Students' application of chemical concepts when solving chemistry problems in different contexts
Karolina
Broman
*a and
Ilka
Parchmann
b
aDepartment of Science and Mathematics Education, Umeå University, Sweden. E-mail: karolina.broman@umu.se
bIPN Leibniz Institute for Science and Mathematics Education, University of Kiel, Germany
First published on 8th April 2014
Abstract
Context-based learning approaches have been implemented in school science over the last 40 years as a way to enhance students' interest in, as well as learning outcomes from, science. Contexts are used to connect science with the students' lives and to provide a frame in which concepts can be learned and applied on a ‘need-to-know’-principle. While effects on interest are coherently reported as positive, they are more diverse regarding cognitive learning outcomes. Hence, the demand for further research on criteria of context-based problems and problem-solving processes has been stated. In this paper, a study is presented investigating students' application of chemical concepts when solving context-based chemistry problems. Tasks for context-based problem solving have been designed systematically, using different combinations of contexts, topics and chemistry concepts in relation to the syllabus. Empirical data were collected using think-aloud interviews where 20 upper secondary students used their chemical content knowledge to solve the problems. The 15 context-based problems raised challenges within organic chemistry where concepts like electronegativity, polarity and solubility had to be applied. The difficulty to differentiate between intra- and intermolecular bonding emphasised in earlier research has also been apparent in this study. Besides the structural formula, which was an important part for the students when solving the tasks, the contextualisation of the problems was often used in the responses; students related their answers to the personal, societal or professional context in different ways. The paper explores the results and gives implications for context-based teaching, learning and assessment.
Introduction
Context-based learning (CBL) approaches have had a strong influence on school science in several countries as a way to both stimulate students' interest and motivation, and to provide more interconnected content knowledge (Nentwig and Waddington, 2005; Bennett et al., 2007; King, 2012). Literature reports positive effects on students' interest and motivation (Bennett et al., 2007; Fechner, 2009), while the impact of CBL on students' cognitive learning outcomes is less coherent (Taasoobshirazi and Carr, 2008; Fechner, 2009). The theoretical background of CBL approaches, for example the German ‘Chemie im Kontext’, is based on the framework of scientific literacy, and theories on interest, motivation and situated learning (Nentwig et al., 2007). According to Nentwig et al. (2007) scientific literacy is perceived as an intersection of knowledge, activities and values. Students' motivation and interest are, as mentioned above, one of the benefits of these approaches', students often find context-based chemistry more interesting and motivating than conventional approaches (Bennett et al., 2007; King, 2012). Since outcomes regarding the cognitive results of CBL are more diverse, Pilot and Bulte (2006) highlight critical aspects important to take into account for the design of CBL approaches; for example the development of suitable assessment, the design of adequate tasks, and the analysis of students' responses to these tasks. These critical aspects are obviously not limited to CBL, still this argues for a more systematic analysis of task criteria and problem-solving processes. As context-based problems combine chemistry content with contexts to make the problem more authentic and relevant, they ask for complex thinking and higher levels of scientific literacy (Hofstein et al., 2011). Therefore the aim of this study is to systematically design context-based problems in demand of higher order thinking where students' responses are systematically analysed. As a consequence, when designing the problems, there have been explicit and intentional starting points regarding both context and content to make it possible to scrutinise how these two parts influence students' responses. The design process of 15 tasks emanated from a pre-study (Broman, submitted; Parchmann et al., submitted) highlighting the importance of a structured design of topic, context and content. Students' responses were collected using semi-structured interviews with think-aloud techniques to get an in-depth qualitative insight on students' chemistry explanations and how they apply chemical concepts in context-based chemistry problems to give implication on teaching and assessment of school chemistry.Background
Chemistry education in Sweden
Since the study was carried out in Sweden the educational background needs to be described as a frame for the study. In Sweden, the upper secondary chemistry syllabus is not explicitly context-based, as in several other countries (e.g. Germany, The Netherlands, UK, US) and chemistry textbooks, which are described as the foundation for teaching (Bergqvist et al., 2013), are more conventional. Previous Swedish research also highlights upper secondary chemistry teaching emanating from the textbook and with a clear emphasis on basic definitions and symbolic representations (Adbo and Taber, 2014). However, connection to everyday life is requested by Swedish upper secondary students to improve school chemistry to make it more meaningful (Broman et al., 2011; Broman and Simon, 2014). This connection to everyday life can be translated into the prospect for CBL approaches foregrounding this study. Even though there is no explicit context-based syllabus in Swedish schools, it is not possible to predict how individual teachers teach chemistry and if the school chemistry students meet might be context-based. Sjöström (2013) concludes that chemistry teachers often try to connect the content with everyday life, however not in a critical way without problematizing the relationship between everyday life and the chemistry content. The notion of ‘everyday life’ is mentioned in the first sentence of the syllabus valid at the time of this investigation; “The subject of Chemistry aims at providing an advanced understanding of chemical processes and a knowledge of the variety of chemical applications and their importance in everyday life, industry, medicine and the living environment” (Swedish National Agency for Education, 2000). This makes it important to ask students how familiar they are with these approaches, in this case, regarding chemistry tasks that are context-based.Context-based learning approaches
The meaning of ‘context’ has been scrutinised from various perspectives, both concerning the notion in general (van Oers, 1998) and within context-based learning (CBL) approaches (Gilbert, 2006; Gilbert et al., 2011). A useful definition applied in several approaches is that the context “is the red thread along which the investigation of the issue in question develops” (Nentwig et al., 2007, p. 1441), not only a mere decoration in the beginning or in the end to illustrate something or motivate students. The context is the starting point from where the teaching proceeds, and the ground principle is that the learner will start off from the context and then be aware of the content knowledge in demand to understand the issue in question on a ‘need-to-know’-principle (Bulte et al., 2006). The relationship between a context and the subject-matter concepts can have varying characteristics; contexts can illustrate concepts, contexts can be used as an application of a concept, and contexts can be seen as a starting point from where teaching can emanate (de Jong, 2006). Nevertheless, the distinction between context and content is not always clear (Häussler et al., 1998; Pilot and Bulte, 2006), e.g. topics like fuels or food are sometimes named contexts while elsewhere, a personal or a societal framing for such topics is defined as context. As a consequence, words like topic, context, modules (Graeber and Lindner, 2008), and themes (Nentwig et al., 2007) sometimes imply the same, sometimes something different. In this study, we have made a clear distinction between the topic regarded as the broad area connected to everyday life, e.g. health, food and fuels; whereas the context is the setting in which the topic is displayed, i.e. personal, societal or professional. Bulte et al. (2006) have emphasised the use of this kind of authentic practices to enhance the relevance of learning about chemistry.New and more unconventional teaching approaches like CBL sometimes get critique for concentrating primarily on engaging students by showing authentic practices that are found relevant, at least from the perspective of the teachers, researchers and textbook authors. Sevian and Talanquer (2014) maintain the need to be aware of the importance of the chemical concepts, not only the context part, where they assert that “in some cases core chemistry ideas and practices get buried under the specific contexts used to motivate and drive learning in the classroom” (Sevian and Talanquer, 2014, p. 11). Furthermore, Marks and Eilks (2010) also challenge context-based chemistry education by claiming it sometimes to be superficial, arguing that contexts not automatically motivate students, and that reflection has to be made when developing these new approaches. Therefore, in this study the chemistry content knowledge is the foundation; the context-based tasks have been designed with chemistry content areas and their concepts as first consideration, the interviews have been conducted by a researcher with upper secondary chemistry teacher experience, and students' responses have always had a clear chemistry focus in the analysis process. Unquestionably, both affective and cognitive parts are intertwined and important to investigate in parallel, hence students' perceived interest for and perceived difficulty of the tasks have also been monitored and will be mentioned briefly here, however analysed further in upcoming publications. The choice to inquire upper secondary students at the Natural Science Programme was also deliberate to emphasise the chemistry content; with older students there is more concentration on the subject itself, and also since this is a programme preparing students for chemistry studies at the university level.
Several research projects investigating CBL approaches have been accomplished over the last 40 years and as mentioned earlier, previous results show mainly positive affective effects whereas the cognitive effects are more complex to draw conclusions from (Ramsden, 1997; Bennett et al., 2007; King, 2012). Most research was conducted in countries where full context-based curricula are implemented or where new teaching units are possible to use. In Australia, using the theoretical framework of Vygotsky's sociocultural perspective in combination with Bourdieu's notion of field, King and Ritchie (2013) have been investigating the links successful students make between concepts and context in an inquiry task on water quality of a local creek. In the Netherlands, Overman et al. (2013) have analysed textbook tasks from both context-based and traditional curricula highlighting the orientation towards traditional chemistry content in the questions from both curricula and although CBL chemistry relates tasks to societal and professional issues, there was not much emphasis on higher order learning. In Germany, Marks et al. (2008) and Marks and Eilks (2010) have developed lesson plans on shower gels and potato crisps from a socio-critical starting point, claiming a potential for higher order thinking skills and meaningful learning. The aforementioned ‘Chemie im Kontext’ is another teaching approach developed and implemented in Germany with modules tested both by secondary teachers and researchers (Parchmann et al., 2006; Nentwig et al., 2007). Since the Swedish chemistry syllabus has no explicit CBL approach, it has not been possible to implement and investigate full teaching units. However, we believe that parts of CBL approaches can be applicable without a full curricula change, making it possible to apply context-based chemistry problems as student tasks without the teaching approach, as these problems in themselves demand the same level of chemistry as the tasks found in upper secondary chemistry textbooks. The benefit of using these problems might be a movement to make school chemistry more relevant for students, a request from both students and teachers, as well as researchers (Nentwig and Waddington, 2005). Another example to implement parts of CBL approaches has been suggested by Christensson and Sjöström (2014) who have analysed on-line thematic chemistry videos from the International Year of Chemistry presenting chemistry in context, where they show a clear message of chemical literacy, a foundation for CBL approaches. In the outlook of this paper we will discuss how parts of a CBL approach, for example context-based tasks, can be applied in chemistry education in a meaningful way even though the curriculum has no explicit context-based focus. This is the opposite of other previous projects where students meet context-based teaching and textbooks whereas their examination tests are more conventional (Bennett et al., 2007).
Chemical explanations and scientific literacy
When investigating students' problem solving of context-based chemistry tasks, the framework of scientific literacy is foregrounding the design process of the problems by developing the tasks with relevant concepts in varying topics and contexts. CBL approaches are not exclusive in relating the framework to scientific literacy; bildung-oriented chemistry education (Sjöström, 2013), socio-scientific issues (Sadler and Zeidler, 2009), and humanistic learning approaches (Aikenhead, 2006) are examples of other learning approaches with the joint goal of scientific literacy. Moreover, the PISA project assesses scientific literacy highlighting its emphasis on today's science education (Bybee et al., 2009). The notion of scientific literacy is a broad expression with composite meaning and is often perceived as the general purpose of science education. By relating to Roberts' (2007) two emphases of scientific literacy, i.e. Vision I and II; the starting point for CBL approaches is Vision II, stressing science in everyday life situations in which science plays a key role. These two visions of scientific literacy, as Roberts stated, represent two “extremes” on a continuum. Therefore, in this study we explore students' application of chemistry concepts when solving context-based problems making it central to consider both Vision I, emphasising the science subject matter itself, in combination with Vision II.Analysis of students' application of chemistry concepts in their responses to context-based problems demands elaboration of what kind of content knowledge these problems require and how we interpret chemical explanations. Taber and Watts (2000) have studied the quality of students' explanations when solving chemistry problems stating three key parameters to consider when valuing the responses to be explanations or not, i.e. if the response has the structure of an explanation by stating words like ‘because’ and ‘therefore’; if the response is logically consistent; and if the response is correct or not. A foundation for chemical explanations are students' ideas about which parts are important to give as a response, in other words what they are used to meet in chemistry education. Smith (2011) highlights the importance of relevant and meaningful learning settings, and claims that the engagement is a crucial factor for successful education. Nevertheless, rote learning has for many years been the foundation for school and university chemistry, where teaching emphasises formal definitions, equations and rules, and where students often get the impression that there is always only one correct answer to every question (Zoller and Pushkin, 2007). School chemistry in most countries has focused rote learning of isolated topics with their concepts and ideas, for instance atomic structure, chemical bonding and thermodynamics (Sevian and Talanquer, 2014). Rote learning has a clear relationship with lower order thinking, for example rote memorization and recall of factual knowledge, which contrasts the idea of context-based authentic problems that ask for complex and higher order thinking. Higher order thinking needs to be learned and trained and therefore students have to meet different kinds of tasks, not only textbook tasks focusing on recall of facts (Bergqvist et al., 2013; Overman et al., 2013). Unquestionably, there is an obvious need for factual knowledge in solving context-based problems; however, the idea here is to not only focus on recall of facts. Higher order thinking relates to meaningful learning by applying non-algorithmic, complex problems with multiple solutions that involve application of multiple criteria and uncertainty (Dori et al., 2003; Zohar and Dori, 2003; Zohar, 2004). Context-based chemistry problems, referring to the goals of scientific literacy, ask for complex thinking when solving the tasks, and is a feasible way to train students to give structured explanations and apply higher order thinking since the problems themselves make it apparent for students that there is not only one single correct answer to every chemistry problem; instead, many different solutions are often conceivable. Many different possible solutions are one central aspect for context-based chemistry problems and we believe this to be an important change for school chemistry (at least in some countries), to make the subject more trustworthy and understandable.
Chemistry problems and concepts
Problem solving has been a significant aspect investigated within science education research for many years (Bodner and McMillen, 1986; Nakhleh and Mitchell, 1993; Bodner and Domin, 2000; Cartrette and Bodner, 2010; Ngu and Yeung, 2012). A prevailing definition of a problem within science education has been claimed by John Hayes “Whenever there is a gap between where you are now and where you want to be, and you don't know how to find a way to cross that gap, you have a problem” (Hayes, 1989, p. xii). It is the second half of the definition that distinguishes problems from exercises, in other words, how familiar the task is. Exercises are solved on routine, whereas problems are novel and demand more from the solver (Bodner and McMillen, 1986). To demand more, other competencies than recall of facts are needed; the “success in solving algorithmic problems does not indicate mastery of the relevant chemical concepts” (Zoller and Dori, 2002, p. 187). Within context-based chemistry, Gilbert highlights the importance of “problems that are clear exemplifications of chemically important concepts, to enable learners to develop a coherent use of specific chemical language” (Gilbert, 2006, p. 970).The syllabus (Swedish National Agency for Education, 2000) is framing the course content, showing which competencies students are supposed to develop during the course. Different content areas are presented in the chemistry syllabus, for instance stoichiometry, acids and bases, redox, organic chemistry, and biochemistry. Regarding organic chemistry, there is an apparent connection to chemical bonding, especially to hydrogen bonds since the properties of many organic compounds can be explained by this kind of intermolecular bonding (Henderleiter et al., 2001). For a long time, chemical bonding has been explained through several diverse models building on physical principles generating alternative conceptions, and even misconceptions, producing learning impediments for students (Taber and Coll, 2002). Students often highlight anthropomorphic explanations for why bonding occurs, for instance that atoms want, need, or search for something and that the ‘octet rule’ is aimed for (Taber and Coll, 2002; Levy Nahum et al., 2010; Adbo and Taber, 2013). Thus, the ‘octet rule’ has often been regarded as a valid foundation for bonding, thereby making it difficult to explain how, for example, sulphur and phosphorus atoms can take part in covalent bonds (Levy Nahum et al., 2010). As a result from preconceptions like this, research recommends a change from the more traditional view on bonding into a new teaching approach of chemical bonding (Levy Nahum et al., 2007). This new bottom-up framework suggested by Levy Nahum et al. (2007, 2008, 2010) relies on concepts like atomic energy levels, Coulomb's law and electronegativity giving a coherent understanding of all types of chemical bonds. In Swedish textbooks and in chemistry classrooms, the traditional view on bonding is prevalent (Bergqvist et al., 2013; Adbo and Taber, 2014), dividing bonding into two distinct groups; i.e. intra-molecular bonds with ionic bonding and covalent bonding, and intermolecular bonds with dipole–dipole-bonding, van der Waals forces and hydrogen bonding. This traditional view makes it interesting to investigate how Swedish students apply chemistry content knowledge on chemical bonding in this study's context-based tasks that demand more complex reasoning regarding chemical bonding.
In summary, context-based problems have the potential to expose the higher order thinking by asking not only for one single correct recalled answer, but instead for different conceivable solutions. However, empirical results have shown different outcomes so far. More research is required on how and when students apply chemistry explanations based on higher order thinking to solve context-based problems successfully and thereby fulfil requirements of the educational framework of scientific literacy. The rationale of our project is to develop a structure for the systematic design and analyses of context-based problems and analyses of students' responses. In this paper the chemistry content knowledge given in the responses according to the different contexts will be explored, whereas the problem-solving process will be scrutinised in a forthcoming paper. The research questions investigated in this study are: (1) How do upper secondary students apply their chemistry content knowledge when solving context-based chemistry problems? (2) Which misconceptions of chemistry content can be revealed when students solve these problems? (3) How do students make use of the contextualization of the task into topics and contexts given in the problems?
Methodology
This study was carried out in Swedish upper secondary schools (school year 10–12) in the Natural Science Programme (NSP). Students choose themselves what programme they want to attend and the NSP is regarded as the programme for the “future scientist”. However, even though most students choose this programme because of prospects for higher education (Anderhag et al., 2013; Broman and Simon, 2014) and most of them also continue to university studies after this programme (SCB, 2012), not everyone aims for tertiary science or chemistry. Still, at the programme chemistry is studied to the highest upper secondary level to prepare for higher education within chemistry. The choice to investigate this population, students that can be seen as the “future scientist”, was deliberate since the chemistry content is focused more at the upper secondary level when compared to compulsory school (purposeful sampling). In this study, we wanted to explore how students who have chosen to read as much chemistry as possible at this level apply their chemistry content knowledge when solving context-based chemistry problems. Therefore, we inquire students' responses to context-based problems, not exercises. 15 context-based tasks representing less pre-structured and more complex conceptual problems to the students (Salta and Tzougraki, 2011) have been designed and developed.Systematic design of context-based problems
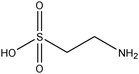
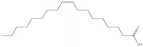
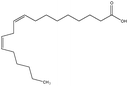
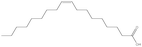
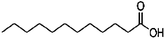
To scrutinise both affective and cognitive challenges with context-based problems, the design of the tasks has been made thoroughly. In a pre-study, ten context-based problems were designed and from this, one exemplary task was studied in-depth to elaborate an analytical framework possible to use both for analysis of the context-based tasks in themselves as well as students' response and process when solving the tasks (Broman, submitted; Parchmann et al., submitted). The pilot study highlighted the apparent need for a more systematic design process where both the content for the tasks as well as the structured development of the tasks have to be carefully considered. Regarding the content for the tasks, results from an exploratory study (Broman et al., 2011) on students' perception of their school chemistry have been applied. For one content area, chemical bonding, students and teachers did not agree on how difficult this was for the students. The students claimed that chemical bonding was quite easy whereas their teachers asserted the content area to be difficult for students. This difference in opinion might be explained by students' unconsciousness about the complexity of chemical bonding. Nevertheless, chemical bonding is a fundamental content area to understand relevant and authentic topics like medical drugs and food. The content is regarded as the chemistry area with its concepts, e.g. in the content area of chemical bonding there are concepts of for instance electronegativity and polarity. The content areas are areas stated in the syllabus (Swedish National Agency for Education, 2000) as well as chapters in the chemistry textbooks used by the students in this study (Henriksson, 2007; Andersson et al., 2008). This distinction between topic, content and context needs to be considered when designing new context-based tasks as well as when investigating students' opinions about these problems to understand which parts of the task explicitly affect students' opinion and influence students' problem solving.With regard to the structured development of the tasks, 15 context-based problems were thus designed with four explicit starting points. First, considering content area for the reason given above, organic chemistry in relation to chemical bonding was chosen, for example problems asking for solubility of different organic compounds. Second, regarding topics, five different topics were selected from results originating from the Relevance of Science Education (ROSE) study (Jidesjö et al., 2009; Sjöberg and Schreiner, 2010). The topics were medical drugs, energy drinks, fats, fuels, and soaps and detergents. Three of them were chosen since they are appreciated by students according to the ROSE study, i.e. medical drugs, energy drinks and fats (in relation to health and food); the topic of fuels was chosen because it is a common area used in context-based courses (e.g.Demuth et al., 2006); and the topic of soaps and detergents was chosen since conclusions are drawn from the ROSE study that students do not appreciate everyday life because they do not value this specific topic (Sjöberg and Schreiner, 2010). Third, the five topics were thereafter framed by three different contexts; a personal, a societal and a professional context (de Jong, 2006). This choice was made to investigate if it is the contextual setting of each topic that affects students' opinion about the problems. Finally, all tasks had two or three structural models included, mainly to accentuate the chemistry focus. An overview of all five topics and their related content areas and molecular structure is presented in Table 1.
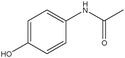
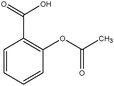
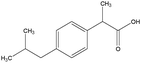
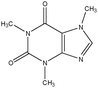
The design process was an iterative process between the English and Swedish languages since the interviewed students had to meet the problems in Swedish whereas the research process and analysis have been carried out in English, hence the wording of the tasks was thoroughly considered. The tasks were not supposed to give clear hints on content areas possible to use and to avoid using chemistry words, the tasks were presented to the students in Swedish using more everyday words in a way that made sense in Swedish. However, the literal translation of the tasks into English, which is presented in Fig. 1 with the three contextualized tasks on the topic of medical drugs, includes words that would not been used in the same way if the task was presented in English. To give an example on what these are, we use the words ‘mix’ and ‘spread’ instead of ‘dissolve’ and ‘solubility’ in the three tasks shown in Fig. 1. It was also important that the text was not too long and complex in the tasks since we wanted to avoid reading comprehension influences.
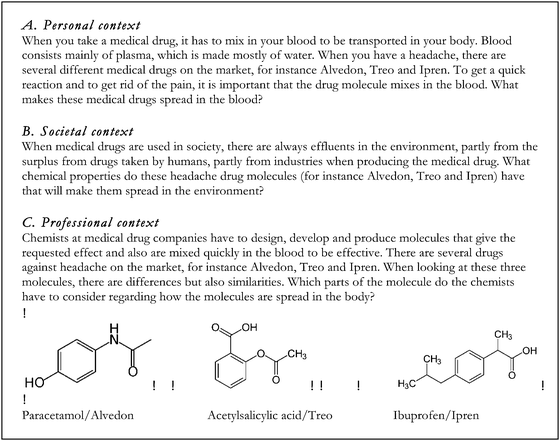 | ||
| Fig. 1 Examples of context-based problems, topic: medical drugs. Literal translation of the tasks from Swedish to English. | ||
To validate the chemistry problems, an interview with a university chemistry professor was accomplished before the students met the tasks. His response was asked to be as broad as possible, to become aware of all possible solutions to the problem. He also had the opportunity to give comments on the tasks to improve them. Besides this, two upper secondary teachers looked at the problems to validate the level of chemistry suitable for upper secondary school students.
Project design, participants and interview guideline
To explore students' applied content when solving context-based chemistry problems, semi-structured interviews with think-aloud techniques were carried out with 20 students from three different schools. The choice to use interviews instead of students' written responses as in one part of the pre-study (Broman, submitted) was to get in-depth insights on both affective and cognitive aspects of CBL approaches and to explicitly investigate the process of problem-solving. The students (aged 18–19 years-old) were in the end of their chemistry schooling at the upper secondary level, and were chosen to solve the context-based tasks. They had completed the two chemistry courses possible to take at the NSP in Sweden. The students could thereafter apply for university studies to become for instance medical doctors, engineers or scientists. The students came from three different schools having different teachers, as a way to minimize teacher effects.All students participated voluntarily, the first contact was taken through the three teachers who gave suggestions on students they thought were willing to participate. The request was students who could take time to do the interview and orally could explain how they think when they solve problems. The students were assessed by their teachers as medium- to high-achievers. The first author contacted the students and informed them about the project. Moreover, the teachers also shared the examination test the students had conducted within their chemistry course, as a way to compare their ordinary tasks with these context-based problems. The interviews were made in Swedish at a place chosen by the individual student, often out-of-school, and lasted about 45–50 minutes each. Every student encountered six tasks, i.e. two topics in three contexts, and was at first asked how these problems resembled tasks they meet in school chemistry. Regarding the cognitive aspects, students solved two tasks, after choosing one context each from both topics.
In the interviews, students always started the problem-solving process on their own, without interference from the interviewer. This was a way to investigate what students come to think about initially. After the students had had the opportunity to express their ideas, the interviewer proceeded by giving hints and asking clarifying questions. If the student gave a broad and extensive answer, clarifying questions were posed, whereas hints were given to students who might elaborate their answers more. The hints were given according to the analytical framework of the Model of Hierarchical Complexity in Chemistry, MHC-C (Bernholt and Parchmann, 2011) which was a helpful analytical tool previously investigated (Broman, submitted; Parchmann et al., submitted), and where the MHC-C-operators “name, describe, explain” were used to structure the hints. To be aware of the solution process and the order and structure of the hints, Fach et al.'s (2007) different stepped supporting tool was used as a technique. When students solved the problems, they used correct, incorrect and incomplete strategies and depending on the strategy, different tools were given by the interviewer. This well-reasoned scaffolding in the interviews was an evident result from the pre-study where it was clear that leading questions and hints affected students' responses (Parchmann et al., submitted). In addition, previous research proposes use of structuring or focusing strategies to foster deeper thinking and not only simple recall, in other words, to use stimulus like for instance tables or formula from which questions are based (Chin and Brown, 2002).
After five interviews, it was apparent that the students at first always concentrated on the structural formula when answering, not noticing the contextualization of the task. As a consequence, a decision was made to make a slight change in the method and not present the structural formula in the beginning of the interview, instead making students solve as much as possible without the formula and thereafter show the formula as a hint. This change of method was noticed when doing the analysis and this different circumstance will mainly be discussed when presenting the results on how the contextualized part affects students' responses. Transcription was made after listening to the interviews, and two months after transcription, the interviews were re-listened and adjusted. Translation from Swedish to English was made in the transcription process to make it possible for the co-author to contribute in the analysis process and enhance inter-rater reliability.
Two exemplary excerpts will be presented here to get an insight about the interview method in relation to the process of problem-solving. The interview process differed between students who were reluctant to elaborate their response, similar to the study of Fach et al. (2007) where several students used only incomplete strategies to solve the problem, and students who solved most of the problem themselves. The less able students needed several hints to solve the problems, for instance general strategic, content-strategic, content-related stepped supporting tools (SST) or by giving students general glossary of important terms. The general strategic SST are hints to extract and concentrate information from the task, content-strategic SST are hints to give ideas on how to solve the task, content-related SST are more clear and explicit hints on how to solve the task, and finally, the general glossary of important terms are hints to give background information about the terms, in this case e.g. bonding and solubility (Fach et al., 2007). On the other end there were students who solved most of the problems on their own and where the interviewer only had to ask clarifying questions. The first excerpt is an example of a student in need of several hints to move forward, trying to solve the task on energy drinks (see Table 1, topic: energy drinks, personal context, task on taurine's and caffeine's solubility in RedBull®) where the hints in the form of MHC-C-operators are given in bold italics, and where ‘…’ means that the student is quiet, thinking for more than 3 seconds:
Student: When I compare caffeine and taurine, I don't know which one is more soluble…
Interviewer: Any idea where to start?
S: No…
I: Any important functional groups? [ name ]
S: Taurine has an OH and an amine, and caffeine carbonyl groups… and caffeine has a cyclic structure… don't know what more to say…
I: Can you describe how these functional groups relate to solubility?
S: OH is soluble in water so… taurine must be soluble…
I: Anything besides the hydroxyl group?
S: It's always OH I look for…
I: Why is OH important?
S: Then it can be hydrogen bonding with water…
I: Is it only OH that can give hydrogen bonding?
S: I think so… or… no… wasn't it N, O, F or something… yes, and nitrogen, then perhaps the NH 2 -group… could that be soluble? Isn't that a base?
I: Could a base explain solubility?
S: Hmm… Yes, yes of course… then it would be an ion, and even more soluble…that's right…
To give one example of a student who solved more of the problem on herself, the following quote represents a more broad response to the question on how the medical drugs can be spread in the environment (see Table 1 and Fig. 1) represents this:
S: I think about that the drugs have to be dissolved in water, then a trick we have learnt is to check the amount of OH-groups since they enhance solubility by hydrogen bonding, but it's also important which part is not soluble in water, not polar, and this part (points at the left side of Ipren), has lots of carbon and this big part will not be dissolved in water as easy as this (points at the oxygen atoms in Treo), and I think it's less polar, but I don't know what happens with this part, the amid or amine or whatever it is, but I think I would go with this who has an OH-group, I mean all has one OH-group but this is smaller, less polar…
I: Do you consider other parts than the OH-group?
S: Perhaps this nitrogen, I really don't know, but if it's something, it must be the N since that's the only thing that's not just carbon and hydrogen, but I'm not sure what it will do, the nitrogen is bonded to a hydrogen, is this important? It might be something like acids and bases, like NH 3 being a base, would this make it easier to spread the drug into the environment? I'm not sure here…
This student exemplifies how the medical drugs are dissolved in the environment by discussing water-solubility and polarity as well as size and she discusses these parts without the need of any hints from the interviewer to move forward, only one clarifying question. By applying Taber and Watts' (2000) key parameters, this response has the structure of an explanation, is logically consistent and is correct regarding content.
Analysis
To scrutinise students' application of chemistry content knowledge, the transcripts from the interviews were analysed using an iterative process by reading the transcripts several times, and thereafter, coding segments were identified. During the coding process, annotated descriptive and interpretive comments were made in the margins of the transcripts. The inductively derived categories that emerged became coding categories by using the content areas from the syllabus (Swedish National Agency for Education, 2000) and their concepts found in the students' chemistry textbooks (Henriksson, 2007; Andersson et al., 2008). Content areas were for example ‘chemical bonding’, ‘structure of matter’ and ‘organic chemistry’, and their concepts were for instance solubility, polarity, size, electronegativity and different kinds of bonding, see Table 2. By using the quote above as an example, the second students' first utterance was first coded into the following concepts: solubility (from the student mentioning dissolved, solubility, soluble), functional groups/organic classes (OH, carbon, amid, amine), size (big part, smaller), polarity (not polar, less polar) and chemical bonding (hydrogen bonding). These concepts were thereafter categorized into the content areas presented in the results section. As a result of every student solving two tasks each, there were 40 student responses, eight for every topic. The coding and categorizing was made by the first author twice, with three months in-between, using an Excel spread sheet where every utterance from both the student and interviewer were coded both by content and by the complexity level. The second author coded parts of the material (8 task solutions with 85 statements out of the 40 solutions) and since the inter-rater reliability was high (Cohen's kappa κ = 0.906) and differences were easily negotiated, the first author's coding was decided to be sufficient.| Content area | Examples of concepts | Topics and contexts |
|---|---|---|
| Structure of matter | Atomic and molecular structure, molecular size | All topics with their contexts |
| Chemical bonding | Electronegativity, polarity, solubility, “lika-loser-lika” | All topics with their contexts |
| Organic chemistry | Classes (e.g. alcohols, carboxylic acids), functional groups | All topics with their contexts |
| Acids and bases | pH, acids, bases | Medical drugs, energy drinks, soaps and detergents with their contexts |
| Energy and enthalpy | Combustion, bond energies | Fuels with their contexts |
Results and discussion
To get background information foregrounding the research questions given in this paper, it was important to investigate how familiar the students were with problems like these. Previous research often have had the design where conclusions are drawn about students' learning outcomes by investigating CBL approaches where the examination tasks are still more conventional (Bennett et al., 2007), however here we study students' responses to less pre-structured and more complex chemistry problems without knowing more about their teaching than looking at their textbook and their ordinary examination test, in other words, the other way around compared to most previous studies. When asked to compare these context-based problems with chemistry tasks the students usually meet in school, students claimed that these problems were unfamiliar. The students were more used to straightforward algebraic tasks in their textbooks, i.e. exercises. When investigating the students' chemistry textbooks (Henriksson, 2007; Andersson et al., 2008), it is evident that these textbooks mainly present exercises asking for lower order thinking like recall of memorized facts or solving repetitive algebraic tasks (Bergqvist et al., 2013). However, in examination tests, sometimes there are a few problems where there is more background information given, presented in this exemplary quote: The tasks I meet in chemistry are not like this, in the textbook the tasks are mostly shorter with a clear, simple question. I just give the correct answer. In a test, there are sometimes one or two tasks with more text, and where I have to write more. But still, I often know what the teacher wants. All three teachers who had students participating in this study shared their examination tests in organic chemistry making it possible to validate the students' opinion that the context-based problems from this study are perceived as new and unfamiliar to the students. The utterance from the quote shown above that you “just have to give the correct answer” relates clearly to the rote learning emphasis common in school. Moreover, the focus on “what the teacher wants” was a very common comment; almost all students related their responses to what they thought their teacher was looking for. This might just be a way of wording, however, the idea that students learn to solve problems in a way to please their teachers is something to consider, what purpose do the students have for their own learning?Students' application of chemistry content
To answer the first research question on how students apply their chemistry content knowledge when solving the context-based problems, it was important to emphasise the chemistry focus in the tasks. As a consequence, all tasks had a focus on combining the content areas ‘organic chemistry’ with ‘chemical bonding’; and all contained structural formula, at least as a hint, as a way to make the students aware of the problem being a chemistry task. After analysis of the content knowledge the students applied when solving the tasks, it was apparent that the students recognized the connection between organic chemistry and chemical bonding. It was obvious in all students' responses that problems on organic chemistry, especially with the structural formula, made them look for functional groups, for instance exemplified by the following quote: When I get structural formula, I always try to find functional groups. If I find OH, then it's soluble in water. However, the student is quite brief in her utterance and the approach to remember factual knowledge is evident, by stating that hydroxyl groups make something soluble in water. The structural formula in the task was a way to ensure that students gave responses using chemistry content knowledge, a clear strategy students had when solving a context-based problem in the pre-study (Parchmann et al., submitted).Some chemistry concepts were common for students to apply, for instance solubility, polarity, and the molecules' size. For an overview of the most common content areas and concepts used to solve the context-based problems, see Table 2. Many tasks concentrate on solubility and therefore the concept of polarity is given as an explanation for solubility in almost all responses. However, it was a Swedish expression called “lika-löser-lika” (translated into English to “like-dissolve-like”) that the students mentioned repeatedly. In Sweden, students meet this expression early in their science schooling, in middle school or at least in lower secondary, and this expression is given by almost all students in their responses, for instance in the quote: If this drug is supposed to be dissolved in the blood, it has to look like blood since it's “lika-löser-lika”, it's always that in chemistry. When asked to elaborate and explain this expression, one student exemplifies water-solubility with an example: Water is H–O–H and to find something that is alike, it's always OH-groups I look for, since the only difference is a hydrogen atom. So if I find OH-groups in a molecule, then it's soluble in water since they look alike. This clear connection between hydroxyl groups and water-solubility was seen in all students' responses. By giving the students a structural formula, they emphasised entirely on hydroxyl groups: I just look for OH, if I find that, then it's polar. If I just find carbon, then it's unpolar. It's as simple as that. These quotes show a clear connection to recall of factual knowledge and the idea that chemistry problems always have ‘one correct answer’.
Besides chemical bonding and polarity, some students mentioned ‘acids and bases’ as an explanation for solubility. This content area was also given as an explanation from the chemistry professor as a possible solution to some of the tasks, for instance to explain how the medical drugs or the molecules in energy drinks are dissolved in the body or the environment. Taurine, one of the ingredients in energy drinks, is a zwitterion, in other words, an overall neutral molecule with both a negative and positive charge, like amino acids. Upper secondary students have not met the term zwitterion in the textbooks and probably not from the teacher. However, some students discussed possibilities for parts of molecules being acids and bases respectively. The connection with acids was molecules containing carboxylic groups (i.e. –COOH) or even sometimes isolated hydroxyl groups (i.e. –OH) where a hydrogen ion was suggested by some students to leave the molecule. Molecules were suggested being bases if they had an amino group (i.e. –NH2), one example already given above when a student explained how taurine could be dissolved in water. The possibility for amino groups to take part in hydrogen bonds was never suggested; hydrogen bonds were only associated with hydroxyl groups, a result also found in the pre-study (Parchmann et al., submitted).
Furthermore, the topic-related aspects of the tasks were often mentioned in the students' responses. The topic of ‘soaps and detergents’ is mentioned by all students, often in relation to what they have discussed at chemistry lessons or read in the chemistry textbooks. When describing fatty acids, the picture of a molecule with a polar head and an unpolar tail was frequently given, for instance in the following quote: We have talked a lot in chemistry class about fatty acids and their polar head and unpolar tail, like hydrophilic and hydrophobic parts, for instance when discussing fats and soaps. The function of soaps and detergents, fuels and fats are presented in all textbooks used by the students, thereby previously potentially known to the students. The topic of energy drinks is not presented in the textbooks; still this topic is mentioned in the responses, mainly by claiming the task to be both interesting and relevant. This relevance of an authentic topic can be exemplified by: Both me and my friends drink a lot of Monster and it's good to see the molecules to understand how it works in the body, I haven't really thought about that before. Now this taurine-molecule makes me curious… It was apparent that the topics encouraged the students to solve the problems and to not only give one short answer. To summarize how the students applied their content knowledge when solving these context-based chemistry problems, the combining of two content areas, i.e. organic chemistry and chemical bonding, was apparent in all students' answers, however mostly by recall of memorized facts. The most common concepts used to combine these areas were solubility and polarity, making these concepts threshold concepts important to master for further understanding of these problems.
Misconceptions of chemistry content
For the second research question, students' misconceptions were scrutinised. The relationship between chemical bonding and the ‘octet rule’ highlighted in previous research (Levy Nahum et al., 2010), and the anthropomorphic explanations (Adbo and Taber, 2013) became apparent here as well. One exemplary quote illustrates this: When I saw S and double bonds in taurine, that made me worried… But in caffeine, there are lots of carbons, that's not soluble in water… I would like to say that taurine is most soluble, it has OH, but the S makes me confused… How can it be two double bonds to the sulphur, and more than four bonds in total? Don't atoms want four bonds? And isn't sulphur bad? I think it's bad for the environment, like in fuels, we don't want to have sulphur compounds. Several students reacted on the sulphur atom; some even questioned if the structural formula was drawn correctly. The anthropomorphic explanations that atoms ‘want’ four bonds were found in several different responses, not only in the task on energy drinks.When students were asked to elaborate their responses on polarity, it was evident that several students have memorized electronegativity values in a way thinking about the atoms themselves as positive or negative without considering the bonding to other atoms. Taagepera and Noori (2000) have found this misconception among university chemistry students, claiming the urge to teach chemical bonding in another way, by asking the ‘why?’ question, not only remembering factual knowledge of Pauling electronegativity numbers. Polarity explains the dislocation of electrons in a covalent bond where one atom can attract the electron pair more than the other. However, it is always elementary to take into account both atoms in a bond, for instance in a bond between a carbon atom and a hydrogen atom, the shared electron pair is relatively equally shared since carbon and hydrogen have almost the same electronegativity value, the bond is therefore unpolar. On the other hand, the bond between a hydrogen and an oxygen atom has a dislocation of the electrons towards the oxygen atom because it attracts the electrons more, this bond is therefore polar. This dislocation of electrons is in chemistry represented using the sign of ∂+ and ∂−. To exemplify this misconception, the following quote is given:
S: Since the medical drug has to be dissolved in water, it has to be polar since water is polar and “lika-löser-lika”…
I: How do you know if a molecule is polar?
S: That's something I remember from last year, when we read about chemical bonding, I know oxygen is negative and hydrogen positive, so I remember that water is polar, we always wrote ∂+ and ∂−…
I: This was regarding water, how about the medical drug molecules?
S: They are more tricky since they are bigger, but I would look for C and O, lots of C then unpolar, lots of O then polar…
I: So you count the number of O to decide if the molecule is polar?
S: Yes, they are ∂−…
I: Always?
S: Yes… aren't they?
In the 15 tasks, almost all students mentioned the content area ‘structure of matter’. Concerning size, the common idea was that small molecules are more soluble in water, because, as one student claimed: the water molecule is so small and has to get close to the molecule it's supposed to dissolve, therefore it's better if the molecule is small. When the task concerned molecules with carbon chains, for instance fatty acids or fuels, the length of the carbon chain was central, mainly to interpret the strength of intermolecular bonding like van der Waals forces. One important translation experience was noticed here since previous research has highlighted difficulties when intermolecular bonds sometimes are named ‘forces’ (Levy Nahum et al., 2010). In the textbooks these students met, both intra- and intermolecular bonding are in Swedish named ‘bonds’. However, both this study and previous Swedish studies (Adbo and Taber, 2014) still emphasise students' misconceptions about bonding types. One exemplary quote discussing fatty acids in fats (see Table 1) describes this confusion: Well, yes, when it's double bonds in the tail, then the bonds between the molecules will be stronger. When there are only single bonds in the tail, there will only be weak van der Waals, the tail will not stick together that much. But if it has a double bond the bond between the tails is stronger. Here the student mixes ideas about strength of intra-molecular bonds, i.e. that double bonds are stronger than single bonds, with the strength of intermolecular bonds, i.e. van der Waals forces.
The discussion about single and double bonds in fatty acids, i.e. saturated and unsaturated molecules, was always on a rote level. Students recalled memorized facts, that double bonds in carbon chains gave a cis and trans structure, one being straight, the other bent. One student started her response to the question in the task on chemical properties of fatty acids: I really don't know what chemical properties are… perhaps solubility… or how they react. Then I know that unsaturated fatty acids are bent, if there's a double bond… you know… our teacher talked a lot about cis and trans… We have learnt that it's bad with saturated fatty acids because they are straight and then they can go into the cells… that's not good… It was common to give reference to what the teacher had said, and also to claim they ‘learn’ and ‘remember’ things. One final quote on structure emphasises this rote memorization regarding structure:
I: You mentioned cis and trans in the beginning, could you enhance you answer?
S: Yes, cis is straight, trans is bent.
I: What does that really mean, can you say anything more?
S: Well, it's the carbon chain I look at, and if it's straight then it's cis, it's on the same side…
I: What is on the same side?
S: … Hmm… It was something about different or same groups on the same side of the double bond… but I really don't remember…
I: You say double bond…
S: Yes, it has to be a double bond to be cis and trans, this I remember…
I: Why?
S: I don't really know, I don't remember…
In summary, misconceptions have been found in some of the students' responses, often related to the two most common applied concepts, i.e. solubility and polarity. These misconceptions have been evident in previous research, therefore we emphasise the need to improve students' understanding of these threshold concepts.
Problem-solving approaches with regard to the contextualization of the tasks
To answer the final research question, the contextualization of the content was focused. The students claimed that both the five topics as well as the three contexts were interesting and relevant, especially the personal context was appreciated; hence the contextualization is found to be important regarding the affective aspects of learning. However, we were also interested in investigating how students might be guided by the contextualization of the problem in two ways; they could be distracted or supported by context-related information. As mentioned earlier, after five interviews there was a slight change in method where the students met the tasks without the structural formula in the beginning, since the structural formula made them look for functional groups and directly discuss factual content knowledge relating to organic chemistry. When saving the structural formula until later in the interview, as a hint, students often came up with more broad explanations in the beginning to the topic and context, not only looking for organic chemistry functional groups. This can be highlighted by an excerpt where a student discusses taurine and caffeine in the energy drink RedBull® (the task mentioned earlier, see Table 1): I don't know what caffeine and taurine looks like, but if they are soluble in water, and they must be since we drink RedBull, they have to be polar, so probably OH-groups… and I would think they are quite small, if they were big, the small water molecule couldn't attach… it could also be an ionic compound, free ions are water-soluble… or it could be an acid or a base depending on pH… This broad answer could also be seen as a way for the students to “be safe” and give as many different responses as possible. However, this was one benefit of this change in method, that some of the students started to think in a more open and broad way suggesting different content areas explaining a chemistry problem.When scrutinising how the students used the contextualised parts in the problems, it was obvious that students mentioned the words used in the task. By relating to the topic of medical drugs, where the three tasks are presented in Fig. 1, the students who chose to solve the personal context mentioned the medical drugs in the body, for instance by discussing blood pH, brain and digestion in the stomach; whereas the students solving the societal context discussed how the molecules are spread in the environment by mentioning sewage plant, filters and biological cleaning with bacteria. Examples of these connections to the context are the two following quotes, one student solving the personal context and the second student solving the societal context from the topic of medical drugs (see Fig. 1): The medical drugs have to dissolve in water and to get into the blood. But it's also important that the molecule can be digested in the stomach, it has to be absorbed, and then it cannot be too big to get out from the stomach to the blood system… and for the societal context: They [the medical drugs] must pass the sewage plant, without being destroyed, I mean, they have biological cleaning with bacteria and then the medical drugs cannot change, and they must have long half-life, and be soluble in water… The first student discusses blood and stomach whereas the second student mentions sewage plants and biological cleaning using bacteria, thereby exemplifying the actual use of the contextualization of the task.
To quantify how common it was to use the contextualization of the task in the responses, Table 3 presents the number of students who discussed the context in their answers one or several times. It is apparent that the personal context, which was most common for students to choose when deciding which task they wanted to solve, had a clear impact on students' responses. The use of the topics was also evident, students related their responses to the topics with one exception, when solving the problem on fuels, only half of the students highlighted the connection to the topic. This use of context-related information was more common after the change of method, inclining the advantage of saving the structural formula as a hint in the process. We therefore argue that the context of the problems actually influences students' responses, for instance by giving them new ideas on how to solve the problem and not only focus on the structural formula and recall of factual knowledge of functional groups. In summary, even though students are not familiar with problems requiring higher order thinking, we argue for the use of these in school chemistry, both as problems to learn from as well as assessment tasks. Still, we need to get more insight on how students make use of this contextualization in the problem-solving process, which will be the focus of our forthcoming paper.
| Topic | Context | Number of responses | Contextual terms mentioned in the response |
|---|---|---|---|
| Medical drugs | Personal | 5 | 5 |
| Societal | 2 | 1 | |
| Professional | 1 | 0 | |
| Energy drinks | Personal | 6 | 6 |
| Societal | 0 | n/a | |
| Professional | 2 | 0 | |
| Fats | Personal | 6 | 5 |
| Societal | 1 | 0 | |
| Professional | 1 | 0 | |
| Fuels | Personal | 6 | 3 |
| Societal | 1 | 1 | |
| Professional | 1 | 0 | |
| Soaps and detergents | Personal | 7 | 7 |
| Societal | 1 | 1 | |
| Professional | 0 | n/a | |
Conclusions and implication
Students' applications of chemistry content when solving context-based problems has been scrutinised to get an in-depth insight on students' explanations when they are given problems where there is not only one single correct answer. The problems in themselves demand complex higher order thinking, something the students need to practice since they are unfamiliar with this from their previous school chemistry. CBL approaches have sometimes been criticized to highlight too much the affective aspects of learning (e.g.Marks and Eilks, 2010; Sevian and Talanquer, 2014), to exclusively make students interested and motivated to study science. Smith (2011) even expresses “considerable suspicion” within a group of professional chemists concerning CBL approaches because of future students' probable lack of factual knowledge. By relating to Roberts' (2007) dichotomy of scientific literacy, this orientation towards Vision II, that science primarily is related to real-life situations, and not on Vision I, with the orientation towards the science subject matter, has been an important starting point for this study where the chemistry content areas with their concepts have been analysed, both regarding the design of the problems in themselves as well as students' responses to the tasks. Although we find it important with Vision II, there has perhaps been too much concentration on the everyday life making the content knowledge invisible, as claimed by Sevian and Talanquer (2014). At the upper secondary level, preparing for university chemistry, the subject matter has to be emphasised, however we think it is possible to do so within the CBL approaches and by applying both Vision I and II, for example by using well-designed context-based chemistry problems for students to solve.When designing these problems, the awareness of the topics, contexts and content areas with specific concepts have been time-consuming, however the design principles have been helpful to both assess students' application of content knowledge as well as emphasising authentic and relevant topics to engage students in the problem-solving process. Our tasks are short enough to solve within existing chemistry courses and thereby possible to use in school chemistry even though the curriculum has no explicit context-based focus. Several previous research projects are more extensive and are carried out in countries with full curricula changes; tasks like those investigated here can be implemented as starting points of a CBL approach. Based on the results we found, we claim that those tasks would engage students to look for connections between the chemical content they have learned (or should learn) and phenomena or questions they meet in their daily-life. Students' apparent use of the contextualization of our tasks, especially regarding the personal context has been supporting and not distracting to the students. This assures that the context in itself is helpful when solving the problem, not being merely a decoration or a motivational trick something Nentwig et al. (2007) warn about. Therefore, we assert the possibility to use these tasks to work on as learning situations, not only as assessment tasks, for students to develop their problem-solving skills and improve their explanations to be more effective and structured. Still, they are suitable to assess students' content knowledge since they ask for broad answers highlighting for students that chemistry problems have more than one single correct answer students are meant to memorize and thereafter recall. Thus we claim that implementation of these kinds of problems when teaching chemistry is a viable route to enhance higher order thinking in chemistry classrooms. One evident example of higher order thinking from this study is represented by students' own question-posing; when the students were encouraged to develop their responses, the students often responded with questions and talked in a dialogic way to move further in their problem solving. It was also apparent that these tasks made the students reason further and develop their responses, not only stating the ‘correct answer’.
Regarding the chemistry content areas, chemical bonding has been found to be a fundamental area important to master to give explanations to these problems. Almost all responses given by our students can be related to chemical bonding, when discussing solubility, chemical reactions or molecular structure. However, since students' misconceptions within this area are common for instance with the central concept of electronegativity, we concur with Levy Nahum et al.'s (2007, 2008, 2010) request for a new bottom-up approach where chemical bonding is taught based on physical elemental principles by applying the idea of continuum of bond strengths and by avoiding the dichotomous classification of bonds as covalent/ionic and intra/intermolecular. In Swedish textbooks, this new bottom-up approach has not been established, and therefore we emphasise the conclusions given by Bergqvist et al. (2013) asserting the need to fill the gap between research and textbook writers. Furthermore, since students made use of the contextualization of the tasks by relating their responses to both the topic and the context, we encourage teachers and textbook writers to connect their chemistry content to everyday life by developing and designing problems with explicit and relevant topics and contexts. Unquestionably, since students are unfamiliar with these kinds of problems, both the design process of the tasks and the problem-solving process need to be implemented in both pre-service and in-service training of chemistry teachers to make teachers confident to change focus from recall of factual knowledge to higher order thinking. Therefore, we would like to close this paper using Henderleiter et al.'s words: “if the goals of chemistry instruction included fostering critical thinking skills and equipping students with the skills and strategies needed to solve problems in contexts beyond those they were taught, then instructional practice must model and apply what is known about how people learn.” (Henderleiter et al., 2001, p. 1129). This was stated several years ago, however, still a valid statement worth highlighting.
References
- Adbo K. and Taber K. S., (2013), Developing chemical understanding in the explanatory vacuum: Swedish high school students' use of an anthropomorphic conceptual framework to make sense of chemical phenomena, in Tsaparlis G. and Sevian H. (ed.), Concepts of Matter in Science Education, Dordrecht: Springer, pp. 347–370.
- Adbo K. and Taber K. S., (2014), Developing an Understanding of Chemistry: A case study of one Swedish student's rich conceptialisation for making sense of upper secondary school chemistry, Int. J. Sci. Educ., 36(7), 1107–1136.
- Aikenhead G. S., (2006), Science Education for Everyday Life: Evidence-based Practice, New York: Teachers College Press.
- Anderhag P., Emanuelsson P., Wickman P.-O. and Hamza K. M., (2013), Students' Choice of Post-Compulsory Science: in search of schools that compensate for the socio-economic background of their students, Int. J. Sci. Educ., 35(18), 3141–3160.
- Andersson S., Sonesson A., Svahn O., Tullberg A. and Rydén L., (2008), Gymnasiekemi B [Chemistry B for upper secondary school], Stockholm: Liber.
- Bennett J., Lubben F. and Hogarth S., (2007), Bringing Science to Life: A Synthesis of the Research Evidence on the Effects of Context-Based and STS Approaches to Science Teaching, Sci. Educ., 91, 347–370.
- Bergqvist A., Drechsler M., de Jong O. and Chang Rundgren S.-N., (2013), Representations of chemical bonding models in school textbooks – help or hindrance for understanding? Chem. Educ. Res. Pract., 14(4), 589–606.
- Bernholt S. and Parchmann I., (2011), Assessing the complexity of students' knowledge in chemistry, Chem. Educ. Res. Pract., 12, 167–173.
- Bodner G. M. and Domin D. S., (2000), Mental Models: The Role of Representations in Problem Solving in Chemistry, Univ. Chem. Educ., 4(1), 24–30.
- Bodner G. M. and McMillen T. L. B., (1986), Cognitive Restructuring as an early stage in problem solving, J. Res. Sci. Teach., 23(8), 727–737.
- Broman K., (submitted), Finding and elaborating frameworks for solving context-based chemistry problems.
- Broman K., Ekborg M. and Johnels D., (2011), Chemistry in crisis? Perspectives on teaching and learning chemistry in Swedish upper secondary schools, Nord. J. Sci. Educ., 7(1), 43–60.
- Broman K. and Simon S., (2014), Upper Secondary School Students' Choice and Their Ideas on How to Improve Chemistry Education, Accepted for publication in Int. J. Sci. Math. Educ., DOI:10.1007/s10763-014-9550-0.
- Bulte A. M. W., Westbroek H. B., de Jong O. and Pilot A., (2006), A Research Approach to Designing Chemistry Education Using Authentic Practices as Contexts, Int. J. Sci. Educ., 28(9), 1063–1086.
- Bybee R., McCrae B. and Laurie R., (2009), PISA 2006: An Assessment of Scientific Literacy, J. Res. Sci. Teach., 46(8), 865–883.
- Cartrette D. P. and Bodner G. M., (2010), Non-Mathematical Problem Solving in Organic Chemistry, J. Res. Sci. Teach., 47(6), 643–660.
- Chin C. and Brown D. E., (2002), Student-generated questions: A meaningful aspect of learning science, Int. J. Sci. Educ., 24(5), 521–549.
- Christensson C. and Sjöström J., (2014), Chemistry in context: analysis of thematic chemistry videos available online, Chem. Educ. Res. Pract., 15(1), 59–69.
- de Jong O., (2006), Making Chemistry Meaningful. Conditions for Successful Context-based Teaching, Educ. Quim., 17, 215–221.
- Demuth R., Parchmann I. and Ralle B., (2006), Chemie im Kontext, Berlin: Cornelsen.
- Dori Y. J., Tal R. T. and Tsaushu M., (2003), Teaching Biotechnology Through Case Studies – Can We Improve Higher Order Thinking Skills of Nonscience Majors? Sci. Educ., 87(6), 767–793.
- Fach M., de Boer T. and Parchmann I., (2007), Results of an interview study as basis for the development of stepped supporting tools for stochiometric problems, Chem. Educ. Res. Pract., 8(1), 13–31.
- Fechner S., (2009), Effects of Context-oriented Learning on Student Interest and Achievement in Chemistry Education, Berlin: Logos Verlag Berlin GmbH.
- Gilbert J. K., (2006), On the Nature of “Context” in Chemical Education, Int. J. Sci. Educ., 28(9), 957–976.
- Gilbert J. K., Bulte A. M. W. and Pilot A., (2011), Concept Development and Transfer in Context-Based Science Education, Int. J. Sci. Educ., 33(6), 817–837.
- Graeber W. and Lindner M., (2008), The impact of the PARSEL way to teach science in Germany on interest, scientific literacy, and German national standards, Sci. Educ. Int., 19(3), 275–284.
- Hayes J. R., (1989), The Complete Problem Solver, 2nd edn, Hillsdale, NJ: Lawrence Erlbaum Associates.
- Häussler P., Hoffman L., Langeheine R., Rost J. and Sievers K., (1998), A typology of students' interest in physics and the distribution of gender and age within each type, Int. J. Sci. Educ., 20(2), 223–238.
- Henderleiter J., Smart R., Anderson J. and Elian O., (2001), How Do Organic Chemistry Students Understand and Apply Hydrogen Bonding? J. Chem. Educ., 78(8), 1126–1130.
- Henriksson A., (2007), Syntes B – kemi för gymnasieskolan [Synthesis B - chemistry for upper secondary school], Malmö: Gleerups.
- Hofstein A., Eilks I. and Bybee R., (2011), Societal Issues and Their Importance for Contemporary Science Education - a pedagogical justification and the state-of-the-art in Israel, Germany, and the USA, Int. J. Sci. Math. Educ., 9, 1459–1484.
- Jidesjö A., Oscarsson M., Karlsson K.-G. and Strömdahl H., (2009), Science for all or science for some: What Swedish students want to learn about in secondary science and technology and their opinions on science lessons, Nord. J. Sci. Educ., 11(2), 213–229.
- King D., (2012), New perspectives on context-based chemistry education: using a dialectical sociocultural approach to view teaching and learning, Stud. Sci. Educ., 48(1), 51–87.
- King D. and Ritchie S. M., (2013), Academic Success in Context-Based Chemistry: Demonstrating fluid transitions between concepts and context, Int. J. Sci. Educ., 35(7), 1159–1182.
- Levy Nahum T., Mamlok-Naaman R., Hofstein A. and Krajcik J., (2007), Developing a New Teaching Approach for the Chemical Bonding Concept Aligned with Current Scientific and Pedagogical Knowledge, Sci. Educ., 91(4), 579–603.
- Levy Nahum T., Mamlok-Naaman R., Hofstein A. and Kronik L., (2008), A New “Bottom-Up” Framework for Teaching Chemical Bonding, J. Chem. Educ., 85(12), 1680–1685.
- Levy Nahum T., Mamlok-Naaman R., Hofstein A. and Taber K. S., (2010), Teaching and learning the concept of chemical bonding, Stud. Sci. Educ., 46(2), 179–207.
- Marks R., Bertram S. and Eilks I., (2008), Learning chemistry and beyond with a lesson plan on potato crisps, which follows a socio-critical and problem-oriented approach to chemistry lessons – a case study, Chem. Educ. Res. Pract., 9(3), 276.
- Marks R. and Eilks I., (2010), Research-based development of a lesson plan on shower gels and musk fragrances following a socio-critical and problem-oriented approach to chemistry teaching, Chem. Educ. Res. Pract., 11(2), 129–141.
- Nakhleh M. B. and Mitchell R. C., (1993), Concept Learning versus Problem Solving, J. Chem. Educ., 70(3), 190–192.
- Nentwig P., Demuth R., Parchmann I., Gräsel C. and Ralle B., (2007), Chemie im Kontext: Situated Learning in Relevant Contexts while Systematically Developing Basic Chemical Concepts, J. Chem. Educ., 84(9), 1439–1444.
- Nentwig P. and Waddington D., (2005), Making it relevant: Context based learning of science, Münster: Waxmann.
- Ngu B. H. and Yeung A. S., (2012), Fostering analogical transfer: The multiple components approach to algebra word problem solving in a chemistry context, Contemp. Educ. Psychol., 37, 14–32.
- Overman M., Vermunt J. D., Meijer P. C., Bulte A. M. W. and Brekelmans M., (2013), Textbook Questions in Context-Based and Traditional Chemistry Curricula Analysed from a Content Perspective and a Learning Activities Perspective, Int. J. Sci. Educ., 35(17), 2954–2978.
- Parchmann I., Broman K., Busker M. and Rudnik J., (submitted), Context-Based Teaching and Learning on School and University Level.
- Parchmann I., Gräsel C., Baer A., Nentwig P., Demuth R. and Ralle B., (2006), “Chemie im Kontext”: A symbiotic implementation of a context-based teaching and learning approach, Int. J. Sci. Educ., 28(9), 1041–1062.
- Pilot A. and Bulte A. M. W., (2006), The Use of “Contexts” as a Challenge for the Chemistry Curriculum: Its successes and the need for further development and understanding, Int. J. Sci. Educ., 28(9), 1087–1112.
- Ramsden J. M., (1997), How does a context-based approach influence understanding of key chemical ideas at 16? Int. J. Sci. Educ., 19(6), 697–710.
- Roberts D. A., (2007), Scientific Literacy/Science Literacy, in Abell S. K. and Lederman N. G. (ed.), Handbook of Research on Science Education, Mahwah, New Jersey: Lawrence Erlbaum Associates, Inc.
- Sadler T. D. and Zeidler D., L., (2009), Scientific Literacy, PISA, and Socioscientific Discourse: Assessment for Progressive Aims of Science Education, J. Res. Sci. Teach., 46(8), 909–921.
- Salta K. and Tzougraki C., (2011), Conceptual Versus Algorithmic Problem-solving: Focusing on Problems Dealing with Conservation of Matter in Chemistry, Res. Sci. Educ., 41, 587–609.
- SCB (2012), Statistics Sweden: Övergång gymnasieskola - högskola [Transition Upper Secondary School - University]. Retrieved 2014-04-01, from http://www.scb.se/sv_/Hitta-statistik/Statistik-efter-amne/Utbildning-och-forskning/Befolkningens-utbildning/Overgang-gymnasieskola-hogskola/Aktuell-pong/2011L12/Behallare-for-Press/Overgang-fran-gymnasieskola-till-hogskola-lasaret-201011/.
- Sevian H. and Talanquer V., (2014), Rethinking chemistry: a learning progression on chemical thinking, Chem. Educ. Res. Pract., 15(1), 10–23.
- Sjöberg S. and Schreiner C., (2010), The ROSE project. An overview and key findings, Oslo: University of Olso, pp. 1–31.
- Sjöström J., (2013), Towards Bildung-Oriented Chemistry Education, Sci. Educ., 22(3), 1873–1890.
- Smith D. K., (2011), From crazy chemists to engaged learners through education, Nat. Chem., 3(9), 681–684.
- Swedish National Agency for Education, (2000), Chemistry syllabus for upper secondary school, Stockholm: The Swedish National Agency for Education.
- Taagepera M. and Noori S., (2000), Mapping Students' Thinking Patterns in Learning Organic Chemistry by the Use of Knowledge Space Theory, J. Chem. Educ., 77(9), 1224–1229.
- Taasoobshirazi G. and Carr M., (2008), A review and critique of context-based physics instruction and assessment, Educ. Res. Rev., 3(2), 155–165.
- Taber K. S. and Coll R. K., (2002), Bonding, in Gilbert J. K., de Jong O., Justi R., Treagust D. F. and van Driel J. H. (ed.), Chemical Education: Towards Research-based Practice, Dordrecht: Kluwer Academic Publishers, pp. 213–234.
- Taber K. S. and Watts M., (2000), Learners' Explanations for Chemical Phenomena, Chem. Educ. Res. Pract., 1(3), 329–353.
- van Oers B., (1998), From Context to Contextualizing, Learn. Instr., 8(6), 473–488.
- Zohar A., (2004), Higher Order Thinking in Science Classrooms: Students' Learning and Teachers' Professional Development, Dordrecht: Kluwer Academic Publishers, vol. 22.
- Zohar A. and Dori Y. J., (2003), Higher Order Thinking Skills and Low-Achieving Students: Are They Mutally Exclusive? J. Learn. Sci., 12(2), 145–181.
- Zoller U. and Dori Y. J., (2002), Algorithmic, LOCS and HOCS (chemistry) exam questions: performance and attitudes of college students, Int. J. Sci. Educ., 24(2), 185–203.
- Zoller U. and Pushkin D. B., (2007), Matching Higher-Order Cognitive Skills (HOCS) promotion goals with problem-based laboratory practice in a freshman organic chemistry course, Chem. Educ. Res. Pract., 8(2), 153–171.
| This journal is © The Royal Society of Chemistry 2014 |