Creating semantic waves: using Legitimation Code Theory as a tool to aid the teaching of chemistry
Margaret A. L.
Blackie
Department of Chemistry and Polymer Science, Private Bag X1, Matieland, South Africa. E-mail: mblackie@sun.ac.za; Fax: +27-21-808-3360; Tel: +27-21-808-3353
First published on 25th August 2014
Abstract
This is a conceptual paper aimed at chemistry educators. The purpose of this paper is to illustrate the use of the semantic code of Legitimation Code Theory in chemistry teaching. Chemistry is an abstract subject which many students struggle to grasp. Legitimation Code Theory provides a way of separating out abstraction from complexity both of which provide substantial challenges to students. These are termed semantic gravity (degree of abstraction) and semantic density (degree of complexity). These ideas are then illustrated using chemical examples in order to demonstrate how they may aid the teaching of chemistry. There is a second pedagogical device which Maton, the developer of Legitimation Code Theory, calls ‘semantic waves’. This is also discussed in the context of chemistry education. The semantic code could be applied to chemistry at all levels.
Introduction
Whenever the inevitable question ‘So, what do you do?’ is offered at a cocktail party I usually offer the answer ‘I am an academic’ rather than ‘I am a chemist’. It doesn't take a trained psychologist to recognise there are probably important identity issues wrapped up in my answer, which could well be fruitfully explored. However, my motivations for the answer are usually to avoid (or at least delay) the inevitable responses of the glazing over of the eyes with the occasional commentary on how much the respondent hated chemistry at school.I have no evidence to support this, but I suspect that part of the reason that so many people struggle with chemistry is that it is a profoundly abstract subject. It is one of the more ‘hidden’ sciences. As a subject in its own right, it took far longer to emerge than the closely related disciplines of physics and biology. This is precisely because the molecular or atomic understanding of matter is neither intuitive nor obvious to the casual observer.
Chemistry is not an easy subject to teach for a number of different reasons which have been discussed in detail by Taber (2001). The work of Johnstone (1982) on pointing out that there are three levels of teaching and learning chemistry, the macroscopic, the microscopic and the symbolic, has been tremendously useful at helping chemistry educators in getting to grips with some of the challenges. Nonetheless, as the science progresses, so too do the challenges in chemistry education (Taber, 2013). A solid grasp of ‘undergraduate chemistry’ requires a skill set which varies from the capacity to provide mathematical proofs to understanding organic reaction mechanisms. Suffice to say, for the purposes of this paper, chemistry is both scientifically and pedagogically complex (Taber, 2013) and it is profoundly abstract.
In this paper I wish to describe an aspect of Legitimation Code Theory (LCT) (Maton, 2014) which I have found to be helpful and illuminating in the task of chemistry education. It has been helpful to me as a teacher, as I have found myself becoming a little more cognizant of the degree of abstraction present in the discipline of chemistry. It has also provided a framework within which I can tease out the different kinds of knowledge which are required for mastery of the subject. There are other tools which have been developed which are aimed at a similar goal, Johnstone's triplet to name just one (Johnstone, 1982; Taber, 2013) Nonetheless, I have found this particular framework to be more useful than others I have used. There are several reasons for this, not least of which is that this framework is not a particular solution to a particular misconception, but rather because it offers a framework which can be applied across any topic (as will be briefly illustrated). Furthermore, this framework has forced me to think about the ways in which I present concepts and the language I use when I am teaching. It has also encouraged me to pay a little more attention to the puzzled looks on the faces of the students. The temptation to ‘power through’ has diminished, and more often than not the extra few minutes used in finding a slightly simpler way of explaining the concept has paid dividends. Moreover, it provides me with two distinct kinds of ‘simplification’, both of which are useful and necessary.
It is important to state clearly at this point that the way in which I am presenting the approach and, indeed, the way in which I have used it so far, has been within sections or topics within an established curriculum. I believe that these ideas could be equally useful in a traditional curriculum of any chemistry domain, as it would be in the kind of spiral curriculum described by Bretz and co-workers (Grove et al., 2008). It may also be applicable across the spectrum from traditional lecture style presentations and peer-to-peer learning (Ryan, 2013).
This paper is divided into several sections. Firstly, a recognition of the complexity of language in the discipline of chemistry. Secondly, a brief description of Legitimation Code Theory including some illustrations of where it has been successfully used as a pedagogical tool in other disciplines. Thirdly, drawing on chemical examples to flesh out the manner in which the theory can be usefully employed in the teaching of chemistry. Finally, some observations and reflections on some of the aspects of the implementation of this approach. As my own speciality is teaching organic chemistry, the majority of the examples will come from this branch of chemistry. I have tried to weave together introducing the ideas of Legitimation Code Theory and the use of examples from the chemistry courses I teach. My intention in so-doing is to aid the reader's familiarization with pedagogical concepts which they may not have encountered before.
Staking out the challenge
As Chomsky pointed out so clearly, the use of language is a characteristic of human beings (Chomsky, 2000). It is language which affords the communication of complex and abstract ideas. Without language much technical and scientific development would be simply impossible. Yet the use of language creates a major hurdle to so many students in chemistry courses (Song and Carheden, 2014). One aspect to this hurdle is the presumption that students mean what we mean when we use a particular term. The very fact that I am using the word ‘term’ in the previous sentence rather than ‘word’ emphasizes the point. Many of the words we use unconsciously in chemistry as expert chemists have everyday meanings that we do not intend to imply, and the terms we use have specific meanings which are precise and not open to interpretation (Song and Carheden, 2014). The idea that the language of chemistry is complex and challenging is nothing new. There is evidence to support the notion that the use of this language by students in conversation aids their understanding of both the vocabulary and the subject (Reingold, 2005). But there is a further problem in chemistry because it is not only the language (which will include the use of symbols), but concepts which are dense with meaning. Lavoisier, who developed a systematic nomenclature for chemical reactions, writes in the preface of his influential work, Elements of Chemistry:“Thus, while I thought myself employed only in forming a Nomenclature, and while I proposed to myself nothing more than to improve the chemical language, my work transformed itself by degrees, without my being able to prevent it, into a treatise upon the Elements of Chemistry. The impossibility of separating the nomenclature of a science from the science itself, is owing to this, that every branch of physical science must consist of three things; the series of facts which are the objects of the science, the ideas which represent these facts, and the words by which these ideas are expressed. Like three impressions of the same seal, the word ought to produce the idea, and the idea to be a picture of the fact. And, as ideas are preserved and communicated by means of words, it necessarily follows that we cannot improve the language of any science without at the same time improving the science itself; neither can we, on the other hand, improve a science, without improving the language or nomenclature which belongs to it. However certain the facts of any science may be, and, however just the ideas we may have formed of these facts, we can only communicate false impressions to others, while we want words by which these may be properly expressed.” (Lavoisier, 1790).
This interplay between ‘words’, ‘ideas’ and ‘facts’, to use Lavoisier's terms, is at the heart of the challenge of teaching chemistry. As the research on ‘teaching chemistry as a second language’ has illustrated, the challenge is not simply that the learning of chemistry is a new language (Song and Carheden, 2014), but rather we must delve into the world of semiotics where the very thing that we are trying to describe has no experiential equivalent in the real world (Taber, 2013; Song and Carheden, 2014).
Legitimation Code Theory
In recent years the development of the ideas of semantic gravity (SG) and semantic density (SD), the semantic code has emerged as part of Legitimation Code Theory (LCT) (Maton, 2014) which is built on a foundation of the work of Bernstein and Bordieu and social realism. Semantics is rooted explicitly in the Bernsteinian idea of horizontal and hierarchical knowledge structures (Bernstein and Solomon, 1999). According to this theory the humanities tend to be horizontal knowledge structures and the natural sciences hierarchical knowledge structures. Any knowledge area has a specific vocabulary which condenses complex ideas into short phrases. In a hierarchical knowledge structure that condensation may require the use of knowledge appropriated at a much lower level (Maton, 2014). For example, in teaching organic chemistry it is presumed that students understand the meaning of a molecular formula, no time is spent explaining what H2SO4 means. We presume that students could identify H2SO4 as sulfuric acid, that this is a strong acid i.e. it has a low pKa value and the implications of what that means. Chemistry, therefore, would be a hierarchical knowledge structure as defined by this framework (Maton, 2014) precisely because a thorough understanding of any aspect of chemistry rests on a massive bulk of unpinning theory, all of which must be assimilated to some degree before any real understanding can be achieved.There is a complex sociological argument which progresses from Bernstein to LCT (Semantics) and is fully developed by Maton (2011, 2014). This paper is aimed primarily at chemistry educators, so I will not expound further on this here, but Maton's recent book ‘Knowledge and Knowers’ (Maton, 2014) will be a useful resource for those who which to pursue the sociological theory. For the purposes of this paper, the ideas of semantic gravity and semantic density coupled with illustrations of the manner in which these concepts can facilitate learning is all that is necessary to use these tools to enhance chemistry education. Herein, I illustrate the manner in which failure to take cognisance of these concepts causes difficulty for chemistry students.
Maton (2011) describes semantic gravity as ‘the degree to which meaning relates to context’ and it can be stronger or weaker i.e. semantic gravity is related to the degree of abstraction. Semantic density is ‘the degree to which meaning is condensed within symbols (terms, concepts, phrases, expressions, gestures, etc.)’ (Maton, 2011)i.e. semantic density is related to the degree of complexity. These two factors are independent of one another and may be relatively stronger or weaker. Maton uses orthogonal axes to represent these, Fig. 1. Note the reversal of the + and − on the semantic gravity axis where a weaker semantic gravity is taken to mean something that is more abstract.
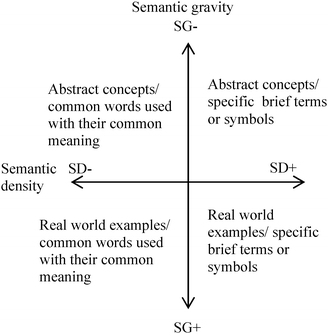 | ||
| Fig. 1 The relationship between semantic gravity and semantic density (Maton, 2011). | ||
The ideas of semantic gravity and semantic density and the related concept of semantic waves (all of which are explained in greater detail herein) have been used to good effect in the context of teaching subjects across the epistemological spectrum for example, journalism (Kilpert and Shay, 2013), nursing (McNamara, 2010), English (Macken-Horarik, 2011) and history (Macnaught et al., 2013; Matruglio et al., 2013). For readers of this paper, the use of these ideas within both physics (Georgiou et al., 2014) and biology education (Macnaught et al., 2013) will be most useful.
Applying the theory to chemistry
I have stated this before, but I think that it is worth repeating again as we try to apply this particular theory to the teaching of chemistry. We do not observe chemistry on a molecular level in our environment. It is not surprising that the science of chemistry was developed a good deal later than mechanics or biology. Furthermore, the language of chemistry is not simply the appropriate usage of specialist words. The vocabulary and symbols all represent entities and processes which we cannot directly observe without the use of instruments. This is to say that a chemical view of the world is not intuitive. In terms of Maton's four quadrants then, the entire subject of chemistry resides in the upper right hand quadrant if the scheme were to be applied to all subjects offered by most universities. Nonetheless, there is still some variation within that quadrant, and the attempt to ground our explanations by the use of simplified terms and more concrete examples is still valuable. Provided, of course, that our simplification does not create unintended misconceptions as has been so valuably highlighted by Taber (2001).If we consider the example:
| NaCl(s) → NaCl(aq) |
The simple chemical equation given above is an example of weak semantic gravity and high semantic density. SG−/SD+ i.e. the upper right hand quadrant. An abstract concept, dissolution, is summarized in element and state symbols. The concept of dissolution has a weak semantic gravity. Understanding dissolution requires at least an understanding of ionic bonding, polar covalent bonding and intermolecular forces. This is an abstract concept – we observe the salt disappearing and we can prove that there have been changes to the water solution as a result of the presence of the salt, but the idea of the ions separating and becoming solvated emerges from trying to make sense of the data, not through direct sensory observation. Dissolution has weak semantic gravity. A full understanding of the process of dissolution is not possible through inference from simple observation. For example, it is not clear that the process of dissolution of table salt in water is not identical to that which happens to sugar. It is only on the application of an electrical current that the chemist will notice that there is something fundamentally different between the two. The element and state symbols have high semantic density. This means that there is a large amount of information condensed into discipline specific vocabulary or symbols. A person without any chemistry background would not be able to intuit the meaning of the equation. Likewise the movement from the bottom left hand quadrant to the bottom right hand quadrant requires the use of appropriate symbols. The bottom right hand quadrant may also be exemplified by the proper use of appropriate vocabulary (Fig. 2).
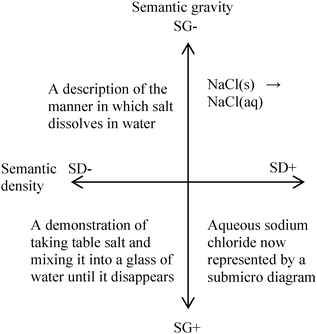 | ||
| Fig. 2 Applying the ideas of semantic gravity and semantic density to the dissolving of sodium chloride in water. | ||
A university level chemistry student could write a paragraph describing the changes observed and the physical and chemical processes involved. A university lecturer could give an entire lecture elaborating on that paragraph. The point is simply that there is a great deal of information embedded in 16 characters.
The challenge in any teaching context is to move from the lower left hand quadrant into the upper right hand quadrant. However, moving obliquely (that is trying to develop the specialist language and the level of conceptual abstraction at the same time) causes problems. The research carried out on teaching chemistry as a second language can be viewed as an attempt to address the issue of increasing semantic density. But it must be noted again, that learning chemistry is a great deal more than just learning new words. It is worth remembering that the science itself is abstract. In terms of chemistry education we remain deeply indebted to researchers such as Johnstone (2000) and Taber (2013) who have helped unpack this complexity.
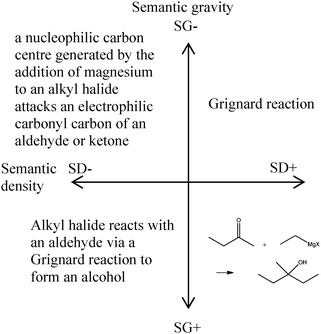
A further point which Maton makes is the use of ‘semantic waves’ (Maton, 2009). This is to say, when one introduces a new term to make sure that one uses the new term interspersed with simpler more familiar, albeit less technically accurate or succinct ways of describing the same phenomenon. This idea of the semantic waves will be returned to later in the paper in more detail. For example, when introducing the idea of SN2 reactions, many introductory organic chemistry textbooks after explaining the symbol, then use the symbol exclusively. I suspect that most of us who teach these sections likewise simply use the term SN2 presuming that because we have explained the symbol once that we can use it without bothering again to speak about a nucleophilic substitution reaction involving two molecules in the rate determining step. This example is relatively trivial because the symbol is almost self-explanatory. Nonetheless, we tend to do exactly the same thing when we introduce named reactions. Once we have explained what a Grignard reagent is, we use the phrase ‘Grignard reaction’ without further thought presuming that the students understand exactly what we are talking about. For Maton, it would be important to shift between the more technical and the less technical several times before adopting the more chemically precise term exclusively (Maton, 2009). I am now going to use the Grignard reaction to give an example of how Maton's framework can be applied.
Extended example using the reaction of a Grignard reagent
An important point to note is that I am using semantic gravity and semantic density scales in a relative way, rather than an absolute way. By this I mean the point where the axes cross one another will vary depending on the person's foundational knowledge. At this stage I am presuming that we are trying to introduce the Grignard reaction to a class of first or second year undergraduate students who have already mastered the fundamentals of chemistry.The Grignard reaction, when it is introduced, is taught as a nucleophilic addition reaction to the carbonyl carbon of an aldehyde or ketone. Organic chemistry by Clayden, Greeves and Warren put it this way: ‘Addition of a Grignard reagent to an aldehyde or ketone gives a stable alkoxide, which can be protonated with an acid to give an alcohol’ (Clayden et al., 2012). If we put this into Maton's diagram it might look like Fig. 3.
SG+/SD− (lower left hand quadrant) – here the language is neither particularly ‘dense’ nor is the concept terribly abstract to an undergraduate chemistry student. The first time a student hears the term ‘Grignard reaction’ they will probably see it as a ‘black box’. By this I mean that the student will not necessarily be able to connect the term ‘Grignard’ with the use of an alkyl magnesium halide reagent. At this point it is reasonable to assume that the student will know what an alkyl halide, an aldehyde or ketone and an alcohol are. The phrase ‘via a Grignard reaction’ is akin to ‘magic happens’. These two reagents add together to form something new. This is also the most common level of engagement of students in an undergraduate laboratory. Follow the instructions and the right product pops out.
SG−/SD− (upper left hand quadrant) – here the ‘black box’ of ‘via a Grignard reaction’ is developed into a description of the Grignard reagent. The terms used are still familiar to the student, but we have shifted to a higher level of abstraction. The mechanism of the reaction is described in language. At this stage the student has developed a way of describing how the reaction might occur. Use of molecular orbital theory to support the formation of the Grignard reagent and to explain the reaction would also fall into this quadrant. Here theory that the student has already appropriated is being used in order to explain this particular reaction. In order to make the transition from the lower left quadrant to the upper left quadrant successfully (weakening semantic gravity) the student must understand what is happening in the reaction.
SG+/SD+ (lower right hand quadrant) – here the chemical reaction symbolism is used, but no mechanistic detail is given. However, the formation of the Grignard reagent is now explicit. Here the student knows what reacts with what. Given the Grignard reagent and the ketone, the student would be able to draw the product. Notice though, that the student may not actually understand yet how the reaction proceeds. This transition can be made through rote learning. The student simply needs to know the particular representation of each of the reagents.
SG+/SD− (upper right hand quadrant) – when the expert organic chemist uses the term ‘Grignard reaction’ he or she is using it as a short hand. It encapsulates the presumption that you can identify which functional groups will react, which reagents and reaction conditions are likely to be used and to give information about the product. Furthermore, the student should be able to accurately draw out a likely mechanism for the reaction.
How does this impact teaching?
Too often those teaching organic chemistry fall into the habit of using the terms and presuming that students understand the full scope of what we are saying. So when we say ‘Grignard reaction’ we are intending to mean everything implied in the upper right hand quadrant, where the majority of students may still be slightly mystified by the ‘black box’ of the lower left hand quadrant perhaps not even connecting the phrase that I persistently pronounce as ‘grinyard’ – to the word that they see in their textbooks!Here, being a little bit more conscious of the development we are seeking can be helpful. We are trying to help students transition from the lower left hand quadrant where chemistry is a mysterious ‘black box’ to the upper right hand quadrant where the student is both able to use the appropriate language and symbolism and understands what is happening in the chemical reaction. Returning now to Maton's idea of semantic waves (Maton, 2009).
Enabling the increase of semantic density
The variation in semantic density can usefully be imagined to be a longitudinal wave (like a sound wave). Here the regions of compression represent periods of time where the correct chemical term is useful exclusively, and the regions of rarefaction represent periods of time where the chemical terms is elaborated upon using more familiar vocabulary. As time goes by the periods of compression can increase in length relative to the periods of rarefaction. In the case of a named chemical reaction, ensuring that one does return to a fuller description of the reaction taking place rather than presuming that the students have assimilated the shorthand of the name is important. For example, using the longer description of a nucleophilic carbon centre generated by the addition of magnesium to an alkyl halide precipitating an addition reaction at an electrophilic carbonyl carbon of an aldehyde or ketone interspersed with the use of the term ‘Grignard reaction’ effectively transitions between lower and higher semantic density. (This transition is represented by the upper left and upper right hand quadrants in the Fig. 3). Again I note that this lowering of the semantic density does not take it all the way down to ‘everyday language’ but it is considerably more accessible to the average first or second year chemistry student who has appropriated the terms nucleophile and electrophile and can accurately draw the general structure of an aldehyde, ketone and alkyl halide.Enabling the decrease in semantic gravity
Here the transition is from lower abstraction to higher abstraction. In chemical terms in this case this means identifying the nucleophile and the electrophile and being to see which two centres are involved in the reaction. (Note: we are not talking about being able to draw the mechanism here.)A second way to ensure that the semantic gravity is being weakened is by showing the reaction (as exemplified in the lower right hand quadrant). If one is teaching with powerpoint, or some equivalent it is easy enough to show the reaction when you refer to the name.
The semantic gravity ‘wave’ refers to lowering the level of abstraction and then raising the level of abstraction again. Here the image of a sinusoidal wave may be most helpful. Perhaps the most obvious way of lowering abstraction is the use of practicals. In this case it would to use a practical session to make a Grignard reagent and then to perform a Grignard reaction. However, for the practical to be effective in the appropriation of the concept, post-practical questions interrogating what has been done and why is important in helping the student to fully grasp what is happening on a molecular level in the flask.
Semantic waves
For Maton, one of the major factors which distinguishes a novice from an expert is whether they reside primarily in the lower left hand quadrant or the upper right hand quadrant (Maton, 2014). As educators, we are trying to help students make that transition towards the upper right hand quadrant, and to stretch them further into the upper right hand sector of the upper right hand quadrant. But in order to help students do that, we need to keep dipping back consciously both to the left and down, so that students can begin to form the mental associations required. We also need to consciously reach upwards and to the right in order to help students appropriate the correct terms. (See Macnaught et al., 2013 for an extended description of this idea in the context of biology education.) If we fail to make these transitions we end up in a situation where we are speaking intending all the meaning of the ‘Grignard reaction’ where the student is not yet even really remembering what is reacting to form which products, nevermind the mechanistic implications.I would suggest that in chemistry, weakening semantic gravity tends to be more challenging than increasing semantic density. Again, using the Grignard example the level of semantic gravity can be tested in two ways. Firstly, requiring the student to describe in words what is happening in a reaction. Secondly, by asking the student to produce the mechanism. Note though, that it is unlikely that the student will grasp the full extent of the intention behind the term ‘Grignard reaction’ unless the lecturer has move from bottom left quadrant to upper right quadrant via both approaches. That is to say going both via the upper left quadrant and via the lower right quadrant.
In organic chemistry tests and exams we tend to focus on semantic route to the upper right hand quadrant which proceeds via the lower right hand quadrant. Questions tend to favour writing out equations, filling in missing products and at the higher level drawing out mechanisms, all use the route via the lower right hand quadrant. Questions requiring a descriptive answer use the route of the upper left hand quadrant. This means that students are gaining the easier to attain higher semantic density rather than the more difficult to attain weaker semantic gravity. As a result they don't remember the abstract concepts from one year to the next, precisely because they never mastered them.
The use of laboratory practicals can help make this transition. Asking questions such as interrogating the logic behind the adding of the reagents – what is added to which flask and when – will help the students move to greater abstraction (weaker semantic gravity). This aids the path via the upper left hand quadrant.
Similarly, occasionally using the simple reaction without requiring mechanistic detail will test whether the foundation of the stronger semantic gravity is present first. Another approach may be to occasionally ask simple questions in class such as – what reacts to give what products in a Grignard reaction will allow for the shift to greater semantic density (the approach via the lower right hand quadrant). To the expert organic chemist these approaches may seem trivial or unnecessarily laborious, but they may well help students to begin the transition into the real assimilation of the abstract i.e. weakening the semantic gravity.
In Maton's description of semantic waves, he uses the poles of ‘real world example’ and abstract concept (Maton, 2011). When applying semantic waves to chemistry, we must acknowledge that we are already working at a relatively high level of abstraction. This is partly the hierarchical or vertical nature of the knowledge structure of chemistry. In a horizontal knowledge structure a well-chosen real world example may be used to illustrate the bulk of the abstract concept in a fairly linear, albeit complex, manner. In a vertical knowledge structure, it isn't quite so simple. A single high level abstract concept may require the incorporation of several different strands of knowledge. For example, understanding reaction mechanisms in organic chemistry requires a level of familiarity with the Periodic Table, with bonding, with hybridization, molecular orbital theory etc. So when we consider the idea of semantic waves in chemistry, we need to consider the temporal wave as well. How does this section of chemistry which I am currently teaching connect with what has gone before, and what is it building towards? As such, it may be worthwhile to revisit the ideas that have gone before in the light of the new theory, and point towards the theory which will follow.
An example of this temporal wave is the way in which I teach the theory of hybridization. In the South African system where I teach first year chemistry, I have the advantage that students have not encountered hybridization at high school but they have encountered some very basic organic chemistry. So they know that ethene is significantly more reactive than ethane. When discussing sp, sp2 and sp3 hybridization I show them the ubiquitous diagrams of these compounds showing the different orbitals involved in bonding. I can then refer back to the reactivity of ethene whilst I have a representation of the hybrid orbitals which shows the sigma and pi bonds and I can relate the highly abstract theory that they are learning back to something that is both a little more concrete and a little more familiar. At the same time I begin to sow the seeds for the ideas of chemical attack by electron poor or electron rich species. I won't use the terms electrophile and nucleophile until we get to mechanism in organic chemistry, but already they have begun to see that there is a connection between chemical reactivity and chemical structure.
Reflections on using LCT in teaching chemistry
Any person teaching any subject at any level will know that there is usually something of a gap between an idealised presentation of a theory and the real-world application. The use of the semantic code of Legitimation Code Theory is no different. There are three key areas where I find these ideas useful. Firstly, in the presentation of new concepts: the awareness of the importance of spending some time in all four quadrants, and actively and explicitly transitioning between them seems to have enhanced my teaching. I think before I began to conceptualise my lectures in this way I was good at transitioning between the lower left and upper left quadrant and the lower left and lower right quadrants, but I presumed that the students were making the transition to the upper right quadrant as a result. This presumption cannot be defended when I am faced with the exams scripts.Secondly, and possibly more importantly, the idea of the semantic wave has changed the way I teach to a certain extent. In that I am much more conscious of intentionally moving between the higher and lower semantic density, and weaker and stronger semantic gravity. I am still ‘finding my rhythm’ with this. It can feel a little forced and even a little patronising, but I suspect that the students don't experience this. It should also be noted here, that this more conscious transition between different levels of semantic gravity and semantic density does not necessarily require a slower pace. Rather it requires a rewording, or the introduction of different kinds of representation, all of which help the student of any calibre to appropriate the material more efficiently.
Thirdly, the setting of exams which utilise all four quadrants. I find it easier to set questions which transition between lower and higher semantic density. These questions also tend to be easier and quicker to mark. Setting questions which transition between weaker and stronger semantic gravity are harder for me to create. They also take longer to mark.
It is the last point that I am finding most challenging and most crucial. It is well established that the form of the assessment has a significant influence on the way in which students learn. One of the ways in which I am trying to train myself to analyse exams more efficiently is to practice using this framework on papers which I have agreed to moderate. At this stage I am still using this for my own learning, but I hope that in time, this framework will be used to inform both my own practice, and the practice of those who have asked me to moderate their courses.
Conclusions
Chemistry is an abstract subject and many students struggle to understand chemistry. I have found that using the ideas of semantic gravity and semantic density which form part of Legitimation Code Theory provides a useful framework within which I can critically engage with my own teaching practice. This also spills over into observing practices in the research environment. It is common practice in many organic synthesis research groups to do ‘problems’ as part of their regular group meetings. In such cases the participants are either given a set of reagents and reaction conditions and are asked to predict the product. Or are given the starting material and product and are asked to give plausible reaction conditions. This process is one of ensuring that organic chemists in training are continually stretching their capacity to go from the lower left hand quadrant to the upper right hand quadrant. Nonetheless, it may be useful to expand the process from simply arrow pushing to the answer, to get the research student to talk their way through the problem too.The point here is simply that from a pedagogical point of view I have found it enormously useful to be a little more conscious of the ideas of semantic density and semantic gravity. I have found it useful to consider the number of concepts a student must have assimilated in order to fully understand the concept I am trying to explain. It has meant that when introducing a new idea I present the new vocabulary and symbols and then drop down to the level that I imagine that the weaker third of the class is operating from. I then use the appropriate chemical terms again, and then introduce visual aids. The purpose here is to consciously move from higher semantic density to lower semantic density and back again, and subsequently from weaker semantic gravity to stronger semantic gravity and back again. This process is iterated several times as different examples are discussed.
Perhaps one of the reasons why chemistry is such a challenging subject for so many students is that study of the subject rarely moves out of the upper right hand quadrant on the semantic graph. We can move from more abstract to less abstract, but frequently the appeal to real world examples is more confusing than helpful. Likewise, we can move from dense symbols to more explicit chemical language, but again we rarely stray into the usage of ordinary words or the ordinary usage to English words for that matter.
I have found the ideas of semantic density and semantic gravity enormously useful in my teaching. It has made me far more conscious of the kinds of complexity which different sections of chemistry require. It has helped the pacing of my teaching, by separating out these different factors and by considering the extent of the leap required by the students at any particular stage. I am also more able to understand where their confusion lies, and to address the specific problem they are facing. I have also been able to apply this model to all aspects of a general chemistry course. The challenge which remains is the development of my assessment in order to properly access the difference between gain in complexity and gain in abstraction.
Ultimately, I believe this is a framework which can be used to enhance good teaching in order to facilitate better learning. In my opinion, many of us do a good job of facilitating increased semantic density in our students. The weakening of semantic gravity is more challenging. I hope that this framework will allow us a way to begin to monitor the gains we might be making in both areas. It remains to be seen whether this framework will be useful across the entire spectrum of chemistry education, but to date, I have used it to good effect in teaching both a general introductory chemistry course and an organic chemistry course. As the framework is independent of the knowledge area, I would expect that it could be used ubiquitously.
Acknowledgements
I would like to acknowledge Prof. Trevor Gaunt and Ms Renée Smit of the University of Cape Town for comments on an early draft of this paper. I would also like to thank Prof. Cecilia Jacobs of Stellenbosch University for her comments. Finally, I would like to thank the two reviewers of this paper for their helpful suggestions.Notes and references
- Bernstein B. and Solomon J., (1999), ‘Pedagogy, Identity and the Construction of a Theory of Symbolic Control’: Basil Bernstein Questioned by Joseph Solomon, Brit. J. Sociol. Educ., 20(2), 265–279.
- Chomsky N., (2000), New Horizons in the study of language and mind, Cambridge: Cambridge University Press.
- Clayden J., Greeves N. and Warren S., (2012), Organic Chemistry, Oxford: Oxford University Press.
- Georgiou H., Maton K. and Sharma M., (2014), Recovering knowledge for science education research: Exploring the ‘Icarus effect’ in student work, Can. J. Sci. Math. Tech. Educ., DOI:10.1080/14926156.2014.935526.
- Grove N. P., Hershberger J. W. and Bretz S. L., (2008), Impact of a spiral organic curriculum on student attrition and learning, Chem. Educ. Res. Pract., 9(2), 157–162.
- Johnstone A. H., (1982), Macro- and micro-chemistry, Sch. Sci. Rev., 64, 377–379.
- Johnstone A. H., (2000), Teaching of chemistry: logical or psychological. Chem. Educ. Res. Pract., 1(1), 9–15.
- Kilpert L. and Shay S., (2013), Kindling fires: examining the potential for cumulative learning in a Journalism curriculum, Teach. High. Educ., 18(1), 40–52.
- Lavoisier A. L., (1790), Elements of Chemistry, in a New Systematic Order: Containing All the Modern Discoveries, Courier Dover Publications.
- Macken-Horarik M., (2011), Building a knowledge structure for English: reflections on the challenges of coherence, cumulative learning, portability and face validity, Aust. J. Educ., 55(3), 197–213.
- Macnaught L., Maton K., Martin J. R. and Matruglio E., (2013), Jointly constructing semantic waves: Implications for teacher training, Linguist. Educ., 24, 50–63.
- Maton K., (2009), Cumulative and segmented learning: exploring the role of curriculum structures in knowledge-building, Brit. J. Sociol. Educ., 30(1), 43–57.
- Maton K., (2011), in Christie F. and Maton K. (ed.), Theories and things: the semantics of discipinarity in Disciplinarity: functional linguistic and sociological perspectives, London: Continuum, pp. 62–84.
- Maton K., (2014), Knowledge & knowers: towards a realist sociology of education, London and New York: Routledge.
- Matruglio, E., Maton K. and Martin J. R. (2013), Time travel: the role of temporality in enabling semantic waves in secondary school teaching, Linguist. Educ., 24, 38–49.
- McNamara, M. S., (2010), What lies beneath? the underlying principles structuring the field of academic nursing in Ireland, J. Prof. Nurs., 26(6), 377–384.
- Reingold, I. D., (2005), Chemistry as a Second Language, Bookend Seminar, vol. 69.
- Ryan, B. J., (2013), Line up, line up: using technology to align and enhance peer learning and assessment in a student centred foundation organic chemistry module, Chem. Educ. Res. Pract., 14(3), 229–238.
- Song, Y. and Carheden S., (2014), Dual meaning vocabulary (DMV) words in learning chemistry, Chem. Educ. Res. Pract., 15(2), 128–141.
- Taber, K. S., (2001), Building the structural concepts of chemistry: some considerations from educational research, Chem. Educ. Res. Pract., 2(2), 123–158.
- Taber, K. S., (2013), Revisiting the chemistry triplet: drawing upon the nature of chemical knowledge and the psychology of learning to inform chemistry education, Chem. Educ. Res. Pract., 14(2), 156–168.
| This journal is © The Royal Society of Chemistry 2014 |