Students' understanding of alkyl halide reactions in undergraduate organic chemistry
Daniel
Cruz-Ramírez de Arellano
a and
Marcy H.
Towns
*b
aUniversity of South Florida, Department of Chemistry, 4202 East Fowler Ave., Tampa, FL 33620, USA
bPurdue University, Department of Chemistry, 560 Oval Drive, West Lafayette, IN 47906, USA. E-mail: mtowns@purdue.edu
First published on 25th March 2014
Abstract
Organic chemistry is an essential subject for many undergraduate students completing degrees in science, engineering, and pre-professional programs. However, students often struggle with the concepts and skills required to successfully solve organic chemistry exercises. Since alkyl halides are traditionally the first functional group that is studied in undergraduate organic chemistry courses, establishing a robust understanding of the concepts and reactions related to them can be beneficial in assuring students' success in organic chemistry courses. Therefore, the purpose of this study was to elucidate and describe students' understanding of alkyl halide reactions in an undergraduate organic chemistry course. Participants were interviewed using a think-aloud protocol in which they were given a set of questions dealing with reactions and mechanisms of alkyl halide molecules in order to shed light on the students' understanding of these reactions and elucidate any gaps in understanding and incorrect warrants that may be present. These interviews were transcribed and analyzed using a qualitative inquiry approach and a modified Toulmin scheme. In general, the findings from this study show that the students exhibited gaps in understanding and incorrect warrants dealing with: (1) classifying substances as bases and/or nucleophiles, (2) assessing the basic or nucleophilic strength of substances, and (3) accurately describing the steps that take place and reactive intermediates that form during alkyl halide reaction mechanisms. In addition, implications for teaching and future research are discussed.
Introduction
Organic chemistry is a course that is not only important and mandatory in chemistry and chemical engineering undergraduate programs, but it is also an integral part in other undergraduate curricula such as biology, biochemistry, medicine, pharmacy, and nanotechnology. There are many instructional strategies that have been developed to aid in the teaching of organic chemistry at the undergraduate level (Bradley, Ulrich, Jones, and Jones, 2002; Browne and Blackburn, 1999; Kingsbury and Schelble, 2001; Horowitz, 2007; Raker and Towns, 2010; 2012a, b). Nonetheless, there is a need for instructional strategies that are based on rigorous research studies that have elucidated student understanding of organic chemistry concepts. If the undergraduate organic chemistry curriculum was developed using research-based approaches and tools, it could be argued that instructors would be more successful in having students truly comprehend the topics of organic chemistry.Student understanding of fundamental organic chemistry concepts
Organic chemistry has historically being considered difficult and pressure-packed (Bradley et al., 2002). It has been suggested that its difficulty originates from the recognition that organic molecular reactivity is a function of multiple and interacting variables, such as steric and electronic variables (Kraft et al., 2010). Student understanding of organic chemistry concepts has been elucidated to some extent for several topics. For the topic of Lewis structures, it has been found that students are often confused about how to construct valid Lewis structures and are unable to recognize the implicit information that can be determined about a molecule by not being able to integrate other knowledge, such as polarity or boiling point, to the explicit information that is shown with Lewis structures (Cooper et al., 2010, 2012). Additionally research has shown that organic chemistry instructors should not take for granted that students will necessarily abstract a salient feature from a pictorial representation of an organic compound (Domin et al., 2008).For the topic of acids and bases in an organic chemistry context, it has been found that students correctly define, give examples, and employ acids and bases according to the Brønsted–Lowry definition, but that they struggle with defining and employing acids and bases according to the Lewis definition, which is the most prevalent one in organic chemistry (Cartrette and Dobberpuhl, 2009; Cartrette and Mayo, 2011). In addition, it has been found that students' mental models of acids and acid strength often rely on heuristic decision-making and superficial characteristics of the molecules as opposed to relying on coherent cognitive constructions of molecular acidity (Bhattacharyya, 2006; McClary and Talanquer, 2011). For the topic of organic reaction mechanisms and the arrow-pushing formalism employed to show electron movement in them, it has been found that the curved arrows used in the electron-pushing formalism hold no physical meaning for many students when they are performing exercises that require their employment (Bhattacharyya and Bodner, 2005; Ferguson and Bodner, 2008).
Some studies have focused on elucidating students' alternative conceptions regarding organic chemistry concepts. An alternative conception, sometimes described as a misconception, means any concept that differs from the commonly accepted scientific understanding of the term (Nakhleh, 1992). A list of alternative conceptions related to organic chemistry that have been identified are: (1) the stability of the final products is more important than the feasibility of the reaction mechanism required to arrive at said products, (2) hydrogen bonds can be induced with hydrocarbons, (3) bond polarities depend on absolute electronegativities of atoms only, whether they are connected or not, (4) the functional group of a molecule determines its acidic strength, (5) when alkyl halides are heated with strong bases in the presence of alcohols, alcohols are generated as the major product, and (6) the addition of water to an alkene in the presence of acid leads to the formations of ketones, aldehydes, and ethers (Taagepera and Noori, 2000; Henderleiter et al., 2001; Rushton et al., 2008; McClary and Bretz, 2012; Şendur, 2012; Şendur and Toprak, 2013).
Several reasons that explain why students struggle to develop a thorough and coherent understanding of organic chemistry have been proposed. Grove and Bretz (2010) proposed that as students progress in an organic chemistry curriculum, they perceive that organic chemistry becomes progressively less straightforward. In particular, this notion was applied to substitution and elimination reactions where more than one product could be produced, thus moving away from an approach where there is one correct answer. As the year progressed, students also encountered synthesis problems where multiple pathways to a targeted product were possible.
Anderson and Bodner (2008) propose that the intense speed with which material is covered in an organic chemistry course, along with the complexity of the material, forces students to become learners who focus on applying memorized rules, often incorrectly, without a coherent understanding of the reasons for the rules or when they should be applied.
Purpose of the study
The aim of this study is to elucidate student understanding of organic chemistry concepts. In this case, the chosen organic chemistry topic is alkyl halide reactions because alkyl halides are the first functional group that is traditionally covered in organic chemistry courses. It is through studying the properties and reactivity of this functional group that students learn about two of the main mechanisms that become a recurring theme throughout the course: substitution reactions and elimination reactions. By assuring a sound understanding of this topic, it could be argued that the foundation upon which the knowledge of other functional groups is constructed has been strengthened. Therefore, this strengthened foundation could assure better performance in the subsequent topics in an organic chemistry course, and possibly in the course as a whole.To examine and elucidate student understanding of alkyl halide reactions in undergraduate organic chemistry, we asked the following research question: Which mistakes or gaps in understanding emerge when students are asked to predict products or mechanisms of reactions involving alkyl halides?
Theoretical framework: personal constructivism
The theoretical framework that was selected for this study is personal constructivism (Geelan, 1997). This framework emphasizes the idea that individuals construct knowledge for themselves through construing the repetition of events, and that knowledge is individual and adaptive rather than objective. Bodner (1986) interpreted personal constructivism in the context of chemistry classrooms. He suggested that individuals use what they already know to organize and make sense of new information, in other words, that “knowledge is constructed in the mind of the learner” (p. 873). Therefore, the only way to elucidate what students know about a certain topic is by directly asking them to describe how they have constructed and connected the concepts relevant to that certain topic.Methods
In order to discover how students had constructed their knowledge of alkyl halide reactions, a study was designed in which participants were asked to solve a series of organic chemistry questions which contained all the essential concepts that are relevant to the topic of alkyl halide reactions. Semi-structured interviews were employed because it gave the researchers the opportunity to see the topic of alkyl halide reactions from the perspective of the participants whose meaning-making was being studied. In addition to using semi-structured interviews, incorporating the use of a think-aloud protocol (Bowen, 1994) during these was essential to understand how the participants had constructed the knowledge of alkyl halide reactions. In a think-aloud protocol, participants are asked to express vocally, or think aloud, what they are considering and how they are proceeding as they solve a given task through initial questions or prompts and follow-up questions or prompts to probe reasoning. This would permit the researchers to understand the participants' thought process in a deep and thorough manner, making it possible to pinpoint both the mistakes and gaps in understanding that lead them to incorrect answers and the correct warrants that lead them to correct answers.Participants and setting
The setting for this study was a large, state-supported, research intensive university in the Midwestern United States of America. The participants were enrolled in a three-credit undergraduate-level organic chemistry course that is offered every semester. The course was the first semester of a two-semester non-majors organic chemistry course. For recruitment, purposeful homogenous sampling was employed. Homogenous sampling (Patton, 2002) aims to understand and describe a particular group in depth. In the case of this study, the group to be described in depth is organic chemistry students enrolled in the chosen course. Twenty-two participants were recruited through electronic mail and personal visitations to laboratory sections by one of the researchers. All the participants were volunteers and were not offered any compensation for participating in the study. The participants were all undergraduate students, ranging from their second to fourth year of study. The majors of the participants were varied, and mostly included biological sciences, pre-professional programs (pre-medicine, pre-veterinary), health sciences, and psychology. There were seventeen participants who self-identified as female and five participants who self-identified as male; all were given pseudonyms to protect their identities. The participants were interviewed immediately after the third exam of the course, which was the one with the relevant content knowledge, during the fall semester of 2011. Approval from the Institutional Review Board (IRB) of the university was received in 2010.Interview questions
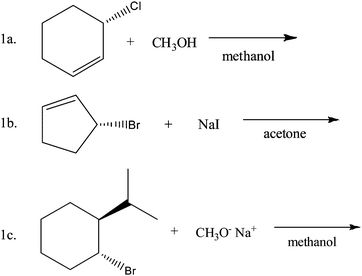
The participants were asked to perform nine organic chemistry questions. These included predicting the major organic product of a given set of reagents, proposing a reasonable mechanism that explained a given organic reaction, and conceptual questions asking about fundamental concepts that are covered prior to alkyl halide reactions. This report focuses on analyzing student responses to questions 1a, 1b, and 1c (the first three of the nine) that are shown in Fig. 1. These questions all require students to predict the product of an alkyl halide reaction.Draw a structural formula for the major organic product of each reaction.
Data sources
All the interviews were recorded using a digital audio recorder and a Livescribe pen (Livescribe, 2010). The Livescribe pen is a “smart pen” with an embedded computer and digital audio recorder. When the pen is used with dot-pattern digital paper it records what it writes for later uploading to a computer. When uploaded, the student's audio and writing are synchronized into one file. All of the interview questions were printed in the dot-pattern digital paper, one question per sheet of paper, and were presented to the participants one question at a time. Each participant proposed an answer for the interview question and the interviewer asked follow-up questions regarding the reasoning behind the participant's answer until the interviewer considered to have a complete picture of the participant's logic and reasoning behind their answer. After all the interviews were conducted, the main researcher listened to them and wrote memos for each of them making general observations. These memos served as a secondary data source.All interviews were transcribed verbatim using the InqScribe© computer software (InqScribe, 2005). The audio recordings and transcriptions served as primary data sources, since they were technically created during the time of the study. In addition, there was a third primary data source in the form of Livescribe© pen worksheets. This primary data source proved to be invaluable because it permitted the researcher to pinpoint exactly what the participants were drawing as they offered their explanations, therefore making sure that their understanding was elucidated with reliability. Linenberger and Bretz (2012) and Harle and Towns (2013) described this technology as a research tool and its usefulness when elucidating student understanding of topics that involve heavy drawing of diagrams or representations of any kind.
Data analysis
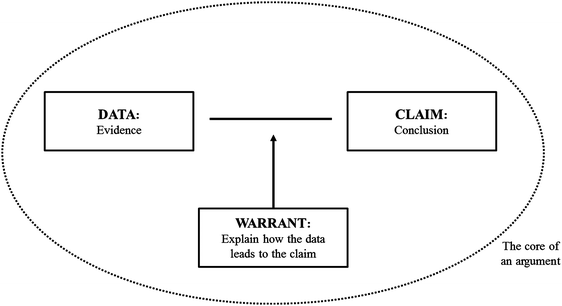
Questions 1a, 1b, and 1c were analyzed using a modified version of Toulmin's model of argumentation as a framework (Toulmin, 1958). This methodology was adapted to chemistry education research from research on undergraduate mathematics education by Cole et al. (2012). Toulmin (1958) created a model to describe the structure and function of argumentation. The model proposes that the core of an argument consists of three parts: the data, the claim, and the warrant. In an argument, the participant makes a claim and presents evidence or data to support that claim. To further improve the strength of the argument, participants often provide more clarification that connects the data to the claim, which serves as a warrant, or a connector between the two. In addition, sometimes rebuttals or qualifiers arise to propel the argument forward. Nonetheless, for the purpose of this analysis, Toulmin's model of argumentation was modified to only include claims, data, and warrants, which was deemed sufficient to describe the arguments presented by the participants. An illustration of the modified Toulmin's model of argumentation can be seen in Fig. 2.Firstly, the interview transcripts for these three questions were coded line by line for all the participants using the three classifications of the modified Toulmin's model of argumentation: claim, data, and warrants. The participants' proposed major organic products were coded as their claim. The different statements they provided to provide evidence or explain their reasoning for proposing the products were coded as either data or warrants, depending on the nature of the statement. An argumentation scheme was generated for each question, for each participant. Secondly, all of the participants' argumentation schemes for each question were analyzed to identify emergent trends and commonalities, in addition to being compared to an expert argumentation scheme generated by the researchers and a faculty expert in organic chemistry. This step in the analysis aided in identifying gaps in understanding and incorrect warrants exhibited by the participants. Thirdly, the trends and commonalities were used to categorize the participants into discrete groups described by the different types of argumentation schemes that were identified. An overview of this methodological approach is shown in Fig. 3.
Reliability and validity
The researchers met with three additional chemistry education colleagues at the beginning of the analysis to collaboratively code a portion (questions 1a and 1b) of the whole transcripts using the modified Toulmin's model of argumentation. This collaborative coding was carried out across multiple participants to achieve a consistent use of the coding scheme and involved discussing each transcript portion until agreement across all coders was achieved. Afterwards, based on that exercise, the main researcher independently coded the remaining of the data. Subsequently, the other researcher and one chemistry education colleague revised the coding done by the main researcher to confirm agreement with the original coding scheme and coherency. The interview protocol and the expert argumentation schemes were prepared by the main researcher and corroborated by an organic chemistry faculty member to assure that they were coherent and correct.Findings and discussion
In order to answer questions 1a, 1b, and 1c, it was expected for the participants to primarily consider the four main reactions of alkyl halides. Two of these reactions are substitution reactions: unimolecular nucleophilic substitution reactions (SN1) and bimolecular nucleophilic substitution reactions (SN2). An SN1 reaction is a two-step interchange of chemical species, with bond breaking preceding bond formation. The first step is ionization to form a carbocation and the second step is the reaction of the carbocation with a nucleophile. An SN2 reaction is a bimolecular concerted displacement of one chemical species by another on an sp3 hybridized carbon atom. The two other main reactions of alkyl halides are elimination reactions: first-order elimination reactions (E1) and second-order elimination reactions (E2). An E1 reaction is a multistep elimination where the leaving group is lost in a slow ionization step and then a proton is lost in a second step. The formation of the most substituted alkene is generally preferred. An E2 reaction is a concerted elimination involving a transition state where the base is abstracting a proton at the same time that the leaving group is leaving. The anti-coplanar transition state is generally preferred (Wade, 2006). The three analyzed questions contained secondary alkyl halides which were purposefully chosen to force the participants to consider the possibility of any of the four aforementioned mechanisms since primary and tertiary alkyl halides, unlike secondary alkyl halides, do not usually possess the possibility of undergoing all four mechanisms. Most participants were able to identify the secondary nature of the alkyl halides; therefore the assessment of that particular characteristic of the substrate was not identified in this study to be a significant gap in understanding.Question 1a
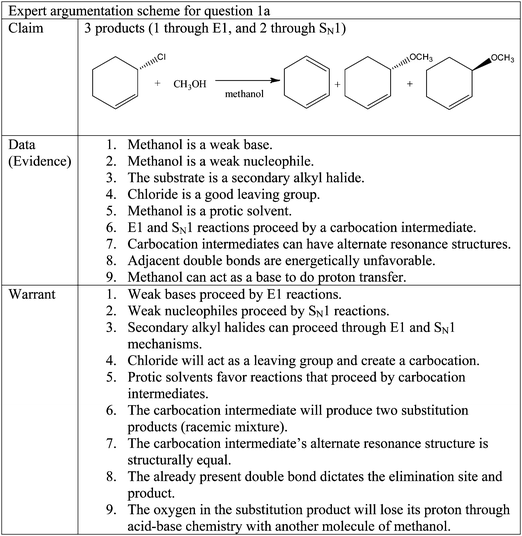
Question 1a, seen in Fig. 1, has three molecules in the correct answer. Cyclohexa-1,3-diene is the product of an E1 reaction while (S)-3-methoxycyclohexene and (R)-3-methoxycyclohexene are the products of an SN1 reaction. The expert argumentation scheme for question 1a is presented in Fig. 4. It is not expected that the student participants in this study will use every data and warrant in their arguments because an expert would have a greater depth and breadth of cognitive resources. In the discussion which follows students are able to construct arguments consisting of a claim, data, and warrants that are reasonable and well supported using fewer pieces of data and warrants than are listed in Fig. 4.The participants' responses to question 1a were categorized in three groups according to their claims. Group 1 had correct claims, Group 2 had partially correct claims, and Group 3 had incorrect claims.
Barbara: …methanol is going to be a weak nucleophile, and also kind of a crappy base, so looks to me like this is gonna be a racemic mixture, or it's gonna go through S N 1 and E1 reaction.
Barbara and Aurora also recognized the formation of a stable carbocation during the mechanism, along with the stereochemical implications that this intermediate would have in the products. Knowing the mechanism proved to be an important aspect of knowing why the SN1 reaction would produce a racemic mixture of products.
Aurora: …I did S N 1 and that has a carbocation which means it [the nucleophile] can come in from either side.
It is interesting that even though these two participants did not mention all of the data and warrants in the expert argumentation scheme such as the nature of the substrate, their reasoning generated a well-thought argument.
Sub-group 2a. Sub-group 2a is composed of three participants, Anna, Dennis, and Isabel, who claimed that an SN1 reaction would occur, yielding the two substitution products shown in the expert argumentation scheme. These participants identified the nucleophilic nature of CH3OH and correctly classified it as a weak nucleophile. In addition, they were also aware of the carbocation intermediate that exists in SN1 reactions, along with the stereochemical implications for the products.
The participants in this sub-group were missing the product produced by the E1 mechanism. The gap in understanding which led to this was that they did not recognize the basic nature of CH3OH. By not classifying CH3OH as a weak base, in addition to a weak nucleophile, they omitted an elimination product. Two incorrect warrants were also identified. These incorrect warrants offer insight as to why these participants did not consider an elimination reaction as an option in their answer. The first incorrect warrant is that the presence of a double bond in the starting material implies that a substitution is the reaction to be done.
Dennis: And I think that I'll do a substitution reaction, uh, just because there's a double bond.
The second incorrect warrant is that the presence of a solvent in the starting materials implies that a substitution is the reaction to be done.
Interviewer: …is there any particular reason why you eliminated the elimination option?
Anna: I'm thinking because there's a solvent, most likely.
These two incorrect warrants demonstrate that participants picked up cues from the question, such as which reagents are used and/or displayed, and attribute to them meanings that were unintended when the question was composed.
Sub-group 2b. Sub-group 2b is composed of six participants, Allison, Gabriel, Carl, Emily, Sonia, and Amy, who claimed that a substitution reaction would take place, yielding one product. The stereochemistry of the product was expressed either as absent or with just one of the enantiomers shown in the expert argumentation scheme. The main piece of data used by these participants was that CH3OH is a nucleophile.
These participants did not recognize the basic nature of CH3OH, leading them to not take into account the formation of the elimination product through E1, exhibiting the same gap in understanding identified in sub-group 2a. In addition, they did not assess the nucleophilic strength of CH3OH. Nonetheless, unlike sub-group 2a, they have gaps in understanding regarding the mechanism and the carbocation intermediate of the substitution reaction. This is why they only suggest the formation of one product, instead of two; they did not consider a carbocation intermediate which would produce two enantiomers as substitution products.
Besides the gaps in understanding, there are several incorrect warrants exhibited by the participants in sub-group 2b. Initially, there are two incorrect warrants which led participants in this group to believe that only a substitution reaction would happen. The first incorrect warrant is that the presence of a protic solvent, as is the case with CH3OH, implies that a substitution must be done.
Gabriel: I thought it was because if you have a protic solvent, it's more likely to be an S N reaction than elimination, for a protic.
The second incorrect warrant is that the presence of the particular CH3OH reagent always means that a substitution reaction must be done.
Interviewer: Why did you choose a substitution over an elimination?
Sonia: I don't think that's what happens with the methanol, I think the methanol would come in and attach…I think it would come in and replace it.
Finally, there are two incorrect warrants dealing with the mechanism and stereochemistry of the SN1 reaction. The first incorrect warrant is that SN1 reactions only cause inversion of stereochemistry, as opposed to the racemic mixture that it really produces. It seems that this participant confused the SN1 and SN2 mechanisms, as what he is describing is actually an SN2 mechanism.
Carl: Because…S N 1, it attacks from the backside so it flips and it makes it go out.
The second incorrect warrant is that a racemic mixture will produce one product through elimination and one product through substitution.
Interviewer: What does racemic mixture mean to you, if anything?
Allison: That one of them is an S N 1, and the other one is an E1.
This warrant is incorrect because in a racemic mixture both products will arise from the substitution mechanism.
Sub-group 2c. Sub-group 2c is composed of two participants, Allison and Robert, who claimed that an elimination reaction would happen through E1 yielding the elimination product shown in the expert argumentation scheme as a result. The main piece of data used by these participants was that CH3OH is a base. Even though these participants were able to make the partially correct claim that an E1 reaction would occur, they relied on rote memorization without providing evidence of deep understanding about the E1 reaction mechanism.
Robert: I'm trying to remember what the chart says. Um, it's a weak base and it would be elimination…it's a weak base so I wanna say that it's E, it's E1.
Robert expressed the correct evidence that CH3OH is a weak base and the correct warrant that weak bases proceed by E1 reactions, but he predicated his answer on a memorized chart, not on chemical and physical characteristics about the mechanism. A similar situation was observed with Allison.
Interviewer: …when you looked at the methanol you immediately said S N 1/E1, what is it about methanol that made you immediately think that?
Allison: Well, in our notes in class she writes that it's methanol, ethanol, and acetone [which do that].
It can be concluded that Allison and Robert exhibited a gap in understanding regarding the mechanism of the E1 reaction, including its carbocation intermediate and stereochemical implications. In addition, Robert exhibited a gap in understanding about CH3OH not stating that it can act as a nucleophile in addition to it being able to act as a base.
Sub-group 3a. Sub-group 3a is composed of eight participants, Danisha, Melissa, Susan, Beth, Sabrina, Maggie, Amber, and Mark, who claimed that a substitution reaction would occur. Nonetheless, they claimed that the functional group that substitutes is a hydroxyl group (OH), and not the correct methoxy group (OCH3), yielding cyclohexa-2-en-1-ol as a product. The main piece of data used by these participants was that CH3OH is a nucleophile.
These participants did not recognize the basic nature of CH3OH. This shows the same gap in understanding regarding the dual (nucleophilic and basic) nature of CH3OH exhibited by all the participants in group 2. In addition, they did not speak about the mechanism of the reaction or about the carbocation intermediate. This shows a significant gap in understanding regarding how substitution reactions take place. The participants' gap in understanding regarding the mechanistic aspects of the reaction is further exemplified by the fact that they substituted with the OH group instead of the OCH3 group. This demonstrates gaps in understanding pertaining to the nature of covalent and ionic bonds, as well as interactions between the reagents at the molecular level.
There are two specific incorrect warrants that have been identified which the participants used to justify their claim that the final product would have an OH group. The first incorrect warrant is that CH3OH behaves as an Arrhenius base.
Interviewer: …it seems like, just by looking at it you know that the OH was the group to be added. How did you know that?
Beth: [the professor says] the ions attached to the front of it aren't important, she just puts [it] in there sometimes and sometimes doesn't, so we kind of only look at the, like, anion part, I guess.
From the way Beth explains her reasoning, it is evident that she views CH3OH as an ionic compound that is able to produce hydroxide ions, instead of a covalent compound. The second incorrect warrant which was identified is that if the nucleophile has an OH group, that one is necessarily the group that substitutes.
Interviewer: …by looking at the methanol, how did you know that the group that was gonna be substituted was gonna be the OH?
Maggie: That's just out of habit from lecture, it was just always OH.
Apart from the OH group issue, two additional incorrect warrants were identified in this group. The first of these incorrect warrants was that if a starting material shows stereochemistry, the product must have an inverted stereochemistry.
Melissa: …since this is shown going one way, I would figure that they're doing S N 2 'cause they want you to do the inversion of stereocenters, so I would just kind of assume that you would do that.
The second of these incorrect warrants is that weak bases proceed by SN2 reactions and strong bases proceed by SN1 reactions.
Danisha: …S N 1 is the one that has the strong base, so I believe it would be S N 2.
Interviewer: It would be S N 2 because it's not [a] super strong base?
Danisha: Yes.
Not only are the actual reaction pathways inverted in this statement, but Danisha uses the word ‘base’ instead of the word ‘nucleophile’ while speaking about nucleophilic substitutions.
Sub-group 3b. Sub-group 3b is composed of two participants, Sally and Brenda, who claimed that a substitution reaction would take place yielding one where the CH3OH molecule bonded through the carbon replaces the chlorine (we note that they created a compound wherein the carbon has formed five bonds). Despite further probing during the interview, participants did not offer any data or warrants to support their claim. The claim offered demonstrate that the students do not understand how to construct proper Lewis structures, which is an essential skill in the undergraduate organic chemistry curriculum. Sally exhibits a disagreement between her verbal explanation and her drawn structure, which exhibits this significant gap in understanding.
Interviewer: …would you tell me what specifically is the basic site in that molecule [methanol]?
Sally: The O.
Even though Sally seems to recognize that the oxygen atom is the nucleophilic site in CH3OH, she draws her Lewis structure as if the carbon is the nucleophilic site. The other participant, Brenda, is more explicit about her confusion.
Brenda: I don't know then how to go about it…the final product to me would be…that's an ugly structure…but I know that's, there's no way that's right.
Brenda seems to be aware that something in her claim is incorrect, but is unable to use her understanding of chemistry to amend it. All of the identified gaps in understanding and incorrect warrants for question 1a are listed in Table 1.
| Question 1a |
|---|
| Gaps in understanding: |
|
1. Do not recognize that CH3OH is a base
2. Do not classify CH3OH as a weak base 3. Do not know the mechanism of the E1 reaction, including its carbocation intermediate and stereochemical implications 4. Do not recognize that CH3OH is a nucleophile 5. Did not classify CH3OH as a weak nucleophile 6. Do not know the mechanism of the SN1 reaction, including its carbocation intermediate and stereochemical implications 7. Do not understand the difference between covalent and ionic bonds 8. Do not understand the acid–base step in the SN1 reaction through which the CH3OH loses its proton to another CH3OH molecule to become the OCH3 group in the final product 9. Do not understand how to construct proper Lewis structures 10. Do not recognize that the carbocation intermediate had an alternate resonance structure |
| Incorrect warrants: |
|
1. The presence of a double bond in the starting material implies that a substitution is the reaction to be performed.
2. The presence of a solvent in the starting materials implies that a substitution is the reaction to be performed. 3. The presence of a protic solvent implies that a substitution reaction must be performed. 4. The presence of CH3OH as a reagent always implies that a substitution reaction must be performed. 5. SN1 reactions only cause inversion of stereochemistry in the product. 6. A racemic mixture will produce one product through an elimination reaction and one product through a substitution reaction. 7. CH3OH behaves as an Arrhenius base 8. If the nucleophile has an OH group, that one is necessarily the group that substitutes in a substitution reaction. 9. If a starting material shows stereochemistry, the product must have an inverted stereochemistry. 10. Weak bases proceed by SN2 reactions and strong bases proceed by SN1 reactions. |
Question 1b
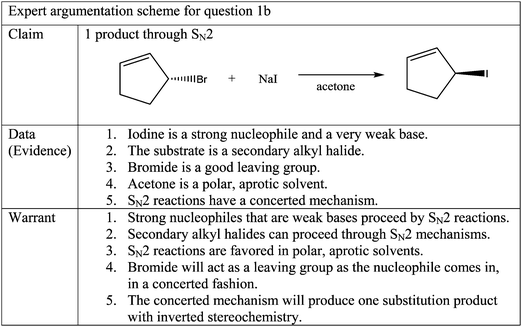
Question 1b, seen in Fig. 1, has one molecule as the correct answer. (S)-3-Iodocyclopentene is the product of an SN2 reaction. The expert argumentation scheme for question 1b can be seen in Fig. 5.Similar to question 1a, the participants' responses to question 1b were categorized into three groups according to their claims. Group 1 had correct claims, Group 2 had partially correct claims, and Group 3 had incorrect claims.
Barbara: So the S N 2 is going to have inversion of the stereochemistry, so we are going to do that, because it kicks, S N 2 is going to come in at the um, to the alpha carbon, at the same time the leaving group gets kicked out causing inversion of stereochemistry.
As it was the case with question 1a, these participants did not mention absolutely all of the data and warrants in the expert argumentation scheme. Although they were still able to provide a correct claim while exhibiting sufficient understanding, there is one significant gap in understanding that was identified. The participants did not mention that in addition to iodide being a strong nucleophile, it is also a weak base. This piece of information is important because it justifies why an elimination reaction does not take place.
Sub-group 2a. Sub-group 2a is composed of five participants Sally, Carl, Beth, Allison, and Mark who claimed was that an SN2 reaction would yield the correct product. These participants classified iodide as a strong nucleophile and expressed the warrant that strong nucleophiles perform SN2 reactions, leading them to make their claim. However, these participants had incorrect or absent stereochemistry in their product, which exposes a gap in understanding regarding the concerted mechanism of SN2 reactions and it stereochemical implications. Two incorrect warrants were identified which explain why the stereochemistry was incorrect in this sub-group's claim. The first incorrect warrant is that SN2 reactions conserve the stereochemistry of the starting material.
Mark: I think the stereochemistry would be the same, I think it's just the iodine switches with the bromine.
Mark believes the iodide and bromide would switch without any stereochemical effects demonstrating an erroneous understanding of the SN2 mechanism. The second incorrect warrant is that elimination reactions are the only ones that affect the stereochemistry of the product.
Beth: …elimination is the one that affects stereochemistry, so maybe in this case I would keep it going back like the Br was, and I would just say that it's S N 2.
Thus, the claims of the students included incorrect or absent stereochemistry because of their lack of understand of the bimolecular nature of the mechanism.
Sub-group 2b. Sub-group 2b is composed of six participants Danisha, Susan, Maggie, Dennis, Amber, and Emily who claimed that a substitution reaction would happen, yielding the correct product with unspecified stereochemistry. Even though they mention that a substitution reaction would take place, they do not specify which kind of substitution reaction. The main aspect that distinguishes this sub-group from sub-group 2a is that these participants did not express any deep understanding or knowledge to justify their claim. Their only piece of shared data across the six participants was that the iodide group reacts with the alkyl halide.
These participants exhibited gaps in understanding regarding most aspects of the reaction. It followed that they did not specify stereochemistry in the product since they did not know the details of the reaction mechanism. These students relied on the superficial aspects or surface features of the representations of the molecules and observed patterns that they recalled from lectures and homework.
Susan: …what's acetone, like I don't know what that is …I'm getting rid of Br 'cause we always get rid of Br…Br's gonna float off somewhere, it's got a plus or a minus, I'm not sure which, I think minus maybe…NaI, it's hard to memorize every single these things and these things which, which one does more likely, like E2 or S N 1 and whatnot…acetone just helps it out somehow by giving it electrons, or taking away electrons, I don't think the whole NaI goes there, it's probably just I.
Interviewer: Why did you just put the I?
Susan: Because, from experience, the NaI generally isn't all there, it's just the I because that's gonna, the protons and electrons are gonna be given to something else somehow, they're gonna be, you know, I don't know what I'm doing.
Strangely, a partially correct claim can be offered even when the participant has very little understanding of the relevant chemical and physical concepts involved in the question.
Interviewer: So what does aprotic mean to you?
Gabriel: To me it means elimination.
Brenda's claim was that sodium would be the species that would substitute, therefore acting as a nucleophile, instead of the iodide.
Brenda: Sodium, let's put sodium here, yup, I'm just gonna do that.
It seemed that Brenda was guessing what to do in the question. Nonetheless, her poor general understanding was evidenced by the fact that she used the sodium cation, an electrophile, as a nucleophile. All of the identified gaps in understanding and incorrect warrants for question 1b are listed in Table 2.
| Question 1b |
|---|
| Gaps in understanding: |
|
1. Do not recognize that iodide is a weak base
2. Do not recognize that polar, aprotic solvents are useful for reactions with concerted mechanisms such as SN2 3. Do not understand the concerted mechanism of SN2 reactions and its stereochemical implications 4. Do not recognize that iodide is a strong nucleophile |
| Incorrect warrants: |
|
1. The solvent plays no role in SN2 reactions.
2. SN2 reactions conserve the stereochemistry of the starting material. 3. Elimination reactions are the only ones that affect the stereochemistry of the product. 4. The presence of an aprotic solvent implies that an elimination reaction is to be performed. 5. A sodium cation can act as a nucleophile. |
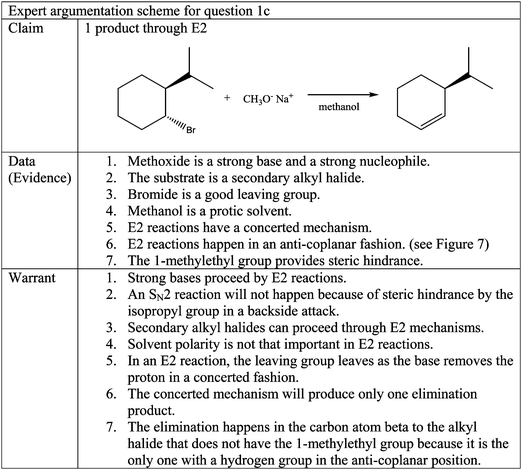
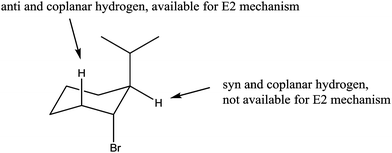
Question 1c
Question 1c, seen in Fig. 1, has one molecule as the correct answer. (S)-3-(1-Methylethyl)cyclohexene is the product of an E2 reaction. The expert argumentation scheme for question 1c can be seen in Fig. 6. The chair confirmation of the alkyl halide is shown in Fig. 7 identifying the anti and coplanar hydrogen available for the E2 mechanism and the syn and coplanar hydrogen which is not. Even though the consulted expert faculty member agreed that the main organic product would be the one shown in Fig. 6, he pointed out that there are other side products that could possibly be formed in the reaction. It was indicated that there is the minor possibility of the bromide group acting as a leaving group which would form a carbocation on the carbon atom which had the bromide group. The resulting carbocation would be secondary; therefore it has the possibility of rearranging to a tertiary carbocation in the carbon atom with the 1-methylethyl group through a 1,2 hydride shift. These two carbocations could give rise to five side products through SN1 and E1 mechanisms. These side products would have been categorized as partially correct claims if they had been justified with the appropriate evidence and warrants. Nonetheless, the participants who proposed any of these side products did not exhibit a thorough understanding of the relevant chemical concepts, as evidenced by incomplete sets of evidence and incorrect warrants. Most importantly, none of these participants mentioned the crucial carbocation intermediate to justify their claim. Therefore, it can be concluded that these participants' claims were not proposed because of the argument offered by the expert faculty member, and their arguments can be analyzed by comparing them to the agreed-upon expert argumentation scheme.As with the other two questions, the participants' responses to question 1c can be categorized in two groups according to their claims. Group 1 had correct claims while Group 2 had incorrect claims.
Sub-group 1a. Sub-group 1a is composed of three participants Aurora, Barbara, and Emily who correctly claimed that there would be one main product through an E2 reaction, as shown in the expert argumentation scheme. They correctly classified methoxide as a strong base and stated that strong bases perform E2 reactions. Furthermore, they correctly expressed that the product would be the least substituted alkene because the proton abstraction happens in an anti-coplanar fashion. Once again, these participants did not mention absolutely all of the data and warrants in the expert argumentation scheme, but were able to express a correct well-reasoned argument. Barbara was the participant who exhibited the most thorough understanding of what happens in the reaction.
Barbara: I look at this and see a secondary carbon…here you have methoxide and sodium, so when I see methoxide, that says strong base…so because it's strong, bases tend to favor elimination so this is gonna go through an E2 mechanism, so we're going to eliminate. So they should be anti and coplanar…so then it should pull off this one because the bromine and the hydrogens are anti and coplanar, so that's the one exception that we learned about.
The exception to which Barbara referred addresses the fact that this reaction produces the least substituted alkene as a product, as opposed to the most substituted alkene which is usually the case due to the increased stability of substituted alkenes. The so-called exception is due to the anti-coplanar fashion of the mechanism, which Barbara and the other two participants mention, but it is also due to the concerted fashion of the mechanism. The notion that the mechanism is concerted may be implied when they express that the mechanism happens in an anti-coplanar fashion, but none of them explicitly mention this concept which suggests a gap in understanding about the concerted fashion of the mechanism. Another gap in understanding exhibited by this sub-group is that they do not mention why an SN2 reaction is not viable in this case, with the exception of Aurora who actually expresses the incorrect warrant that strong bases perform both E2 and SN2 reactions simultaneously, without taking into consideration the steric effects of the 1-methylethyl group. Moreover, she chooses the SN2 product as the main one in her proposed E2–SN2 mixture.
Aurora: I know that this is a strong base…I would do S N 2 and E2.
Interviewer: Ok. If you had to choose between, a main product between the S N 2 product and the E2 product, what would you choose? Or you can say that they would both be equal, that's also acceptable.
Aurora: I would choose S N 2.
Interviewer: Is there any particular reason why?
Aurora: I don't know, I always just think of E2 being bulky bases to eliminate.
From this interaction it can be seen that Aurora also exhibits the incorrect warrant that E2 reactions always employ bulky bases. Even though Aurora chose the SN2 product as the main one when forced to choose, she was classified in this sub-group because she did propose the correct product as one of her options and, most importantly, because she expressed the correct warrant for proposing that product, which is the anti-coplanar fashion of E2 mechanisms. Another incorrect warrant expressed by Emily demonstrated a lack of understanding about the E2 reaction mechanism.
Emily: …the Br is going to be my leaving group, the two electrons in here are gonna come off, and then this Na plus is going to attract the Br negative and they'll form that bond…so I'm gonna have a positive, I guess, carbocation there, and the CH 3 O minus.
Emily proposed that the mechanism has a carbocation intermediate, even though she expressed that the reaction proceeded by an E2 pathway.
Sub-group 1b. Sub-group 1b is composed of three participants Maggie, Carl, and Robert who correctly claimed that there would be one main product through an elimination reaction, as shown in the expert argumentation scheme. The participants made the correct claim and justified it using only one piece of data, that methoxide is a base. None of the students expressed any correct warrants connecting the claim and data. These participants exhibited gaps in understanding regarding most aspects of the reaction. The first incorrect warrant is that during the elimination the hydrogen atom is abstracted from the carbon atom that has the most hydrogen atoms bonded to it.
Interviewer: Is there any reason why you put the double bond between these two carbons [forming the least substituted alkene] instead of these two carbons [forming the most substituted alkene]?
Maggie: I think it's easier to take off that hydrogen, 'cause there's more hydrogens on that carbon.
This warrant is incorrect because the decision of which hydrogen atom to abstract should be based on the geometry of the transition state and reactive intermediates of the reaction, and on the stability of the resulting alkene. The second incorrect warrant is that the presence of stereochemistry in the alkyl halide implies that the least substituted alkene must be formed as a product.
Robert: So then, I remember something in our notes, it said normally you would take the hydrogen off the most substituted, um, substituent, but if you have stereochemistry then I believe it's off the opposite one.
The final incorrect warrant that was identified is that the elimination reaction must happen as far away as possible from the most substituted carbon atom.
Interviewer: Ok, so what made you decide to do the elimination in the carbon far, in the carbon farthest away from the isopropyl group, instead with the one who has the isopropyl group?
Carl: Um, because you want the alpha carbon to be further away from the more substituted group, so instead of having it right here, with these two coming off, you put it down here, where it's less substituted.
This warrant is also incorrect because it does not take into consideration the reaction mechanism or the stability of the resulting product. Across this set of participants there is confusion about the way in which this concerted mechanism takes place and they do not recognize that the H and the leaving group must be anti-coplanar position. The findings from sub-groups 1a and 1b for question 1c provide more evidence for the assertion that it is possible for participants to offer acceptable claims while exhibiting gaps in understanding and incorrect warrants about fundamental concepts of the reaction in the question.
Sub-group 2a. Sub-group 2a is composed of six participants Allison, Sabrina, Anna, Sonia, Mark, and Amy who claimed that an elimination reaction would produce the most substituted alkene, between the carbon atom that had the bromide group and the carbon atom with the 1-methylethyl group. These participants recognized the basic nature of the methoxide group. The only warrant these participants had in common was that the most substituted alkene must always be formed when performing an elimination reaction. While it is correct that the most substituted alkene would be the more stable elimination product, it is not always the product that will be formed given that there are mechanistic and steric issues that must be taken into consideration. Given the reaction conditions in question 1c, the most favored pathway was an E2 reaction. Through this pathway, the only mechanistically viable product is the least substituted alkene. Nonetheless, all of the participants in sub-group 2a expressed the incorrect warrant that the most stable alkene will always be formed.
Sonia: I put the double bond between these two because it's, this side has the most substituents, so it wants to form between the more stable.
This incorrect warrant brought to light several gaps in understanding these participants have about elimination reactions. The main one is a general lack of knowledge regarding the mechanism of E2 reactions, including the transition state, the anti-coplanar proton abstraction, and the stereochemical implications of these two aspects. Furthermore, there is no assessment of the strength of the base and no association of base strength with type of elimination reaction to be performed.
Additionally, there are two other incorrect warrants identified in this sub-group. The first one is that the absence of a double bond in the alkyl halide implies that an elimination reaction is to be performed.
Amy: Ok, um, so in this case unlike the other cases, I guess I wouldn't immediately think substitution.
Interviewer: Why?
Amy: Um, I guess because…just the fact that this doesn't have a double bond.
The second incorrect warrant is that any structurally viable alkene is possible when performing an elimination reaction.
Interviewer: Ok, let me ask you this, in your elimination product, did you realize you had two options? You could've done the double bond here or here.
Allison: Yes.
Interviewer: Why did you choose that spot?
Allison: I guess you could put it both ways; it doesn't really matter because you need four bonds to complete it, and [in] both areas, that's available.
Interviewer: So then you're saying that both alkenes would've been valid?
Allison: Mmhmm [yes].
Allison did not consider what mechanistic pathway the reaction could take; she is only considered the structural viability of the end product.
Sub-group 2b. Sub-group 2b is composed of ten participants Isabel, Melissa, Dennis, Brenda, Beth, Danisha, Amber, Gabriel, Susan, and Sally who claimed was that the methoxide group would act as a nucleophile in a substitution reaction. These participants recognized that methoxide can act as a nucleophile but they did not recognize the more probable basic behavior of this reagent. Therefore, they opted to use methoxide as a nucleophile in a substitution reaction instead of as a base in an elimination reaction. They had no expressed warrants in common; even though all of them performed a substitution reaction, there is no coherent, common reasoning for this claim. Since substitution reactions are routinely taught before elimination reactions, it might be the case that the participants defaulted to a substitution reaction just because they were unsure of how to proceed; nonetheless, the participants did not explicitly mention this as the reason for their claim.
Three incorrect warrants were identified that help explain why the participants in this group chose to do a substitution reaction over an elimination reaction. The first incorrect warrant is that the explicit depiction of separate charges in the sodium methoxide reagent implied that a substitution must occur.
Dennis: …it has the charges separate, and I remember looking in the book and when the charges were separate, we did the substitution.
This incorrect warrant seemed to be another example in which a participant makes an incorrect generalization from other questions they have seen in lecture and homework. It also demonstrates the fragmented nature of the tapestry of knowledge that the students construct. The second incorrect warrant is that if stereochemistry is shown in the alkyl halide, then an SN2 reaction is to be performed.
Gabriel: In this case it would be S N 2.
Interviewer: Why would you choose to do an S N 2?
Gabriel: Uh, like the first time it was very familiar with the solvent being protic, and then with the stereochemistry being shown here I just assume that it'd be an S N 2 again.
Gabriel was also a member of sub-group 2b for question 1a, in which it was shown that he expressed the incorrect warrant that protic solvents imply a substitution reaction must be made. In this case, he used that warrant again and added the fact that the stereochemistry is shown in the alkyl halide as a second warrant to justify his claim that a substitution reaction is going to be produced. Finally, the third incorrect warrant that was identified is that eliminations only happen with bulky bases.
Isabel: …it would've been elimination but it's not sterically hindered.
Interviewer: And bases used for elimination usually should be sterically hindered?
Isabel: Yeah, the nucleophile should be sterically hindered.
As Isabel correctly assessed, methoxide is not a sterically hindered base. Nonetheless, her warrant is incorrect because elimination reactions can proceed with both sterically hindered and unhindered bases. All of the identified gaps in understanding and incorrect warrants for question 1c are listed in Table 3.
| Question 1c |
|---|
| Gaps in understanding: |
|
1. Do not understand the concerted fashion of the E2 mechanism
2. Do not understand the anti-coplanar proton abstraction in the E2 mechanism 3. Do not classify the bromide as a good leaving group 4. Do not recognize that an SN2 reaction is not viable because of steric hindrance 5. Do not recognize that OCH3 is a base 6. Do not classify OCH3 as a strong base 7. Do not recognize that strong bases proceed through E2 reactions |
| Incorrect warrants: |
|
1. Strong bases perform both E2 and SN2 reactions simultaneously, regardless of steric hindrance.
2. E2 reactions always employ bulky bases. 3. E2 reactions have a carbocation intermediate. 4. During an elimination reaction, the hydrogen atom is abstracted from the carbon atom that has the most hydrogen atoms bonded to it. 5. The presence of stereochemistry in the alkyl halide implies that the least substituted alkene must be formed as a product. 6. Elimination reactions must happen as far away as possible from the most substituted carbon atom. 7. The most stable alkene will always be formed during an elimination reaction. 8. The absence of a double bond in the alkyl halide implies that an elimination reaction is to be performed. 9. Any structurally viable alkene is possible when performing an elimination reaction. 10. The explicit depiction of separate charges in an ionic reagent implies that a substitution reaction must be performed. 11. The presence of stereochemistry in the alkyl halide implies that an SN2 reaction is to be performed. 12. Elimination reactions only happen with bulky bases. |
Concluding remarks
The objective of this study was to examine and elucidate students' understanding of alkyl halide reactions in undergraduate organic chemistry. This study provides support for this objective by identifying gaps in understanding and incorrect warrants exhibited by undergraduate students when solving organic chemistry questions about alkyl halides. There are several conclusions regarding how the students constructed their knowledge of alkyl halide reactions that can be reached from the findings of the study.One of the most important aspects of understanding alkyl halide reactions is being able to classify substances either as bases that are able to abstract a proton in elimination reactions and/or as nucleophiles that are able to react with an electrophilic carbon atom in nucleophilic substitution reactions; this includes the ability to distinguish between basicity and nucleophilicity. In addition, it is important to have the ability to assess the strength of the bases and nucleophiles by making the distinction between strong and weak species. The inclusion of these pieces of data proved to be a distinguishing factor between groups with correct answers and groups with incorrect answers. In addition, the arguments for the groups with correct answers contained the correct warrants that connected the kind of reagent with the kind of reaction it performs (e.g. weak nucleophiles perform SN1 reactions), while the arguments for the groups with incorrect answers contained either incorrect or no warrants about this connection. Therefore, it can be concluded that the inclusion of this specific type of correct warrant that connects the kind of reagent with its inherent physical and chemical characteristics with the type of reaction it performs is a distinguishing factor between groups with correct answers and groups with incorrect answers.
Another important aspect of understanding alkyl halide reactions is being able to describe the steps of the mechanisms, including the reactive intermediates or transition states. The arguments for the groups with incorrect answers contained little or no information about the steps of the mechanisms of the reactions. Therefore, the inclusion of specific information about the mechanisms of the reactions, including the reactive intermediates and transition states, is a distinguishing factor between groups with correct answers and groups with partially correct or incorrect answers.
Another conclusion that can be reached when looking across the incorrect warrants voiced by the students is that they often apply rules memorized from particular examples to new questions which they deem to be similar based upon surface features of the molecules. By following these heuristics, these students incorrectly apply rules that are not generalizable to other questions, which lead them to incorrect answers.
Implications for instruction
In conclusion, the findings from this study suggest that students who propose incorrect answers to organic chemistry questions about alkyl halide reactions exhibit significant gaps in understanding and incorrect warrants mainly dealing with: (1) classifying substances as bases and/or nucleophiles, (2) assessing the basic or nucleophilic strength of substances, and (3) accurately describing the steps that take place and reactive intermediates that form during alkyl halide reaction mechanisms.The findings from this work demonstrate that students have difficulties assessing the acidic/basic or electrophilic/nucleophilic nature and strength of the reagent. To scaffold students' developing understanding of reaction mechanisms, it is important that instructors help students learn how to make these assessments and distinctions in detail, before teaching specific reaction mechanisms involving alkyl halides. Effective pedagogical approaches include small group discussions where students concepts and understandings of acids/bases and electrophiles/nucleophiles are considered. As students describe their understandings and compare them across group members they have the opportunity to refine their knowledge to a more sophisticated understanding.
The state of their evolving understanding could be assessed by using an tool that contains a list of ten to fifteen varied reagents, including acids, bases, electrophiles, and nucleophiles, and asks students not only to classify them using one of those four categories and assess their strength, but also to provide an example of a chemical reaction that illustrates the classification that they assigned to the reagent. Again, this instrument could be used as a basis for classroom discussion and refinement of concepts. When instructors explain mechanisms, it would be beneficial to require students to explicitly make these classifications when answering a question that asks them to propose the major organic product or mechanism of a reaction involving alkyl halides. Thus, connecting the classifications in the assessments, instructors could better pinpoint and address the source of any mistakes by the students.
In addition, this study demonstrates that students have difficulties when connecting which types of reagents perform specific types of reactions (e.g. weak nucleophiles perform SN1 reactions). It would be beneficial to ask students to provide reasons which support a proposed major product or mechanism particularly the chemical and physical characteristics of molecules and the steps and intermediates of reaction mechanisms. That way, instructors can identify any incorrect warrants exhibited by the students and respond to them in a timely fashion that helps to refine student knowledge. It is also important to provide pedagogical support for the notion that one starting compound reacting with one reagent can yield multiple products as Grove and Bretz (2010) have noted. Classroom discussions where students consider possible products accompanied by reasons why they would be produced would allow students to contextualize ad refine their knowledge.
This study also demonstrates that students can propose a correct major organic product for a given set of reagents without understanding completely the physical and chemical characteristics of the molecules and the mechanism through which the product was formed. If the learning objectives involve students understanding the mechanism along with being able to identify the major products, then requiring the students to propose a mechanism which details their reasoning would be a more valid assessment than simply suggesting major products. Setting the classroom expectation that reasoning is required and discussion is part of the normal activities should help students refine and reorganize their knowledge of organic chemistry.
Implications for research
This study elucidated students' understanding of alkyl halide reactions in undergraduate organic chemistry. After such elucidation, a possible next logical step in research could be to use the findings described in this study to build a diagnostic instrument for alkyl halide reactions. The instrument could be constructed employing multiple-choice questions by using the gaps in understanding and incorrect warrants that have been identified as a guide, incorporating student quotes from this study as distractors in the test items. This design would enable instructors to identify the corresponding incorrect warrant that prompted the students to make an incorrect choice, so that they can correct it in a timely fashion. Once the instrument is designed and validated, subsequent research projects could verify if the usage of such an instrument by an instructor improves student performance in the first semester of undergraduate organic chemistry courses.Beyond instrument design, this study points the way towards possible pedagogical initiatives based upon scaffolding student learning of alkyl halide reactions and their associated mechanisms. Using this study to design more effective and evaluate pedagogies, whether they be learning progressions or portions of a spiral curriculum, and to evaluate these pedagogical approaches is another area for further research.
In addition, this study showed that the methodological approach described by Cole et al. (2012) can be applied to chemistry education research projects aimed at elucidating students' understanding of topics that require students to make a claim and support it with evidence and warrants, such as proposing the major product of an organic reaction. By applying a modified version of Toulmin's model of argumentation, gaps in understanding and incorrect warrants that lead students to make mistakes when answering organic chemistry questions were identified. In the same manner, future research projects can apply this methodology to elucidate students' understanding of the reactions of any other functional group in organic chemistry, as well as of any other topic in chemistry that in some manner requires students to make claims and support them with evidence and warrants.
References
- Anderson T. L. and Bodner G. M., (2008), What can we do about ‘Parker’? A case study of a good student who didn't ‘get’ organic chemistry, Chem. Educ. Res. Pract., 9, 93–101.
- Bhattacharyya G., (2006), Practitioner development in organic chemistry: how graduate students conceptualize organic acids, Chem. Educ. Res. Pract., 7, 240–247.
- Bhattacharyya G. and Bodner G. M., (2005), “It gets me to the product”: how students propose organic mechanisms, J. Chem. Educ., 82, 1402–1407.
- Bodner G. M., (1986), Constructivism: a theory of knowledge, J. Chem. Educ., 63, 873–878.
- Bowen C. W., (1994), Think-aloud methods in chemistry education: understanding student thinking, J. Chem. Educ., 71, 184–190.
- Bradley A., Ulrich S. M., Jones M. and Jones S. M., (2002), Teaching the sophomore organic course without a lecture. Are you crazy? J. Chem. Educ., 79, 514–519.
- Browne L. M. and Blackburn E. V., (1999), Teaching introductory organic chemistry: a problem-solving and collaborative-learning approach, J. Chem. Educ., 76, 1104–1107.
- Cartrette D. P. and Dobberpuhl M. R., (2009), The concept inventory as a diagnostic of understanding and achievement in a year-long organic chemistry course, Chem. Educ., 14, 1–10.
- Cartrette D. P. and Mayo P. M., (2011), Students' understanding of acids/bases in organic chemistry contexts, Chem. Educ. Res. Pract., 12, 29–39.
- Cole R., Becker N., Towns M., Sweeney G., Wawro M. and Rasmussen C., (2012), Adapting a methodology from mathematics education research to chemistry education research: documenting collective activity, Int. J. Sci. Math. Educ., 10, 193–211.
- Cooper M. M., Grove N., Underwood S. M. and Klymkowsky M. W., (2010), Lost in Lewis structures: an investigation of student difficulties in developing representational competence, J. Chem. Educ., 87, 869–874.
- Cooper M. M., Underwood S. M. and Hilley C. Z., (2012), Development and validation of the implicit information from Lewis structures instrument (IILSI): do students connect structures with properties? Chem. Educ. Res. Pract., 13, 195–200.
- Domin D. S., Al-Masum M. and Mensah J., (2008), Students' categorization of organic compounds, Chem. Educ. Res. Pract., 9, 114–121.
- Ferguson R. and Bodner G. M., (2008), Making sense of the arrow-pushing formalism among chemistry majors enrolled in organic chemistry, Chem. Educ. Res. Pract., 9, 102–113.
- Geelan D. R., (1997), Epistemological anarchy and the many forms of constructivism, Sci. Educ., 6, 15–28.
- Grove N. P. and Bretz S. L., (2010), Perry's scheme of intellectual and epistemological development as a framework for describing student difficulties in learning organic chemistry, Chem. Educ. Res. Pract., 11, 207–211.
- Harle M. and Towns M. H., (2013), Students' understanding of primary and secondary protein structure: drawing secondary protein structure reveals student understanding better than simple recognition of structures, Biochem. Mol. Biol. Educ., 41(6), 396–376. DOI 10.1002/bmb.20719.
- Henderleiter J., Smart R., Anderson J. and Elian O., (2001), How do organic chemistry students understand and apply hydrogen bonding? J. Chem. Educ., 78, 1126–1130.
- Horowitz G., (2007), The state of organic teaching laboratories, J. Chem. Educ., 84, 346–353.
- InqScribe, (2005), http://www.inqscribe.com/.
- Kingsbury C. and Schelble S., (2001), Teaching chemistry in the new century: organic chemistry, J. Chem. Educ., 78, 1172–1173.
- Kraft A., Strickland A. M. and Bhattacharyya G., (2010), Reasonable reasoning: multi-variate problem-solving in organic chemistry, Chem. Educ. Res. Pract., 11, 281–292.
- Linenberger K. J. and Bretz S. L., (2012), A novel technology to investigate students' understandings of enzyme representations, J. Coll. Sci. Teach., 42(1), 45–49.
- Livescribe, (2010), http://www.Liverscribe.com/.
- McClary L. M. and Bretz S. L., (2012), Development and assessment of a diagnostic tool to identify organic chemistry students' alternative conceptions related to acid strength, Int. J. Sci. Educ., 34(15), 2317–2341.
- McClary L. and Talanquer V., (2011), College chemistry students' mental models of acids and acid strength, J. Res. Sci. Teach., 48(4), 396–413.
- Nakhleh M. B., (1992), Why come students don't learn chemistry: chemical misconceptions, J. Chem. Educ., 69, 191–196.
- Patton M. Q., (2002), Qualitative Research and Evaluation Methods, 3rd edn, Thousand Oaks, CA: Sage Publications.
- Raker J. R. and Towns M. H., (2010), Benchmarking problems used in second year level organic chemistry instruction, Chem. Educ. Res. Pract., 11, 25–32.
- Raker J. R. and Towns M. H., (2012a), Problem types in synthetic organic chemistry research: Implications for the development of curricular problems for second-year level organic chemistry instruction, Chem. Educ. Res. Pract., 13, 179–185.
- Raker J. R. and Towns M. H., (2012b), Designing undergraduate-level organic chemistry instructional problems: Seven ideas from a problem-solving study of practicing synthetic organic chemists, Chem. Educ. Res. Pract., 13, 277–285.
- Rushton G. T., Hardy R. C., Gwaltney K. P. and Lewis S. E., (2008), Alternative conceptions of organic chemistry topics among fourth year chemistry students, Chem. Educ. Res. Pract., 9, 122–130.
- Şendur G., (2012), Prospective science teachers' misconceptions in organic chemistry: the case of alkenes, J. Turkish Sci. Educ., 9(3), 160–185.
- Şendur G. and Toprak M., (2013), The role of conceptual change texts to improve students' understanding of alkenes, Chem. Educ. Res. Pract., 14, 431–449. DOI: 10.1039/c3rp00019b.
- Taagepera M. and Noori S., (2000), Mapping students' thinking patterns in learning organic chemistry by the use of knowledge space theory, J. Chem. Educ., 77, 1224–1229.
- Toulmin S. E., (1958), The Uses of Argument, Cambridge: Cambridge University Press.
- Wade Jr., L. G., (2006), Organic Chemistry, 6th edn, Upper Saddle River, NJ: Pearson Prentice Hall.
| This journal is © The Royal Society of Chemistry 2014 |