Examination of the topic-specific nature of pedagogical content knowledge in teaching electrochemical cells and nuclear reactions
Sevgi
Aydin
*a,
Patricia M.
Friedrichsen
b,
Yezdan
Boz
c and
Deborah L.
Hanuscin
b
aSecondary Science and Mathematics Education (SSME) Department, College of Education, Yuzuncu Yil University, 65080, Van, Turkey. E-mail: sevgi.aydin45@hotmail.com
bMU Science Education Center, University of Missouri, Columbia, MO 65211, USA
cSSME, College of Education, Middle East Technical University, 06800, Ankara, Turkey
First published on 9th July 2014
Abstract
The purpose of this study was to examine experienced chemistry teachers' pedagogical content knowledge (PCK) for two different topics in chemistry to better understand how PCK is specific to topic, including whether all components of PCK are topic-specific and to what degree. To explore the topic-specific nature of PCK, we examined two experienced teachers' PCK using a case study methodology. Multiple data collection strategies were used, including a card-sorting activity, Content Representation (CoRe), semi-structured interviews, observations, and field notes. The data collected were analyzed both deductively and inductively. Results revealed that the teachers used more content-based and teacher-centered instruction to teach electrochemistry, whereas their instruction was less teacher-centered, and included Science–Technology–Society–Environment discussions and implicit NOS instruction to teach nuclear reactions. The teachers also varied in the extent of their knowledge of learners and curriculum in comparing their PCK for each topic. In regard to assessment, the teachers' assessment practices were at the general PK level; they lacked topic-specific PCK for either topic. We provided recommendations for professional development programs, pre-service teacher education programs, and curriculum developers to support teachers in developing topic-specific PCK.
Introduction
In science education research, pedagogical content knowledge (PCK) is a useful theoretical framework for investigating teachers' knowledge (Abell, 2007). Shulman (1987) described PCK as a unique mixture of content and pedagogical knowledge necessary for teaching a topic in an understandable way to students. Since Shulman's introduction of the construct of PCK, researchers have studied the components and sources of PCK, and how PCK develops. Based on a growing body of literature, there is widespread agreement that PCK is topic-specific (Abell, 2007, 2008; Loughran et al., 2004; Magnusson et al., 1999); however, there are differing perspectives as to whether PCK is also domain or discipline-specific (Davis and Krajcik, 2005; Veal and MaKinster, 1999) and questions remain regarding how teachers transform their subject matter knowledge (SMK) of different topics into PCK for teaching different topics in the same discipline (Abell, 2008). PCK is a complex construct in the sense that it comprises the interplay of the different knowledge and belief components; the degree to which these components themselves are topic-specific is yet to be explored. Do teachers use different instructional and assessment strategies for teaching different topics, or is the nature of PCK specific to a topic in regard to the quality, quantity, or interplay of components? These are important questions that can inform the design of pre-service teacher education programs and professional development activities. Despite our growing body of research on PCK, there remains a need for PCK research within the complexity of classrooms to determine how teachers transform their SMK into pedagogically powerful representations to support student learning of different topics (Abell, 2008; Avraamidou and Zembal-Saul, 2005).While examining teachers' PCK for teaching a single topic (e.g., genetics) provides valuable information, focusing on the same teachers' PCK for different topics in the same discipline will help researchers better understand the nature of PCK. By this comparison, we can learn why and how components of teachers' PCK (e.g., knowledge of instructional strategies and curriculum) differ or show similarities across topics. Studies of this kind have the potential to answer Abell's (2008) question as to why some topics are more difficult to teach than others. In order to reach a shared conception of the PCK, a closer examination of teachers' PCK in regard to different topics is necessary. In this study, we ask How is two experienced chemistry teachers' PCK different and/or similar for teaching the topics of electrochemical cells and nuclear reactions? The approach we take goes beyond merely describing the nature of teachers' PCK for a particular topic. Rather, the purpose of our study is to compare and contrast experienced teachers' PCK for different topics within the same discipline and to better understand the extent to which the component knowledge bases informing their PCK are topic and/or discipline-specific.
Theoretical framework
This study was based on Shulman's conception of PCK as “the special amalgam of content and pedagogy that is uniquely the province of teachers, their own special form of professional understanding” (1987, p. 8). The PCK model proposed by Magnusson and her colleagues (1999) is one of the most commonly utilized models in science education. This model identifies subject matter knowledge, pedagogical knowledge, and knowledge of context as the domains of teacher knowledge that influence PCK.Within this model, Magnusson and her colleagues conceptualize PCK as having five sub-components: orientations to science teaching (OST), knowledge of curriculum (KoC), knowledge of learners (KoL), knowledge of assessment (KoA), and knowledge of instructional strategies (KoIS). OST is defined as a teacher's knowledge and beliefs about the goals and purposes of science teaching at a specific grade level. A teacher's OST acts like a filter for the other PCK components (Magnusson et al., 1999). KoC includes knowledge about mandated goals and objectives, and specific curricular programs and materials. KoL consists of knowledge about the learners' prior knowledge and difficulties that learners face. KoA includes knowledge of dimensions of science learning: what to assess and how to assess. Finally, KoIS includes two sub-categories: knowledge of discipline-specific strategies and knowledge of topic-specific strategies. Knowledge of topic-specific strategies consists of teachers' knowledge about appropriate strategies for particular topics, with two sub-categories: topic-specific representations and activities. Teachers should know when and how to use the representations (e.g., analogies, models, illustration, and examples) and how to create representations to help learners understand the topic. The activities include knowledge of simulations, demonstrations, and experiments to help learners construct science knowledge and understand relationships between them.
For this study, we modified Magnusson et al.'s (1999) PCK model in light of related literature and the results of a pilot study of an experienced chemistry teacher's teaching of “Matter and Measurement” and “Atomic Models”. For the pilot study, the participant teacher's teaching was observed for two weeks for each topic and interviews were conducted with the teacher in order to understand the reasons for her instruction. Both the pilot study and the recommendations of Friedrichsen et al. (2009) and Friedrichsen et al. (2011) helped us represent OSTs with central and peripheral goals. We also noted differences in teachers' ideal (i.e., guided inquiry orientation stated in the interview) and working teaching conceptions (i.e., didactic orientation enriched with activities) based on the work of Samuelowicz and Bain (1992). Within the KoC category, we added the following sub-components based on our pilot study: horizontal and vertical relations to the other topics in the same discipline, and altering the sequence of the sub-topics in the curriculum. Related literature also supported these alterations. For example, Magnusson et al. (1999) mention vertical curriculum alignment; however, it was not included as a distinct sub-category of KoC in their model. Rather, it was included in the goals and purposes sub-category. We drew on Grossman (1990) and included horizontal and vertical alignment as a separate sub-category of KoC. Additionally, regarding the ‘altering the sequence of the sub-topics in the curriculum’, Friedrichsen et al. (2009) referred to this as “altering the curriculum” and we have included this sub-component in our PCK model. Finally, the pilot study also led us to augment the KoA component with a ‘purpose of assessment’ sub-component that includes why the teachers assess.
Literature review
In this section, we review the literature on the relationship between SMK and PCK, the nature of PCK, and the role of teaching experience and reflection on PCK development. At the end of this section, we identify gaps in the literature.Relationship between SMK and PCK
For the sake of clarity, we need to differentiate content knowledge and subject matter knowledge before examining PCK further. Content knowledge (CK) is teachers' knowledge about the subject matter to be learned or taught. (Koehler and Mishra, 2009, p. 63) However, in the literature, these terms are used interchangeably. For instance, in the Handbook of Research on Science Teaching, Abell (2007) stated that she would not make any differentiation between the two so the reader could understand the either one. Likewise, we adapted to the same strategy regarding the difference between CK and SMK. Due to the fact that Magnusson and her colleagues used SMK in the model, we preferred to use it in this study. Additionally, we think that it would be suitable because most of the studies examined here did not discuss the CK and SMK difference.Studies examining teachers' PCK for teaching topics with strong or weak SMK show when teachers' SMK was robust, teachers also had rich knowledge of learners (Sanders et al., 1993). Although teachers had a rich archive of classroom activities, and a rich understanding of how to plan a lesson in their area of specialization, teachers stated they had difficulties in planning when their SMK was weak. Limitations in SMK created obstacles for teachers in determining learning goals, identifying key concepts, activities to use, and how to teach. In a yearlong study, Carlsen (1993) examined the influence of SMK on teachers' discourse in class and reported that teachers had a tendency to ask low-level questions when they were unfamiliar with the topic.
Other research, however, indicates the relationship between teachers' SMK and PCK is not straightforward. Ingber (2009) reported that teachers with strong SMK were more adept in using terminology and relating concepts to other topics. Additionally, they had a richer repertoire of instructional resources. However, there was no difference regarding teachers' instructional strategy use when they had strong or weak SMK. Ingber (2009) concluded that the instructional strategy choice was teacher-specific and was not influenced by teachers' level of SMK. Similar to Ingber, Newton and Newton (2010) reported that powerful SMK did not guarantee the teacher would ask high-level questions. Finally, Rollnick et al. (2008) examined the influence of SMK on teaching the mole concept and reported that teachers' shallow understanding of the topic made it hard for them to relate the conceptual and algorithmic parts of the mole topic. Teachers with weak SMK preferred to teach the mole by algorithm only; whereas, teachers with robust SMK taught at both conceptual and algorithmic levels. Rollnick et al. (2008) concluded that although SMK has a major influence on teachers' PCK, focusing only on SMK and its development is not a realistic approach to study teacher knowledge. Other factors should be considered, including the context in which teachers teach.
Nature of PCK
Researchers have compared and contrasted teachers' PCK in a variety of ways, including comparing (a) PCK for the same topic taught in different science disciplines, (b) different teachers' PCK for the same topic in the same discipline, and (c) the nature of the integration of PCK components for teachers' PCK for teaching two different topics in the same discipline. Veal and Kubasko (2003) compared teachers' PCK for teaching the same topic in different disciplines. They reported that geology teachers tended to teach evolution with an empirical approach by drawing on rocks, geographical shapes, and the time necessary for change. In contrast, biology teachers had an evolutionary perspective and drew on fossil analysis, adaptation, and living organisms.For the same topic in the same discipline, teachers may develop different types of PCK Henze et al. (2008) reported that teachers had two different types of PCK for teaching ‘Models of the Solar System and the Universe’. Type A PCK focused only on the content of models, whereas, Type B focused on the models and model development as well as the model content. Additionally, each type of PCK had its own development and interaction among the sub-components. The development of Type A PCK occurred when teachers had inadequate SMK and positivist views of the models. Type B PCK development occurred in teachers with adequate SMK, relativist and instrumentalist views of models.
Park and Chen (2012) examined biology teachers' PCK for teaching two different topics, photosynthesis and heredity, focusing on the nature of the integration among PCK components (i.e., OST, KoIS, KoA, KoL, and KoC). Integration was identified as the number of connections teachers made among the PCK components. Quantitative analysis showed that the number of the interactions among the PCK components was much higher for photosynthesis in comparison to heredity. For example, the researchers reported one teacher made 11 connections between KoL and KoIS when teaching and reflecting on photosynthesis, while only 4 connections were identified for teaching heredity. Furthermore, three of the four participants could not integrate KoC and KoA, and KoL and KoC components while teaching heredity whereas those three teachers did integrate those components while teaching photosynthesis. For both topics, KoL and KoIS integrations were central while KoA and KoC had limited roles in teachers' thinking and practice. Although Park and Chen (2012) explored the topic-specificity of the integration of PCK components, the empirical evidence for how and why teachers teach topics in different ways was missing in their study. Therefore, research on how and why components of PCK show differences/similarities for teaching different topics demands attention.
PCK development: role of teaching experience and reflection
Researchers have explored teaching experience as a factor influencing PCK development. Pre-service and novice teachers generally do not have robust PCK (Magnusson, et al., 1999; De Jong and van Driel, 2001, Halim and Meerah, 2002). With the help of teaching experience, teachers develop ‘curricular saliency’, that is expertise in when to teach the topic and how to relate it to other topics (Geddis et al., 1993). However, teaching experience alone, in the absence of teacher education coursework, was not enough to develop robust PCK (Friedrichsen et al., 2009). Therefore, to support PCK development of teachers, teacher education programs should provide discipline-specific courses to help teachers how to diagnose learners' difficulties and develop a rich repertoire of instructional strategies to address those difficulties during teaching. In addition to that, reflection on teaching is an important factor influencing PCK development positively. The development of PCK occurred through the teachers' reflection-in-action which occurs in the case of unexpected events or results during teaching and reflection-in action which is made through thinking on the practice after teaching (Schön, 1983, 1987, as cited in Park and Oliver, 2008).In summary, teaching experience supported by well-constructed teacher education programs and teachers' reflections are vital for developing robust PCK. Therefore, research on experienced teachers with those characteristics promises to provide richer examples of teachers' PCK and how it influences their practice.
Contribution of the study
Since the introduction of PCK, the literature has focused on the interaction between SMK and PCK, the nature of PCK, and the role of teaching experience and reflection on PCK development. One of assertions in the literature is that PCK is topic-specific. However, few studies have established this topic-specificity by contrasting the PCK that a single teacher has developed for two different topics. To address this gap in the literature, the purpose of this study was to compare and contrast experienced chemistry teachers' PCK for teaching two different topics. The overall research question was: “How are two experienced chemistry teachers' PCK different and/or similar for teaching the topics of electrochemical cells and nuclear reactions?” This study contributes to the literature by more deeply probing the extent to which teachers' PCK is topic-specific.Methodology
We utilize a case study methodology (Merriam, 1998) to explore teachers' PCK for different topics. Case studies provide comprehensive information related to an event, a subject or a setting (Merriam, 1998). In order to be a case, the boundedness of the system is essential. Merriam (2009) states “for it to be a case study, one particular program or one particular classroom of learners (a bounded system) or one particular older learner selected on the basis of typicality, uniqueness, success, and so forth would be the unit of analysis” (p. 41). In this study, the case is defined as two experienced chemistry teachers working in the same high school teaching two different topics, electrochemical cells and nuclear reactions.Participant selection
We purposefully selected two teachers who had the potential to provide rich data that would allow us to explore and compare their PCK in-depth (Patton, 2002). Using criteria from the literature (e.g., Berliner, 2001; Friedrichsen and Dana, 2005), we sought experienced chemistry teachers at the secondary level (at least 5 years teaching experience) who taught in student-centered ways, held chemistry teaching certification for the high school level, participated in professional development, and were teaching chemistry in a conceptual way rather than just emphasizing the algorithmic calculations (Table 1).| Participant | Teaching experience | Master/PhD | Other job experiences | School type | PDs and training experience |
|---|---|---|---|---|---|
| Mr Demir | 15 years | — |
Electrical technician in a factory,
Elementary science teaching in elementary school for three years, |
Private School |
Performance-based assessment,
Introducing new chemistry curriculum, |
| Mrs Ertan | 8 years | Master | Tutoring | Private School |
Performance-based assessment,
Introducing new chemistry curriculum, |
In public schools in [the country], the instruction of most teachers is didactic in nature and focused on algorithmic calculations. Therefore, we decided to select teachers working in private schools due to their tendency to teach chemistry more conceptually than teachers do in public schools. We also decided to focus on one private school in order to control for influence of the school context on teachers' practice. Moreover, another issue we considered in the participant selection was the weekly schedules of the teachers. Since the observation of teachers' practice was essential, overlap in their schedules would be prohibitive to data collection. Using these criteria (e.g., school type, schedule), we selected two teachers who taught at a private high school with a student enrollment of 450–500 students (16–18 years of age). The school was located in a large city in [the country]. The socio-economic status of students in the school was high and the school was well equipped with over-head projectors, computers, and smart boards in the classrooms. The chemistry classrooms reflected a typical laboratory setting with benches and cupboards. A chemistry technician prepared the laboratory materials in advance. The participants' class sizes ranged from 20–24 students.
Research activities were carried out with the approval of the Institutional Review Board (IRB) to ensure the rights and protections of the participants. Participation was voluntary, and pseudonyms are used throughout the manuscript to preserve confidentiality.
Selection of the subject matter and the topics
Both participants in the study taught 11th grade. In the chemistry curriculum for 11th grade in [country], there are units focused on energy and chemical change, reaction rate, chemical equilibrium, electrochemistry, and radioactivity. The first three are strongly related to each other. Therefore, electrochemistry and radioactivity units were chosen to examine teachers' PCK for different topics. From those two units, we selected the topics of electrochemical cells and nuclear reactions. The former includes both electrochemical cells under non-standard conditions, and electrolytic cell types. The latter includes fission and fusion reactions as sub-topics. We selected these two topics because they are not conceptually linked to each other, and have also been noted as difficult for students to learn (De Jong et al., 1995; Alsop et al., 1998; De Jong and Treagust, 2002; Nakiboğlu and Tekin, 2006). Given the well-documented research on students' misconceptions and difficulties about electrochemical cells (Garnett and Treagust, 1992a,b; Ogude and Bradley, 1994; Sanger and Greenbowe, 1997a,b; De Jong and Treagust, 2002; Schmidt et al., 2007) and nuclear reactions (Millar et al., 1990; Alsop et al., 1998; Prather, 2005; Nakiboğlu and Tekin, 2006) topics, the study contributes to both chemistry education and science teacher education.Data collection
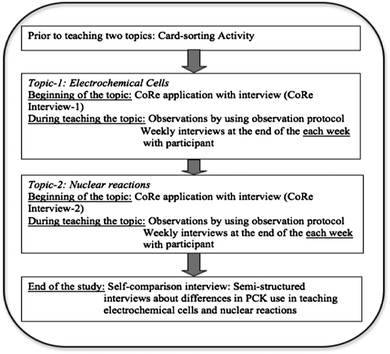
Fig. 1 shows the data collection strategies of the study, which were carried out by the first author.To capture teachers' PCK, researchers need to collect data for an extended time through the use of multiple data sources (Loughran et al., 2004; Abell, 2007). Therefore, multiple data collection strategies were used, including a card-sorting activity, Content Representation (CoRe) tool (Loughran et al., 2004), semi-structured interviews, observations, and field notes. We achieved data triangulation by using multiple data sources (e.g., card-sorting activity, interview transcripts, field notes, and CoRe) (Patton, 2002).
| Types of interviews | Purpose & description | Time |
|---|---|---|
| Interview-1: weekly interviews |
Purpose: to gather information about how teachers' PCK is different for teaching different topics
Participants were asked about their teaching practice, clarifying researcher's questions |
Time: at the end of each week for each topic (in total; eight interviews per teacher)
Length: 30 minutes |
| Interview-2: self-comparison interview | Purpose: to elicit teachers' ideas about the differences and similarities in their PCK for the topics |
Time: at the end of the study
Length: 30 minutes |
Data analysis
Our initial codebook included deductive codes based on our revision of Magnusson et al.'s (1999) PCK model; however, we remained open to other emerging codes. For instance, when coding the field notes and interview transcripts, we noted that teachers altered the curriculum at some points, so we added that code. In this manner, we created a codebook by analyzing one participant's entire data set, then used this to analyze the second participant's data.In the second phase of the analysis, codes were organized into categories and sub-categories of PCK for each participant and topic, and checked for consistency across data sources. For instance, for analysis of OST, we analyzed the data collected through the card-sorting activity. The central and peripheral goals of teachers were determined. Additionally, data collected through observation and interviews were helpful in understanding the participants' OST. Therefore, we triangulated our findings from multiple data sources.
In the third phase, we created summary tables for each topic taught by each teacher, for each of the PCK components: instructional strategies, learner, curriculum, and assessment. In total, we created 16 summary tables, 8 for each participant.
In order to be able to compare and contrast teachers' PCK for the two topics, we focused on one PCK component and a single participant at a time, comparing tables for each of the topics. For example, we compared Mr Demir's knowledge of learner tables for teaching electrochemical cells and nuclear reactions, looking for differences and similarities. Similar analyses were carried out for each PCK component and for each participant. After finishing the comparison of each teacher's PCK in different topics, we compared and contrasted both participants' PCK to check if there were any differences between them. Cross-case analysis revealed that both teachers' teaching were quite similar. As a result, we decided to report both teachers' PCK combined rather than individually.
At the final step, we labeled the categories inductively (Table 3) (e.g., for KoA; PK-coherent assessment use in the electrochemical cells topic). For instance, in the electrochemical cells topic, both participants assessed learners' understanding at the beginning (e.g., assessment of prior knowledge), during (e.g., assessment of to what extent they learned through quizzes), and at the end of the topic (e.g., test) using both formal (e.g., quiz) and informal (e.g., informal questioning) ways. Therefore, the KoA category for the electrochemical cells topic was labeled, “PK coherent assessment”.
| PCK component | Codes | Categories | Label | Description |
|---|---|---|---|---|
| KoA for teaching the electrochemical cells topic | Informal questioning, quiz, observing learners' performance | How to assess | PK-coherent assessment use in the electrochemical cells topic | The important characteristics of the coherent assessment were use of multiple assessment strategies (e.g. informal questioning, quiz, observing learners' performance, and test), for different purposes (e.g. to elicit learners' prior knowledge, to check how much learners learn, and to grade), and through teaching the topic (e.g. at the beginning, during, and at the end of the topic). |
| To elicit learners' prior knowledge, to check how much learners learn, and to grade | Purposes | |||
| Calculation of cell potential, determining anode and cathode by the use of half-cell potentials | What to assess |
For labeling each PCK component, a summary table (see Table 5) was created which shows all the assigned labels. The description of all labels is provided in the Results section.
To ensure credibility and trustworthiness, two colleagues, experienced in qualitative research and PCK, engaged in peer debriefing (Merriam, 1998) with the first author. In addition to the first author, they also coded half of the data independently (i.e., data belonging to the one of the participants). Then, they discussed inconsistencies in data coding, and reached consensus. Furthermore, during member checking with the participants in the final interview, both teachers agreed with our interpretations.
Results
In this part, comparison of teachers' PCK for two topics was given for PCK components. Before presenting the results, we wanted to highlight an important point regarding the teachers' knowledge and practice that may be quite different from each other. In this research, we focused on teachers' practice rather than their knowledge. Therefore, we provided specific examples from teachers' practice by the use of field notes and observations, and then teachers' views, goals, or reasons for enacting instructional/assessment activities based on our interview data.Comparison of the data revealed areas of difference as well as similarity—while the two teachers had generally similar PCK to each other, there were striking differences between their PCK for the two topics. In addition, we noted that despite these differences, these were less pronounced for particular subcomponents. Below, we compare and contrast each teacher's PCK components for the two topics.
Orientation to science teaching (OSTs)
Both teachers had a didactic OST, reflected in their lecture-based teaching approaches. However, they enriched their lectures with demonstrations, analogies, and activities. Both Mr Demir and Mrs Ertan had conflicts between their personal beliefs about the purposes of teaching high school chemistry (i.e., to help learners discover rather than providing knowledge) and the realities of the [country] education system (i.e., packed curriculum and high-stakes university entrance exam). In Table 4, we present their personal beliefs, ideal purposes, and their working purposes. The first was determined from the card-sorting activity, while the latter was determined through classroom observations and weekly interviews.| Participants | Purpose type | Ideal purposes | Working purposes |
|---|---|---|---|
| Mr Demir | Central purposes |
To relate chemistry to daily life.
To help learners discover rather than providing knowledge. To develop science-process skills. |
To deliver the content. To prepare learners for university entrance exam. To relate chemistry to daily life. |
| Peripheral purposes |
To facilitate learners' interest in chemistry.
To develop environmental consciousness. To provide historical development of concepts. |
To facilitate learners' interest in chemistry. To develop environmental consciousness. | |
| Mrs Ertan | Central purposes |
To develop higher order thinking skills (e.g. critical thinking).
To relate chemistry to daily-life. To develop scientific literacy. |
To deliver the content. To prepare learners for university entrance exam. To relate chemistry to daily-life. |
| Peripheral purposes | To facilitate learners' interest in chemistry. | To facilitate learners' interest in chemistry. | |
There was some overlap between the teachers' ideal purposes (espoused goals) and their working purposes (goals reflected in classroom practice) for teaching chemistry. One overlap was the central purpose of “relating chemistry to daily-life”. Due to Mr Demir's background in vocational high school and in industry, he made many links between chemistry and daily life. Mrs Ertan had the same central purpose, but for a different reason. She valued relating chemistry to daily life because she had perceived her own high school chemistry courses as boring. Her former chemistry teachers did not link the topics in chemistry to life. Even though there were time constraints due to a packed curriculum, both Mr Demir and Mrs Ertan found time to talk about where and how we use the phenomena taught in the class.
Although there was some overlap between teachers' ideal and working purposes, there were more discrepancies between the two (see Table 4). For instance, in the card-sorting activity, Mr Demir stated that it was important to provide knowledge about the history of the development of chemistry concepts; however, this was not reflected in his practice. When asked about them, he explained:
Curriculum is too loaded. It is stated that learners should learn by doing, through making projects and research. However, it is impossible to do that because of the curriculum load. If I used all of them [suggested activities], I think, I would teach one third of the 11th grade curriculum… Although we focus on mandated objectives in the curriculum, we do not know whether questions will be asked from all of them in the [university entrance exam]. We do focus on objectives, but… parents will complain about our teaching and want us to teach for that exam… So, it seems the purpose is… preparing them for the exam (Mr Demir, Card-sorting activity).
Moreover, in the card sorting activity, Mr Demir stated that one of his central goals was to help learners discover rather than provide knowledge to them; however, he lectured in most of his classes. He did use a lab activity for determining the relative reactivity of metals in electrochemistry; however, the lab was very teacher-directed. He gave the purpose of the lab activity, the procedure, and how to collect data. Although he stated that he wanted to use discovery strategies in his class, none were observed.
Similar discrepancies were noted between Mrs Ertan's ideal and working purposes. During the card sorting activity, she placed the “didactic teaching” scenario in her ‘not agree’ category. However, she was observed to teach fission and fusion reactions in exactly the same way as were described on the card she discarded during Card Sorting Activity. For the first two days of the nuclear reaction topic, she used lectures to teach fission and fusion. On the last day of the week, she used an interactive demonstration to show how fission was used in atomic bombs and nuclear reactors. The activity was used after she delivered the content through lectures.
In summary, both teachers had a didactic orientation for teaching 11th grade chemistry. However, it is not purely didactic. By the use of hands-on activities, analogies, animations, and discussions on environmental issues, it was supplemented. They stated that due to time limitations caused by the packed curriculum and university entrance exam, they had to shift from their ideal purpose for teaching chemistry to purposes that helped them handle the reality of the [country] education system.
Knowledge of instructional strategies (KoIS)
Upon comparing the two topics, we identified two different types of instruction that characterized teachers' approaches. The ‘content-based and teacher-centered instruction’ was utilized to teach electrochemical cells, whereas, ‘less teacher-centered instruction enriched with implicit nature of science (NOS) and Science-Technology-Society-Environment (STSE)’ was used for teaching the topic of nuclear reactions. In the following section, we describe each type of instruction separately.Discipline-specific strategies. In the electrochemical cells topic, neither of the teachers used discipline-specific strategies (e.g., 5E or inquiry), but rather relied on more general pedagogical knowledge. At the beginning of the topic, they presented the content through didactic teaching. After that, both participants solved a problem on the students' worksheet. The teachers always modeled how to solve the first problem and stressed the important points that learners should be careful about. Then, they had students complete the rest of the problems on the board. We identify this strategy as a general pedagogical strategy, as a mathematics teacher might use the same strategy.
Topic-specific strategies. Following the lectures and worksheet problems, the teachers used several different activities to increase student interest and to help them understand the topic. They used a structured cookbook laboratory related to reactivity of metals and ions in which students sequenced different metals and ions (e.g., zinc (Zn), copper (Cu), iron (Fe), magnesium (Mg) and hydrogen ion (H+) after observing reactions). In addition, both Mr Demir and Mrs Ertan used two teacher demonstrations and one hands-on activity. One teacher demonstration showed the color change during redox reactions between Zn metal and the Cu(NO3)2 electrolyte. The second demonstration was used to introduce electrochemical cells (e.g., Zn–Cu cell). Mr Demir showed how to make an electrochemical cell by the use of Cu- and Zn-electrodes, Zn(NO3)2 and Cu(NO3)2 electrolytes, and a salt bridge filled with KNO3 solution. He mentioned oxidation of zinc electrode from zinc atom (Zn0) to zinc ion (Zn2+) and the reduction of cupper ion (Cu2+) to cupper atom (Cu0). Both participants had a ‘verification-type’ approach to laboratories. The purpose of the demonstrations was to make the topic more concrete and to help the students remember it.
In addition to activities, the teachers used representations to make the content more concrete and visual. The teachers also showed a video illustrating how to make an electrochemical cell and its components. The teachers used a lot of topic-specific analogies in their teaching. Mrs Ertan used a waterfall analogy to illustrate the spontaneity of the reactions occurring in the electrochemical cells. She stated, “As in waterfall, there is a flow from high potential to low one. The direction of electron flow is from anode to cathode. Then, the potential of anode is higher than that of cathode”. (Mrs Ertan, field notes) When asked the reason for using this analogy, she said:
…to help learners understand that the reactions focused in the cells are spontaneous, which is similar to movement of water from higher point to lower one. The electrons move from electrode with higher potential to other with lower potential. They can visualize it better with the analogy (Mrs Ertan, weekly interview).
In addition to analogies, both participants also developed varying representations to help learners understand the topic. For instance, Mr Demir and Mrs Ertan used a representation (Fig. 2) in which they compared the oxidation number of the substance before and after the reactions. Due to the fact that students had difficulty in determining the oxidized and reduced species in the cell (i.e., this difficulty was stated by De Jong and Treagust, 2002), they both used this representation. Both teachers focused on whether the substances received or gave electrons.
Finally, teachers discussed the use of electrochemical cells in daily life. They talked about the difference between rechargeable nickel–cadmium batteries and non-chargeable ones, dry cells, and lead storage batteries used in cars. Also, they mentioned industrial uses of cells, including the electro refining of copper metal, and aluminum production. Both teachers used PowerPoint slides to provide details about the cell ingredients and show photos of the different types of electrochemical cells at the macroscopic level.
In summary, both Mr Demir's and Mrs Ertan's instruction was similar in that they reflected transmission of knowledge from teacher to learners rather than sharing the responsibility with learners. Also, they both focused primarily on the science content in the electrochemical cells topic and missed the opportunity of teaching NOS and discussing environmental issues regarding the electrochemical cells.
Discipline-specific strategy use. Similar to the other topic, none of the teachers used discipline-specific strategies to teach nuclear reactions (e.g., 5E or inquiry). Although their teaching was more learner-centered and included different aspects than the content (e.g., NOS and environmental issues), the discipline-specific strategy use was still missing.
Topic-specific strategy use. Didactic teaching was used at the very beginning of the topic to deliver content knowledge; however, overall this topic was less teacher-centered and included more student participation. Both teachers spent much less time lecturing than they did in the previous topic and incorporated discussions to satisfy learners' curiosity (i.e., students asked so many questions about nuclear energy), and to provide a scientific information on nuclear energy rather than telling his/her personal views. Moreover, while teaching this topic, the teachers took less control of the learning process in comparison to the electrochemical cells topic. Students were more active participants in the learning activities, as they engaged in discussions regarding the effectiveness, cost, and effect of nuclear energy on the environment, people, and society. Questioning and discussions were integrated in the nuclear reactions topic, as shown in the excerpt below:
Mr Demir: How do we benefit from fission reactions?
Students (Stds):….
Mr Demir: In which areas?
Std-1: To make atomic bomb.
Mr Demir: And?
Std-2: Hydrogen bomb?
Mr Demir: No, it is fusion reaction…. If the energy released during fission is controlled, it is nuclear power plant. How is it controlled in nuclear reactors?
Std-3: We can make it in thick lead blocks.
Mr Demir: How can you control energy with lead block?
Std-4: They use cold water in reactors.
Mr Demir: It is used for energy transfer. What I want to ask is that how we can use the atomic bomb reaction in the reactor? It is a huge amount of energy but it is not released all of a sudden. How can scientists achieve it?
Std-3: We can use less amount of uranium. If we use less uranium, the energy released would be less too.
Std-5: We can use isotopes of uranium.
Std-4: The neutrons produced have to be caught.
Mr Demir: The thing that you should do is catching the neutrons produced in order to impede them to collide with other uranium atoms.
Std-3: It is decreasing the amount of energy released.
Mr Demir: To do it, control rods are used (Mr Demir, field notes).
In a similar way, Mrs Ertan and the students in her class also discussed those issues. She realized that her students were very interested in nuclear energy and atomic bombs, and the effects on people and the environment. One of the students asked permission to make a presentation on World War II (WW-II), the atomic bomb, and the Chernobyl accident. She allowed him to make a 40 minute presentation on these topics. At the end of his presentation, Mrs Ertan helped the students summarize the information.
Second, both teachers introduced the NOS aspect, ‘scientific knowledge is subject to change’, implicitly when teaching the topic of nuclear reactions. After talking about the students' prior knowledge (e.g., atom, nucleus and the particles forming the nucleus of atom), Mrs Ertan added:
A short time ago scientists thought that proton, neutron, and electron were the smallest particles of the atom. However, recent research, you remember we talked about research has been conducted in CERN [The European Organization for Nuclear Research], showed that there are sub-atomic particles smaller than those (Mrs Ertan, field notes).
Then, one of the students asked whether it is possible to convert a proton into a neutron by changing the quarks in the proton. Mrs Ertan said that she did not know. She added that scientists may not be able to do it now but they may be able to achieve it in the near future. Throughout the topic on nuclear reactions, both teachers stressed that in light of the results gathered from ongoing research, the knowledge we have now might be replaced with new knowledge. However, the teachers were not explicit that scientific knowledge is subject to change, nor did the students explicitly discuss this NOS aspect.
Third, there were no algorithmic calculations included in this topic. The teachers spent a great deal of time modeling how to do calculations and having students work problems in the previous topic; however, in the nuclear reactions topic, the teachers spend a great deal of time in questioning and discussions about nuclear reactions and energy, which may be related to the nature of the topic.
Fourth, the teacher supplemented their instruction with representations that helped students visualize the scientific concepts. For instance, Mr Demir showed the chain reaction's symbolic and sub-microscopic representations when asked how the energy released during the fission reaction could be controlled in nuclear power plants. The teachers spent time explaining representations showing the reactants and products of the fission reaction. Although Mr Demir used representations to make electrochemical cells more concrete in the previous topic, he used representations differently in this topic in that he used representations to facilitate class discussion.
Additionally, in the nuclear reactions topic, both Mr Demir and Mrs Ertan used a “Domino Activity” to help students understand how fission reactions in atomic bombs and nuclear reactors are different. The teachers explained how to set up the activity, and the students worked in groups of 4–5. First, the students built a straight line of dominoes and then knocked them down. They timed how long it took for all the dominoes to fall. Second, with the same number of dominoes, they built a pyramid shape. Again, students timed how long it took for all the dominoes to fall. Teachers asked the students to compare the times measured for both arrangements of dominoes. At the end of the activity, both teachers asked which formation represented an atomic bomb. The students concluded that the straight-line formation represents a nuclear reactor whereas the second formation represents an atomic bomb. This representation is clearly a topic-specific strategy for teaching nuclear reactions. When asked in the weekly interview, Mr Demir stated that the purpose was to help student remember different types of nuclear reactions.
It was to show what chain reaction is and how they can occur in different ways. It was for those purposes. I had taught that they could occur in different ways before so it was for making the knowledge more permanent. I believe that now it is more permanent (Mr Demir, weekly interview).
Finally, to help students see connections between nuclear reactions and daily life, both teachers discussed how we use nuclear reactions in our lives. They discussed the use of radioactivity with X-rays in medicine, food irradiation, external radiation therapy, radiocarbon dating in archeology, and nuclear power plants. Like in the electrochemical cells, both teachers talked about the daily life use of nuclear reactions and energy.
Knowledge of curriculum (KoC)
Across the two topics, the teachers' KoC varied. In the electrochemical cells, the teachers had a highly integrated knowledge of how topics were integrated across the horizontal and vertical curriculum. In contrast, in the nuclear reactions topic, teachers had a limited network of topic integration.Relations to other topics and disciplines. Mr Demir and Mrs Ertan connected the electrochemical cells topic to the topics taught in earlier science courses (e.g., types of chemical reactions, and how to assign oxidation number), to topics taught earlier in the academic year (e.g., spontaneity of chemical reactions, chemical equilibrium, and spectator ions), and to topics taught in the physics course (e.g., electricity). It was obvious that both teachers drew upon content taught in physics and earlier in the chemistry course. For instance, Mrs Ertan related electrochemical cells to the chemical equilibrium topic taught earlier in the semester. She wrote the reaction between Zn and Cu2+ ion:
| Zn(s) + Cu2+(aq) ⇆ Zn2+(aq) + Cu(s) |
Then, Mrs Ertan asked: “When the cell reaction reached to equilibrium, how can I write the equilibrium constant of it, K?” Then, she wrote the K constant with help of the students:
| K = [Zn2+]/[Cu2+] |
They talked about the effect of changes in the concentrations of Zn2+ and Cu2+ ions and how they would influence the cell potential. During the interview, she stated that the link between chemical equilibrium and cell potential for non-standard conditions would make learning about electrochemical cells easier:
If the cells under non-standard conditions are taught by the use of chemical equilibrium, it is so simple to learn for learners. If not, students do try to memorize it. Because they have already known the equilibrium topic, they do not have difficulty in understanding the cells under non-standard conditions (Mrs Ertan, weekly interview).
When asked about the curricular connections, teachers stated that they wanted to help students remember previous topics and relate new content to previous content.
Altering the curriculum sequence. Both Mr Demir and Mrs Ertan drew on their curricular knowledge to make changes in the sequencing of concepts within the topic of electrochemical cells. For instance, both participants altered the sequence of “Calculations in Faraday's Law in Electrolytic Cells” after teaching the electrolytic cell. The national curriculum suggests that it should be taught at the very beginning of the topic. When asked, they both stated that teaching the electrochemical cells first and then teaching Faraday's law and its calculations made more sense to them. In the past, their students had difficulty with the suggested sequence, so the teachers altered the sequence.
Knowledge of the national curriculum. Mr Demir and Mrs Ertan were knowledgeable about the chemistry curriculum (i.e., regarding the sequence of the topics and how topics are related to others). Moreover, they also paid attention to the objectives and the specific warnings stated in it. In the national high school chemistry curriculum, objectives and the suggestions are given as to what extent they should teach the topic. Participants were aware of all the objectives and suggestions provided in the curriculum. For instance, the use of reduction potentials to determine anode and cathode is suggested. They were aware of it before teaching and used reduction potentials.
Relations to other topics and disciplines. Both Mr Demir and Mrs Ertan started the nuclear reactions with an atom, its structure, and sub-components. This was review material taught in 9th grade chemistry. They picked an atom (e.g., carbon-12) and asked students the atomic number, mass number, and nucleon. However, no horizontal curriculum connections were made to topics taught earlier in the course or in the physics course.
Altering the curriculum sequence. Although teachers made some changes in the sequence of concepts in the previous topic, they were not critical of the sequence of concepts in the topic on nuclear reactions, and followed the sequence suggested in the national curriculum.
Knowledge of the national curriculum. Finally, with regard to being aware of the goals and objectives stated in the national curriculum, both teachers paid attention to the goals and objectives, and suggestions given in the curriculum. For instance, in the curriculum material, it stated:
The real examples should be used in the calculations of atomic and mass numbers during nuclear changes and hypothetical examples of nuclear changes should not be used in assessment (Curriculum material, 2011, p. 77).
The purpose of the explanation provided in the curriculum was to prevent teachers from writing unreal equations to represent nuclear reactions. Some teachers write equations by using X, Y and Z to represent elements in the nuclear reactions rather than utilizing the real radioactive elements (e.g., U, Po, and Th). In summary, both participants were aware of and followed the objectives and suggestions provided in the national curriculum.
Knowledge of learners (KoL)
Knowledge of learners involves teachers' awareness of learners' difficulties, misconceptions, and pre-requisite knowledge that learners should have before learning the new topic. Upon comparing the two topics, the teachers differed in the extent of their knowledge of learners and learning difficulties. In the electrochemical cells topic, the teachers had more knowledge of learners and potential misconceptions, while they lacked a similar level of knowledge in the nuclear reactions topic.Learning difficulties. All the difficulties mentioned by teachers during the CoRe interview-1 were observed in the class. For instance, both Mrs Ertan and Mr Demir stated that learners find it hard to learn how the signs of anode and cathode are determined in electrochemical and electrolytic cells (CoRe interview-1). During classroom observations, this learning difficulty was apparent. The teachers also knew learners would have difficulty in identifying the reduced and oxidized species in the cell reactions.
Misconceptions. The participants' knowledge of student misconceptions regarding electrochemical cells was not as rich as their knowledge of learners' difficulties for the same topic. Mr Demir and Mrs Ertan could not state any specific misconception in the electrochemical cells in the CoRe interview-1. However, when they started teaching the topic, they realized some misconceptions. For example, Mr Demir and his students were solving problems. Mr Demir drew the cell figure including two half cells on the board:
Std: The first one is anode, right?
Mr Demir: No, it is not. There is no rule like that. It is only in the writing the [short-hand] cell notation (Mr Demir, field note).
When asked in the interview, Mr Demir stated that he detected it in previous years as well. He thought that learners had this misconception because teachers always say “oxidation and reduction” therefore, students think that oxidation is first and the anode would always be first on the left side of the diagram. He thought this misconception was further re-enforced because, in most problems, the anode is the first half-cell (Mr Demir, weekly interview). The same misconception was observed in Mrs Ertan's class. When one of the students asked Mrs Ertan if the anode was the first half-cell, she told students not to focus on the physical placement of the cells. This misconception was stated in the literature by Sanger and Greenbowe (1997a).
Pre-requisite knowledge. Both Mr Demir and Mrs Ertan were able to describe pre-requisite knowledge that learners would need for electrochemical cells in the CoRe interview-1. They stated that learners should be able to understand chemical reactions, chemical calculations, oxidation number, rate and heat of reactions, and chemical equilibrium topics. For instance, in electrolytic cells, both teachers required students to remember the spectator ion that does not react with the species in the solution. It is necessary to know the spectator ion concept to determine which species would be oxidized and reduced in the cell reaction (field notes).
Difficulties. When asked in the CoRe interview-2, Mr Demir stated that learners generally could not visualize nuclear reactions because of the abstract nature of the phenomenon. Another difficulty mentioned by both teachers was that students have difficulty discriminating between chemical and nuclear reactions. This point was stated by Nakiboğlu and Tekin (2006). Additionally, Mrs Ertan stated that learners have difficulty in discriminating artificial and natural nuclear reactions. When asked the reasons for these difficulties, she said:
Till now they have learned chemical reactions. But from now on they are going to learn nuclear ones. They [the nuclear and chemical reactions] are really different from each other. Atoms reacting and mass are saved in chemical reactions but it is not the case in nuclear ones (Mrs Ertan, weekly interview).
The participants were unaware of any other difficulties students might have learning about nuclear reactions.
Misconceptions. Mr Demir and Mrs Ertan had little knowledge of learners' misconceptions in this topic. Mr Demir described only one misconception in the CoRe interview-2: “Nuclear reactions are bad and dangerous”. He thought that students generally have more difficulties than misconceptions in this topic. Mrs Ertan could not identify any student misconceptions in the CoRe interview-2. However, the first author identified several student misconceptions during instruction. For instance, while Mrs Ertan was teaching, some learners stated that fission is natural whereas fusion is artificial nuclear reaction. She stated that students also thought “both fission and fusion occur in nuclear reactors”. Although the researcher detected them, the teachers did not specify those in the CoRe interview-2.
Pre-requisite knowledge. Mr Demir and Mrs Ertan identified little pre-requisite knowledge necessary for learning about nuclear reactions. They stated that learners need to understand atomic structure and isotopes but not much else. They asked students to recall what an isotope was at the beginning of the topic.
Knowledge of assessment (KoA)
When compared to other PCK components, the teachers did not have topic-specific PCK for assessment. Although they used different assessment techniques (e.g., informal questioning, quiz, and test) for assessing learners' understanding, neither the purpose of assessment nor the methods of assessment were specific to the topics taught. Most of the examples given below are general pedagogical knowledge (PK) – assessment knowledge rather than topic-specific. Teachers differed in the extent of their use of assessment in the two topics. For the electrochemical cells topic, the teachers had more coherent assessment, while it was fragmented in nuclear reactions.How to assess and purpose of assessment. Throughout the electrochemical cells topic, both teachers used a variety of general assessment methods. For instance, they used informal questioning, quizzes, observing learners performing exercises, and a test to assess learners' understanding. They used formative assessment techniques for the purpose of assessing students' progress toward the learning goals. However, when asked in the weekly interview, they did not specify any topic-specific reason (i.e., that is for why they use those in the electrochemical cells topic). Additionally, both Mr Demir and Mrs Ertan started the electrochemical cells topic with eliciting learners' prior knowledge about electrochemistry and the cells. They asked: “What do you know about electrochemistry?”, “what is a chemical cell?” (field notes) when asked the reason why, both teachers stated they began all topics this way in order to understand their learners' prior knowledge and to be able to take these ideas into account. However, the teachers did not discuss any topic-specific diagnostic assessment strategies.
In addition to the general diagnostic questions used at the beginning of the topic, they used various formative assessment techniques to determine to what extent learners understood specific concepts. For instance, after teaching how to determine the anode and cathode in electrochemical cells and how to form a cell, the teachers had students work problems, labeling the electrodes as anode and cathode in electrochemical cells. While learners were labeling the electrodes, teachers observed their performance, stressed some important points that learners missed, and gave feedback about their performance (field notes). In light of students' performance, teachers decided to perform more exercises, focusing on where students had difficulty. Additionally, Mr Demir and Mrs Ertan gave two quizzes in this topic. The graded quizzes were returned to students, indicating what mistakes they had made. At the end of the electrochemical cells topic, teachers used a paper–pencil test to assess learners' understanding. They used open-ended items and multiple-choice items. When teachers seek to see learners' performance step by step, teachers utilized open-ended items in which learners construct their answers. To illustrate, by using the standard oxidation potentials given in the question, students were required to decide first the anode and cathode. Then they determine the electron flow, ion flow through salt bridge, potential difference between the anode and cathode, and write oxidation and reduction half-cell reactions and cell reaction. In this topic, the teachers used diagnostic, formative, and summative assessments.
What to assess. Mr Demir and Mrs Ertan assessed only the science content in this topic. Teachers focused on assessing whether students learn to balance redox reactions, label the electrodes as anode and cathode in electrochemical cells, determine the electron flow, ion flow through salt bridge, potential difference between the anode and cathode, and write oxidation and reduction half-cell reactions and cell reaction. However, they did not assess the students' NOS understandings or the use of electrochemical cells in daily life.
In summary, Mr Demir and Mrs Ertan's assessment in this topic was coherent regarding the purpose of assessment and how to assess sub-components. They used diagnostic, formative, and summative assessments. The feedback they received from formative assessments informed their decisions to re-teach content or to give students more practice problems. However, their assessment strategies were at the general PK level and not specific to the topic of electrochemical cells.
How to assess and purpose of the assessment. Mr Demir and Mrs Ertan used only two types of assessment strategies: informal questioning to elicit prior knowledge (i.e., eliciting learners' prior knowledge about isotopes) and a summative test to evaluate students' understanding. An example of eliciting learners' prior knowledge was observed at the beginning of the topic.
Mr Demir: What does isotope mean?
Stds: They have the same number of protons but their mass numbers are different.
Mr Demir: What do I mean when I say U-238, U-235, and U-234?
Std-1: Their mass numbers are different.
Std-2: They have the same number of protons.
Mr Demir: They are the different isotopes of uranium. What do we use them to discriminate from each other? Mass numbers. (Mr Demir, field notes)
Neither teacher used formative assessment strategies during the topic. Both assessed learners' prior knowledge at the beginning of the topic and graded them on the test at the end. The test consisted of only multiple-choice items, and no open-ended items. Thus, we characterized their assessment as fragmented for this topic.
What to assess. Similar to the first topic, teachers only assessed the science content in this topic. Although there were many discussions on nuclear energy use and daily use of nuclear reactions, the teachers did not assess students' understanding of these connections. Likewise, NOS was mentioned during instruction (i.e., the idea that ‘science is subject to change’ was mentioned implicitly) but was not included in the topic test.
Discussion
PCK is widely conceptualized as being ‘topic-specific’, yet the extent to which this notion applies to teachers' PCK remains somewhat unclear. Does ‘topic specific’ refer to PCK as a whole, or are the particular subcomponents of PCK also specific to topic? Or rather, is PCK specific to topic regarding the quality, quantity, or interplay of components? Researchers have not answered this question so far. In this study, we probe the topic-specific nature of PCK by examining two experienced chemistry teachers' PCK as enacted in teaching two different topics. In Table 5, the results were summarized.| PCK components | Topics | |
|---|---|---|
| Electrochemical cells | Nuclear reactions | |
| OST | Didactic orientation | |
| KoIS | Content-based and teacher-centered instruction | Less teacher-centered Instruction enriched with implicit NOS and discussion on STSE |
| KoL | Robust | Less robust |
| KoC | Network of topics | Limited network of topics |
| KoA | PK-coherent | PK-fragmented |
We found that, though they held similar orientations and had similar PCK when compared to each other, teachers exhibited differences in their PCK in terms of teaching two different topics. Our data suggest that teachers' knowledge of instructional strategies, learner and curriculum were topic-specific whereas their knowledge of assessment, instructional sequence, and orientations were not topic-specific. In summary, teachers' OSTs (i.e., didactic) were consistent across the two topics, yet, the nature of the other PCK components differed between the two topics (Table 5).
Orientations to science teaching (OST)
Both participants held primarily didactic teaching orientations, which did not shift from one topic to the other. This finding is consistent with Magnusson et al. (1999), who proposed that OSTs were course-specific, not topic-specific. (Friedrichsen, et al., 2009) in their study of three experienced biology teachers confirmed that the participants' OSTs were course-specific, with central and peripheral components shifting for different courses. On a pragmatic level, one would not expect teachers to hold radically different teaching beliefs for different topics within the same course. What is interesting to note, however, is that despite consistency in their orientations, the teachers took two very different instructional approaches to teaching different topics (i.e., teacher centered versus student centered). Prior research suggests that a teacher's fundamental beliefs about the nature of science support certain orientations, and in turn that certain orientations may be consistent with reform-based practices, such as inquiry (Volkmann and Zgagacz, 2004). Our data suggest that teachers' adoption of particular instructional approaches is not merely a matter of orientations, but also a question of context and outside pressures. Although the participants in this study were eager to use reformed-based instructional strategies (e.g., inquiry), they stated that the educational system in [the country] was a significant barrier to achieving this goal. Nargund-Joshi et al. (2011) and Zhang et al. (2003) reported a similar situation in India and China, respectively.Differences in teachers' PCK for different topics
We noted several differences in teachers' PCK for teaching electrochemical cells and nuclear reactions; however, these appear to be related to teachers' SMK, the structure of the national curriculum, and the nature of the topics themselves.SMK is critical for developing robust PCK (Magnusson et al., 1999; Abell, 2007). Although we initially assumed the teachers' SMK was equally strong for both topics, as we observed the teachers we found this was not the case. Both teachers' SMK was deep in regard to electrochemical cells, whereas, consistent with prior research (Atwood and Sheline, 1989; Nakiboğlu and Tekin, 2006), both teachers had weaker SMK for nuclear reactions. Though strong SMK does not guarantee rich PCK (Lee et al., 2007; Kind, 2009), in the present study, this played a role in teachers' ability to make meaningful alterations in the curriculum and their plans.
A second consideration in explaining differences in teachers' PCK for these two topics is the emphasis of the national curriculum guide, which has been criticized in that “[i]nstruction in nuclear chemistry is limited or lacking in the chemistry curriculum” (Nakiboğlu and Tekin, 2006, p. 1712). Nuclear reactions are taught toward the end of the school year, which may lead teachers to ignore it or teach it superficially (Atwood and Sheline, 1989). However, in our study, the teachers did not skip over the topic, but chose to teach it using a more student-centered approach via discussions. The teachers in this study believed there was inadequate curriculum support for the nuclear reactions topic and this may have contributed to their limited knowledge of horizontal and vertical curriculum connections and learners' difficulties. Davis and Krajcik (2005) stated that educative curriculum materials that give information about students' difficulties, the reasons for those and suggestions to deal with students' difficulties, and suggest some teaching materials and activities that teachers can use for the specific topic.
Our data also provide evidence that the nature of the topics is an important consideration. It has been reported that teachers who have strong SMK for a topic allow their students to talk more than when the teacher lacks SMK (Carlsen, 1993; Garmston, 1998). However, in this study, we found the opposite; the teachers let learners talk less during the topic for which they had stronger SMK (electrochemical cells). The differences may be related to the sequential nature of the electrochemical cells topic. It includes clear-cut sub-parts, which makes the topic sequential. In order to be able to learn the subsequent parts, the previous ones play a pre-requisite role. Also, learners also need to have considerable pre-requisite knowledge of chemistry and physics. In addition, the topic includes multiple concepts (e.g., half reactions and standard potentials) (De Jong and Treagust, 2002), which makes learning the topic difficult. In contrast, the nuclear reactions topic requires little pre-requisite knowledge (i.e., atom, isotope, atomic and mass number) (Nakiboğlu and Tekin, 2006) and is more integrated in nature, in that there are no clear-cut sub-parts.
The nature of the two topics may also explain the differences in instruction. Nuclear reactions topic is a controversial topic on which scientists, media, society, and politicians have debates –especially after the tsunami in Japan. Parallel to discussions in the media, teachers used discussions to examine the different aspects (e.g., energy and environment) of the topic. The teachers also encouraged students to be more engaged with the topic of nuclear reactions and to share ideas. Although differences were observed in the participants' teaching of the two topics, the instructional sequence including lecturing, using activities, and performing exercises were similar. DeBoer (1991) stated that this type of instructional sequence has been the chronic illness of teachers' instruction for a long time. Likewise, Friedrichsen et al. (2009) observed the use of a similar instructional sequence in their study. This type of instructional sequence may be only one that they have experienced. Similarly, for both topics, none of the teachers used discipline-specific strategies (e.g., conceptual change, 5E, inquiry). This may be related to the lack of knowledge about how to implement those strategies (Settlage, 2000) and the lack of experience teaching in that way (Flick, 1996). Ingber (2009) and De Jong et al. (1995) revealed that the lack of discipline-specific strategies might be explained by teacher-specific teaching rather than a topic-specific one. Teachers may have a tendency to implement similar types of activities with the same purpose and the same sequence without considering the topic being taught. In other words, teachers may develop their own styles of teaching (e.g., delivering the content through didactic teaching) and teach most of the topics in that way. Therefore, teachers' instructional sequencing may not be a reflection of topic-specific PCK, but rather teacher or course-specific habits.
On the other hand, the sequential versus integrated nature of the topics may also provide explanation for the differences in KoC. As mentioned above, electrochemical cells require pre-requisite knowledge in chemistry (e.g., chemical reactions, periodic table, chemical equilibrium, oxidation number- charge, etc.) and physics (e.g., circuits, electron flow, etc.). For effective teaching of the former, teachers need to be aware of the chemistry curriculum in regard to the sequence of concepts, as well as horizontal and vertical connections. In contrast, the nuclear reactions topic only requires minimal pre-requisite knowledge (e.g., atom, isotope, atomic and mass number) (Nakiboğlu and Tekin, 2006) and does not require connecting topics as much as the electrochemical cells topic does. The differing nature of the two topics may explain why teachers' KoC was more extensive in the electrochemical topic but was limited for nuclear reactions. Moreover, teachers' robust SMK in the electrochemical cells topic may be influential in understanding how this topic is effectively linked to other chemistry topics. Another possible explanation for the difference in KoC may relate to differences in teachers' knowledge of learners' difficulties. Since teachers were more aware of students' difficulties and their pre-requisite knowledge regarding electrochemical cells, they may have paid more attention to make both horizontal and vertical links for that topic.
The sequential versus integrated nature of the two topics may have also shaped the teachers' assessment practices. Although both teachers' assessment knowledge was at the general PK level, their assessment practices varied between the two topics of study. Coherent assessment use may be related to the sequential nature of the electrochemical cells topic (i.e., De Jong and Treagust (2002) also mentioned the sequential nature of the electrochemical cells topic). In order to move to the next part of a lesson, teachers needed to check whether learners understood the previous part (Sirhan, 2007). In contrast, while teaching nuclear reactions, the teachers may not have considered formative assessment necessary. Though we expect experienced, reflective teachers to have robust PCK (Grossman, 1990; van Driel et al., 2002), in this study, the two teachers did not develop topic-specific assessment strategies. None of the teachers could give any topic-specific explanations for why they used those assessment strategies when it was prompted in the weekly-interviews. Rather, they tried to explain it by the use of general purposes (e.g., to follow learners' understanding) and general strategies (e.g., quiz) without specifying why it is suitable to implement. Our findings parallel those of Henze et al. (2008), who reported that teachers had limited knowledge of assessment although they developed a rich repertoire of instructional strategies for teaching ‘Models of the Solar System and Universe’. Development of knowledge of assessment might take more time than the development of other PCK components (Hanuscin et al., 2011); Henze et al., 2008). Topic-specific PCK for assessment may require targeted professional development. Alternatively, perhaps PCK for assessment is not topic-specific at all and only develops at the subject or discipline level. In terms of KoL, both teachers were highly knowledgeable about learners' difficulties and misconceptions in electrochemical cells. Moreover, they were aware of the pre-requisite knowledge necessary to learn electrochemical cells. However, in regard to nuclear reactions, teachers had little knowledge of student difficulties, misconceptions, or the pre-requisite knowledge necessary to learn the topic well. The reason for the difference in teachers' KoL may be related to teachers' limited SMK in nuclear reactions. It seems that if SMK is robust for a topic, it may assist their understanding of pre-requisite knowledge, and possible difficulties and misconceptions.
The difficulty of the topic perceived by students may also result in the differences in teachers' PCK for each of the topic. The topic of electrochemical cells requires much pre-requisite knowledge and comprehension of multiple concepts (De Jong and Treagust, 2002); it is a conceptually difficult topic, and one with which students struggle. Therefore, teachers may have spent more time reflecting on ways to make electrochemical cells easier for students to understand. As a result of seeing students struggle, teachers may have utilized more formative assessments in order to make sure that students understand.
Conclusions and implications
The findings of this study contribute to the literature in several ways. First, while PCK has been characterized as topic-specific in the literature (e.g., Magnusson et al., 1999), the extent of the topic-specificity of PCK has not been a focus of previous research. To explore this question, we compared two teachers' PCK for teaching two different topics. This study provides evidence that, in addition to SMK, the nature and complexity of a topic may influence teachers' development of PCK. The two teachers in this study did have topic-specific KoIS, KoL, and their knowledge was more robust for the topic of electrochemical cells, which was perceived as a more difficult topic for students to learn. However, the teachers did not possess topic-specific knowledge for instructional sequencing or for assessment for either of the two topics. This suggests that more general domains of teacher knowledge are manifested in the enactment of teachers' PCK. That is, the topic-specific nature of PCK should be interpreted more cautiously. Due to the limited numbers of participants working in a single context, however, the results of the study should also be interpreted carefully. Though a case study approach provides a detailed illustration of how PCK is specific to a given topic, these results may not be generalized to different teachers, contexts and topics.Despite these limitations, however, our study suggests several implications for professional developers and teacher educators, curriculum developers, and researchers. First, our findings indicate a need for targeted professional development to support teachers in developing topic-specific PCK, with particular attention paid to topic-specific instructional strategies and assessment strategies. Although both participants participated in professional development activities, because these activities were not specific to chemistry topics (e.g., teaching electrochemical cells or nuclear reactions), previous trainings and experiences did not play a significant role in supporting their PCK development in this topic. Therefore, our study supports the notion that professional development should not only be specific to discipline (e.g., how to use performance-based assessment in chemistry) but also specific to topics within that discipline (e.g., how to assess learners' understanding in electrochemical cells, what to assess regarding learners' understanding in nuclear reactions, etc.). Hence, professional development activities should take into account the topic-specific nature of PCK. In addition to the professional development activities, pre-service teacher education programs and induction year mentoring programs should focus on how pre-service teachers and/or novice teachers develop topic-specific PCK in addition to discipline specific one. An explicit attention for relating subject and topic-specific PCK is the key for enriching teachers' PCK for teaching specific topics (Sickle, 2012). Furthermore, the science teaching method course may be one of the best contexts for teaching pre-service teachers topic-specific PCK. In some of the countries (e.g., US), teacher education programs prepare teachers who are going to specialize in a variety of disciplines—all together at once. Due to the nature of the disciplines, teachers may not be able to transfer their knowledge and practice from one discipline to another. In light of the results and the literature (Henze et al., 2008; Sickle, 2012), teachers need special support and training for teaching the topics. We recommend that content-specific methods courses should be offered to future biology, chemistry, and physics teachers in order to support their PCK development. Teachers may need more support for developing some components than the others. Hence, teacher educators should also include topic-specific assessment strategies for assessing learners' understanding both in-service and pre-service teacher education programs. Moreover, the use of good examples of CoRes and PaP-eRs prepared by experienced teachers (i.e., some examples are provided in Loughran et al., 2006) would be beneficial for pre-service and novice teachers.
In regard to curriculum, teachers would benefit from educative curriculum materials that provide teacher background information to help build teachers' PCK and SMK (Davis and Krajcik, 2005). Curriculum materials should provide information about student learning difficulties, pre-requisite knowledge necessary for the specific topic, suggested instructional activities and strategies, as well as assessment strategies. Our study suggests that educative curriculum materials are especially critical for topics in which teachers tend to have weak SMK (e.g., nuclear reactions). The materials should also stress the nature of the topic and offer topic-specific activities to teachers especially for the abstract topics and the ones in which teachers have difficulty in using hands-on activities and real materials (e.g., nuclear reactions).
In regard to research, our study calls attention to a need for additional studies conducted to examine teachers' PCK for different topics within the same discipline (both disparate and closely related) in order to further explore the topic-specific nature of PCK. Magnusson et al.'s model (1999) proposed KoA as topic-specific; however, our findings add to previous studies (e.g., Hanuscin et al., 2011) that challenge this notion. Targeting professional development efforts on particular subcomponents of PCK may provide one venue to explore this further.
Appendix
Observation protocol
The observer(s) will have selected 3–5 interesting instances to discuss. What constitutes an interesting instance? For instance:Knowledge of assessment (KoA)
Teacher implements assessment to ascertain student prior knowledge.The teacher recognizes that the students are having difficulty with a particular idea.
The teacher uses a low-level assessment strategy such as providing an exit-slip that requires students to define rather than explain or synthesize.
The teacher acts on data collected during student assessment.
References
- Abell S. K., (2007), Research on science teacher knowledge, in Abell S. K. and Lederman N. G. (ed.), Handbook of Research on Science Education, New Jersey: Lawrence Erlbaum Associates, pp. 1105–1151.
- Abell S. K., (2008), Twenty years later: Does pedagogical content knowledge remain a useful idea?, Int. J. Sci. Educ., 30, 1405–1416, DOI: http://10.1080/09500690802187041.
- Alsop S. J., Hanson J. and Watts D. M., (1998), Pupils' perceptions of radiation and radioactivity: the wary meet the unsavoury, Sch. Sci. Rev., 72, 75–80.
- Atwood C. H. and Sheline R. K., (1989), Nuclear chemistry: include it in your curriculum, J. Chem. Educ., 5(66), 389–393.
- Avraamidou L. and Zembal-Saul C., (2005), Giving priority to evidence in science teaching: a first-year elementary teacher's specialized practices and knowledge, J. Res. Sci. Teach., 42(9), 965–986, DOI: http://10.1002/tea.20081.
- Berliner D. C., (2001), Learning about and learning from expert teachers, Int. J. Ed. R., 35, 463–482.
- Carlsen W. S., (1993), Teacher knowledge and discourse control: quantitative evidence from novice biology teachers' classrooms, J. Res. Sci. Teach., 30, 471–481.
- Curriculum Material, (2011).
- Davis E. A. and Krajcik J. S., (2005), Designing educative curriculum materials to promote teacher learning, Educational Researcher, 34(3), 3–14, DOI: http://10.3102/0013189X034003003.
- DeBoer G. E., (1991), A history of ideas in science education: Implications for practice, New York, NY: Teachers College Press.
- De Jong O., Acampo J. and Verdonk A., (1995), Problems in teaching the topic of redox reactions, J. Res. Sci. Teach., 32(10), 1097–1110.
- De Jong O. and Treagust D., (2002), The teaching and learning of electrochemistry, in Gilbert J. K., De Jong O., Justi R., Treagust D. F. and van Driel J. H. (ed.), Chemical education: Towards research-based practice, Dordrecht: Kluwer Academic Publishers, pp. 317–337.
- De Jong O. and Van Driel J., (2001), The development of prospective teachers' concerns about teaching chemistry topics at a macro-micro-symbolic interface, in Behrednt H., Dahncke H., Duit R., Gräber W., Komorek M., Kross A. and Reiska P. (ed.), Research in science education: Past, present and future, Dordrecht, The Netherlands: Kluwer Academic, pp. 271–276.
- Flick L. B., (1996), Understanding a generative learning model of instruction: a case study of elementary teacher planning, Journal of Science Teacher Education, 7(2), 95–122.
- Friedrichsen P. M. and Dana T. M., (2003), Using a card-sorting task to elicit and clarify science-teaching orientations, Journal of Science Teacher Education, 14(4), 291–309.
- Friedrichsen P. M. and Dana T. M., (2005), Substantive-level theory of highly regarded secondary biology teachers' science teaching orientations, J. Res. Sci. Teach., 42(2), 218–244, DOI: http://10.1002/tea.20046.
- Friedrichsen P., Abell S. K., Enrique P. M., Brown P. L., Lankford D. M. and Volkmann M. J., (2009), Does teaching experience matter? Examining biology teachers' prior knowledge for teaching in an alternative certification program, J. Res. Sci. Teach., 46(4), 357–383, DOI: http://10.1002/tea.20283.
- Friedrichsen P., Van Driel J. and Abell S., (2011), Taking a closer look at science teaching orientations, Sci. Educ., 95, 358–376, DOI: http://10.1002/sce.20428.
- Garmston R. J., (1998), The persuasive Art of Presenting: becoming expert teachers-Part I, Journal of Staff Development, 19(1), 60–63.
- Garnett P. J. and Treagust D. F., (1992a), Conceptual difficulties experienced by senior high school students of electrochemistry: electrochemical (galvanic) and electrolytic cells, J. Res. Sci. Teach., 29, 1079–1099.
- Garnett P. J. and Treagust D. F., (1992b), Conceptual difficulties experienced by senior high school students of electrochemistry: electric circuits and oxidation-reduction equations, J. Res. Sci. Teach., 29, 121–142.
- Geddis A. N., Onslow B., Beynon C. and Oesch J., (1993), Transforming content knowledge: learning to teach about isotopes, Sci. Educ., 77, 575–591.
- Grossman P., (1990), The Making of a Teacher, New York: Teachers College Press.
- Halim L. and Meerah S. M., (2002), Science teachers' pedagogical content knowledge and its influence on physics teaching, Res. Sci. Technol. Educ., 20(2), 215–225.
- Hanuscin D. L., Lee M. H. and Akerson V. L., (2011), Elementary teachers' pedagogical content knowledge for teaching the nature of science, Sci. Educ., 95(1), 145–167, DOI: http://10.1002/sce.20404.
- Henze I., van Driel J. H. and Verloop N., (2008), Development of experienced science teachers' pedagogical content knowledge of models of the solar system and the universe', Int. J. Sci. Educ., 30(10), 1321–1342, DOI: http://10.1080/09500690802187017.
- Ingber J., (2009), A comparison of teachers' pedagogical content knowledge while planning in and out of their science expertise, Unpublished doctoral dissertation, Columbia University, NY, USA.
- Kind V., (2009), Pedagogical content knowledge in science education: potential and perspectives for progress, Stud. Sci. Educ., 45(2), 169–204, DOI: http://10.1080/03057260903142285.
- Koehler M. J. and Mishra P., (2009), What is technological pedagogical content knowledge?, Contemporary Issues in Technology and Teacher Education, 9(1), 60–70. Retrieved from http://www.citejournal.org/vol9/iss1/general/article1.cfm.
- Lee E., Brown M. N., Luft J. A. and Roehrig G. H., (2007) Assessing beginning Secondary science teachers' PCK: Pilot year results, Sch. Sci. Math., 107(2), 52–60, DOI: http://10.1111/j.1949-8594.2007.tb17768.x.
- Loughran J., Mulhall P. and Berry A., (2004), In search of pedagogical content knowledge I science: developing ways of articulating and documenting professional practice, J. Res. Sci. Teach., 41, 370–391, DOI: http://10.1002/tea.20007.
- Loughran J., Berry A. and Mulhall P., (2006), Understanding and Developing Science Teachers' Pedagogical Content Knowledge, Rotterdam: Sense Publishers.
- Magnusson S., Krajcik J. and Borko H., (1999), Nature, sources and development of pedagogical content knowledge for science teaching, in Gess-Newsome J. and Lederman N. G. (ed.), Examining pedagogical content knowledge: The construct and its implications for science education, Boston: Kluwer, pp. 95–132.
- Merriam S. B., (1998), Qualitative research and case study applications in education, London: Sage.
- Merriam S., (2009), Qualitative research: A guide to design and implementation, San Francisco, CA: Jossey-Bass.
- Millar R., Eijkelhof H. and Klaassen K., (1990), Teaching about radioactivity and ionizing radiation: an alternative approach, Phys. Educ., 25(6), 338–342.
- Nakiboğlu C. and Tekin B. B., (2006), Identifying students' misconceptions about nuclear chemistry. A study of Turkish high school students, J. Chem. Educ., 83(11), 1712–1718.
- Nargund-Joshi V., Park-Rogers M. A. and Akerson V., (2011), Exploring Indian secondary teachers' orientation and practice for teaching science in an era of reform, J. Res. Sci. Teach., 48(6), 624–647, DOI: http://10.1002/tea.20429.
- Newton L. D. and Newton D. P., (2010), What teachers see as creative incidents in elementary science lessons, Int. J. Sci. Educ., 32(15), 1989–2005, DOI: http://10.1080/09500690903233249.
- Ogude N. A. and Bradley J. D., (1994), Ionic conduction and electrical neutrality in operating electrochemical cells. Pre-college and college student interpretations, J. Chem. Educ., 71(1), 29–34.
- Park S. and Chen Y., (2012), Mapping out the integration of the components of pedagogical content knowledge (PCK): examples from high school biology classrooms, J. Res. Sci. Teach.. 49(7), 922–941, DOI: http://10.1002/tea.21022.
- Park S. and Oliver J. S., (2008), Revisiting the conceptualization of pedagogical content knowledge (PCK): PCK as a conceptual tool to understand teachers as professionals, Res. Sci. Educ., 38, 261–284, DOI: http://10.1007/s11165-007-9049-6.
- Patton M. Q., (2002), Qualitative evaluation and research methods, 3rd edn, Thousand Oaks, CA: Sage.
- Prather E., (2005) Students' beliefs about the role of atoms in radioactive decay and half-life, J. Geosci. Educ., 53, 345–354.
- Rollnick M., Bennett J., Rhemtula M., Dharsey N. and Ndlovu T., (2008), The place of subject matter knowledge in pedagogical content knowledge: a case study of South African teachers teaching the amount of substance and chemical equilibrium, Int. J. Sci. Educ., 30(10), 1365–1387, DOI: http://10.1080/09500690802187025.
- Samuelowicz K. and Bain J. D., (1992), Conceptions of teaching held by academic teachers, Higher Education, 24(93), 93–111.
- Sanders L. R., Borko H. and Lockard J. D., (1993), Secondary science teachers' knowledge base when teaching science courses in and out of their area of certification, J. Res. Sci. Teach., 30(7), 723–736.
- Sanger M. J. and Greenbowe T. J., (1997a), Common student Misconceptions in electrochemistry: galvanic, electrolytic, and concentration cells, J. Res. Sci. Teach., 34, 377–398.
- Sanger M. J. and Greenbowe T. J., (1997b), Common student misconception in electrochemistry: current flow in electrolyte solutions and the salt bridge, J. Res. Sci. Teach., 30, 111–126.
- Settlage J., (2000), Understanding to learning cycle: influences on abilities to embrace the approach by preservice elementary school teachers, Sci. Educ., 84, 43–50.
- Shulman L. S., (1987), Knowledge and training: foundations of the new reform, Hardward Educational Review, 57, 1–22.
- Sickle A. J., (2012), Examining beginning biology teachers' knowledge, beliefs, and practice for teaching natural selection. Unpublished doctoral dissertation, University of Missouri-Columbia, MO, USA.
- Sirhan G., (2007), Learning difficulties in chemistry: an overview, Journal of Turkish Science Education, 4(2), 2–20.
- Schmidt H., Marohn A. and Harrison A. G., (2007), Factors that prevent learning in electrochemistry, J. Res. Sci. Teach., 44(2), 258–283.
- Van Driel J. H., de Jong O. and Verloop N., (2002), The development of preservice chemistry teachers' pedagogical content knowledge, Sci. Educ., 86, 572–590, DOI: http://10.1002/sce.10010.
- Veal W. R. and Kubasko D. S., (2003), Domain specific pedagogical content knowledge of evolution held by biology and geology teachers, Journal of Curriculum and Supervision, 18(4), 334–352.
- Veal W. R. and MaKinster J. G., (1999), Pedagogical content knowledge taxonomies, Electron. J. Sci. Educ., 3(4), available at: http://unr.edu/homepage/crowther/ejse/ejsev3n4.html.
- Volkmann M. J. and Zgagacz M., (2004), Learning to teach physics through inquiry: the lived experience of a graduate teaching assistant, J. Res. Sci. Teach., 41(6), 584–602, DOI: http://10.1002/tea.20017.
- Zhang B., Krajcik J., Sutherland L. M., Wang L., Wu J. and Qiang Y., (2003), Opportunities and challenges of China's inquiry-based education reform in middle and high school: perspectives of science teachers and teacher educators, Int. J. Sci. Math. Educ., 1, 477–503.
| This journal is © The Royal Society of Chemistry 2014 |