrecht: Springer.
- Taber K. S., (2014a), Prior Knowledge, in Gunstone R. (ed.), Encyclopedia of Science Education, Berlin-Heidelberg: Springer-Verlag.
- Taber K. S., (2014b), Student Thinking and Learning in Science: Perspectives on the Nature and Development of Learners' Ideas, New York: Routledge.
- Taber K. S. and García Franco A., (2010), Learning processes in chemistry: Drawing upon cognitive resources to learn about the particulate structure of matter, J. Learn. Sci., 19(1), 99–142.
- Taber K. S. and Tan K. C. D., (2011), The insidious nature of ‘hard core’ alternative conceptions: implications for the constructivist research programme of patterns in high school students' and pre-service teachers' thinking about ionisation energy, Int. J. Sci. Educ., 33(2), 259–297, DOI: http://10.1080/09500691003709880.
- Talanquer V., (2006), Common sense chemistry: a model for understanding students' alternative conceptions, J. Chem. Educ., 85(5), 811–816.
- Talanquer V., (2007), Explanations and Teleology in Chemistry Education, Int. J. Sci. Educ., 29(7), 853–870.
- Talanquer V., (2013), How do students reason about chemical substances and reactions? in Tsaparlis G. and Sevian H. (ed.), Concepts of Matter in Science Education, Dordrecht: Springer, pp. 331–346.
- Tan K.-C. D. and Taber K. S., (2005), What comes after stable octet? Stable sub-shell!, Journal of Science and Mathematics Education in Southeast Asia, 28(1), 81–102.
- Tan K.-C. D., Taber K. S., Liu X., Coll R. K., Lorenzo M., Li J., Chia L. S., (2008), Students' conceptions of ionisation energy: a cross-cultural study, Int. J. Sci. Educ., 30(2), 263–283, DOI: http://10.1080/09500690701385258.
- Treagust D. F., (1995), Diagnostic assessment of students' science knowledge, in Glynn S. M. and Duit R. (ed.), Learning Science in the Schools: Research Reforming Practice, Mahwah, New Jersey: Lawrence Erlbaum Associates, pp. 327–346.
- Treagust D. F., (2006), Diagnostic assessment in science as a means to improving teaching, learning and retention, Paper presented at the UniServe Science Assessment Symposium Proceedings, http://openjournals.library.usyd.edu.au/index.php/IISME/article/view/6375.
- Tsaparlis G. and Sevian H. (ed.), (2013), Concepts of Matter in Science Education, Dordecht: Springer.
- van Eijck M. and Roth W.-M., (2007), Keeping the local local: recalibrating the status of science and Traditional Ecological Knowledge (TEK) in education, Sci. Educ., 91(6), 926–947.
- van Merriënboer J. J. G., (2013), Perspectives on problem solving and instruction, Comput. Educ., 64(0), 153–160, DOI: http://10.1016/j.compedu.2012.11.025.
- Vosniadou S., (2002), On the Nature of Naïve Physics, in Limón M. and Mason L. (ed.), Reconsidering Conceptual Change: Issues in Theory and Practice, Netherlands: Springer, pp. 61–76.
- Vosniadou S., (2014), Examining cognitive development from a conceptual change point of view: the framework theory approach, Eur. J. Dev. Psychol., 1–17, DOI: http://10.1080/17405629.2014.<?ccdc_no 921153?><?ccdc_no 921153?>921153<?ccdc END?><?ccdc END?>.
- Vosniadou S., Vamvakoussi X. and Skopeliti I., (2008), The framework theory approach to the problem of conceptual change, in Vosniadou S. (ed.), International Handbook of Research on Conceptual Change, New York: Routledge, pp. 3–34.
- Vygotsky L. S., (1934/1994), The development of academic concepts in school aged children, in van der Veer R. and Valsiner J. (ed.), The Vygotsky Reader, Oxford: Blackwell, pp. 355–370.
- Watts M. and Taber K. S., (1996), An explanatory gestalt of essence: students' conceptions of the ‘natural’ in physical phenomena, Int. J. Sci. Educ., 18(8), 939–954.
- Wellman H. M., (2011), Developing a theory of mind, in Goswami U. (ed.), The Wiley-Blackwell Handbook of Childhood Cognitive Development, 2nd edn, Chichester, West Sussex: Wiley-Blackwell, pp. 258–284.
- White R. T. and Gunstone R. F., (1992), Probing Understanding, London: Falmer Press.
- Wilensky U. and Resnick M., (1999), Thinking in Levels: A Dynamic Systems Approach to Making Sense of the World, J. Sci. Educ. Technol., 8(1), 3–19.
Footnotes |
| † Learning is taken here to be a change in the behavioural repertoire, in the sense that after learning some new behavioural option (e.g. stating niobium is a metallic element) becomes possible or likely that was not available (or at least not likely to be activated) before (Taber, 2009). |
| ‡ A project to explore ‘Young Children's Reasoning About Everyday Chemistry’, funded by the Leverhulme Foundation, is underway at the University of Cambridge and University College London, UK. |
| § In terms of Lakatos' (1970) notion of a scientific research programme these are referred to as hard core assumptions: those assumptions which are taken for granted and not questioned within the research programme. |
| ¶ In terms of Lakatos' (1970) notion of a scientific research programme, the knowledge-in-pieces perspective can be understood as a ‘refutable variant’ of the (‘constructivist’) research programme into the contingent nature of learning in science - that is a particular theoretical idea consistent with the hard core commitments of the programme and suggesting a particular direction for research (Taber, 2009). |
| This journal is © The Royal Society of Chemistry 2014 |