Teaching chemical change modeling to Tunisian students: an “expanded chemistry triplet” for analyzing teachers' discourse
Alain
Dumon
* and
Imene
Mzoughi-Khadhraoui
IUFM d'Aquitaine, bd. du recteur J. Sarrailh, Pau 64000, France. E-mail: alain.dumon@neuf.fr
First published on 8th November 2013
Abstract
Through a comparative analysis of the chemical content of three teachers' discourse; we propose to give answers to the question: “how is the connection between the experiential level and the generally accepted representation of the three levels of chemistry presented by teachers to Tunisian students, during their first contact with chemical change modeling”. We chose to perform the analysis using an “expanded chemistry triplet” to represent the different levels and their possible connections to the elements of knowledge taught. These elements of knowledge, called facets, included definitions, representations and rules used by the teachers to convey the meaning. While there was some difference in priorities between the three teachers with regard to teaching content, the connection between the experiential field and the three levels of representation of chemistry was only partially taught by all. It is the description, the interpretation and the representation of objects and events of the experiential field at the macroscopic level that are strongly in the majority. On the other hand, interest in the sub-microscopic level and clarification of the procedures to implement for the quantitative treatment of chemical reactions seems insufficient. The responsibility is thus left to the students to acquire a large amount of knowledge and expertise. These observations led us to formulate proposals for improving the teaching of chemical reactions and teacher training.
Introduction
Following the work of Johnstone (1982, 1991, 2000), it is generally accepted that the interpretation and understanding of chemical phenomena requires the linking of three levels of representation: macroscopic, submicroscopic and symbolic levels. Talanquer (2011, p. 179) says: “The idea that chemical knowledge can be represented in three main ways: macro, submicro, and symbolic (chemistry triplet) has become paradigmatic in chemistry and science education.”During the learning of chemistry, the constant interplay between these three levels of thought, “the triplet relationship” as it has recently been called by Gilbert and Treagust (2009), presents a real difficulty for students (see for example: Johnstone, 2000; Kozma, 2003; Treagust et al., 2003; De Jong and Van Driel, 2004; Onwu and Randall, 2006; Ramnarain and Joseph, 2012). In particular, we found (Mzoughi-Khadhraoui and Dumon, 2012) that the majority of beginners are not able to make sense of literal and symbolic representations of chemical reactions. They fail to coordinate the different knowledge levels in order to achieve this (Taber and Bricheno, 2009).The encountered difficulties may be attributed, either to the fact that beginner students do not have a clear understanding of what represents a chemical reaction, or they have not mastered the rules that they have been taught or the fact that the rules are not always applicable, or they lack chemical knowledge (Yarroch, 1985; Ben-Zvi et al., 1988; Friedel and Maloney, 1992; Abraham et al., 1994; Fillon, 1997; Chambenois et al., 2003; Taber, 2009). But starting from the idea that the kind of teaching is one determining factor in school learning (Mercier and Buty, 2004; Taber, 2012), we can also examine the role of the received teaching. In order to precisely highlight what the teacher brings to students in the subject area they are studying, what the teacher explains and what he leaves to the responsibility of the students (Robert, 2001; Chappet Pariès, 2004), we are interested in the teachers’ discourse during the initial teaching in French to Tunisian students of chemical change modeling. (In the present manuscript, all the dialogue presented has been translated into English.)
The “expanded chemistry triplet”
Modeling can be defined as the passage from a first description of objects and events perceived in the experiential field of reference (phenomenology) to a second description using the world of theories and models (concepts, models, laws, formalism, etc.) (Tiberghien, 1994). A characteristic of chemistry is that the modeling activity does not just give a possible description of objects and phenomena of the perceptible world (natural gas, air, combustion, for example), it changes the objects and works on new objects and events, so-called reconstruction, at both the macroscopic (methane, oxygen in the air, chemical reaction, for example) and submicroscopic (methane and oxygen molecules, conservation of atoms, and so one) levels, in a theoretical approach (Le Maréchal, 1999). Another characteristic is the use of a specific language (where the symbolic register occupies an important place) based on rules and/or conventions. Language that allows the representation and communication of concepts and models developed at these two levels (Taber, 2013) (e.g., CH4, methane gas or methane molecule, O2, oxygen in the air or oxygen molecule).How is the empirical field and the world of theories and models represented? Firstly, we can adopt the suggestion of some authors (Kermen and Méheut, 2009; Talanquer, 2011; Taber, 2013) to split the macro corner of the Johnstone chemistry triplet into two apexes: the experiential level corresponding to the chemical phenomenon observed and the experiential device implemented to achieve it, and the macroscopic models that interpret it (substance, chemical species, element or compound, reaction/chemical change, reactants and product, molar mass, for example). In agreement with Taber (2013) and other authors, we consider that the observed phenomena is reconceptualized not only at the macroscopic level but also in terms of the theoretical models of the structure of matter at the submicroscopic level (atom, molecule, ion, atomic and molecular masses, for example).
In order to represent the concepts of these different levels, chemists use conventional formalisms: chemical symbols and formulae, particulate drawings, molecular models, drawings of chemical apparatus, etc. We can according to Taber (2013), consider “that the symbolic knowledge domain cannot be readily separated from the macroscopic and sub-microscopic domains as a discrete level of chemical knowledge. Then, as “the symbolic is inherent in how we think about chemistry”, “the processes of learning, teaching and applying chemistry which involve re-descriptions into and between components of the specialized symbolic ‘language’ used to describe chemical ideas at the two levels” (p. 165) is represented by the “revisited chemistry triplet” of Fig. 1.
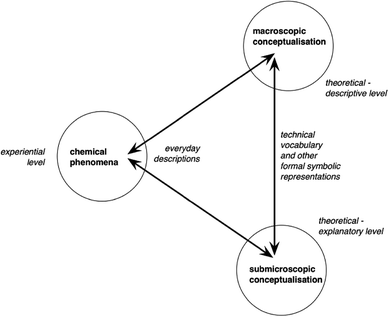 | ||
| Fig. 1 Taber's representation of the process of learning, teaching and applying chemistry (reproduced from Taber, 2013). | ||
But we can also consider that, particularly for beginner students, the “formal symbolic representations” is an additional level of knowledge in learning chemistry (Johnstone, 1991). For Talanquer (2011), this level, includes not only the symbolic representation but also all the visual signs used to facilitate qualitative and quantitative thinking and communication about both experiments and models in chemistry. So he named it, the “visualization” level. We consider that the symbolic/visualization level is one component of the world of theories and models.
Finally, inspired by the tetrahedron proposed by Kermen and Méheut (2009) to represent the connection between the experiential field and model (macroscopic and sub-microscopic) levels for symbolic representations, we have chosen to represent the connection between the experiential level and the world of theories and models (macroscopic, submicroscopic and visualization levels) by a “quadruplet” (or expanded chemistry triplet) (Fig. 2). This expanded chemistry triplet shows the different interactions that may occur during teaching between the four levels of description and interpretation of chemical phenomena.
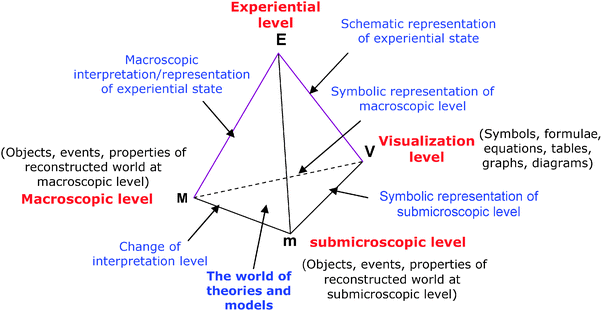 | ||
| Fig. 2 Schematic representation of the connection between the experiential level and the word of theories and models in chemistry. | ||
To illustrate how this quadruplet can be used to analyze how teachers move from one level to another, we will take an example: the combustion of a hydrocarbon gas used as a experiential state of reference in the three chapters devoted to the chemical “reaction”. Common language is used in the first description to the novice of this experiential state (apex E) and involves only perceptual phenomena: butane gas (known by the students) burns in air, we observe a flame and heat. The language used evolves subsequently by integrating concepts of macroscopic modeling levels previously learned (Martinand, 1995).
The first step in modeling is to enable students to describe the experiential fact using the language of chemistry (EM side): the butane gas burns in the presence of oxygen in air; it forms carbon dioxide and water (the experiential state will be so described in these terms to introduce the chemical word equation). While neglecting a number of perceptual phenomena (flame, heat, burning match, speed) to focus on the change in the kinds of chemicals. In the next step the change will be visualized by a chemical word equation (called reaction scheme in Tunisia) between reactants and products, butane + dioxygen → carbon dioxide + water (first level of symbolization, VM side). The syntactic rule to meet at this teaching level to write or read such a representation is: the product names are derived from those of the reactants. But this is not always so simple, especially when the name of the chemical species (butane, water, ammonia, for example) gives no details of their composition (Taber, 2009). The language used for these descriptions and representations of the chemical reaction using chemicals nomenclature is based on more or less arbitrary choices that have evolved throughout history (Mzoughi-Khadhraoui and Dumon, 2012).
Then, after representing the chemicals by their symbols (apex V), the reaction is visualized by an equation showing the proportions in which the reagents react and products form (CH4 + 2O2 → CO2 + 2H2O) (second level of symbolization, VM side), the chemical equation which reflects the mass conservation. The EV side corresponds to the representation of the experiment by a drawing, the MEV triplet to the representation of the experiential field at the macroscopic level.
However, in order to help students to understand the reaction equation, passage through the submicroscopic level is necessary. Thus, in the Vm side of the tetrahedron, the chemical equation symbolizes the principle of atoms conservation in kind and number. Several authors agree that the symbolic language is similar to a real language (Laszlo, 1993; Barlet, 1999; Jacob, 2001; Taber, 2009). It has an alphabet (the symbols of the atoms) with which we can form words (formulae of chemicals) in agreement with some spelling rules (the symbols' order, the indices related to atoms) based on atoms' properties (electronegativity, valence). Words can be combined to form sentences (chemical equations) according to some semantic rules (the meaning of signs + and →) and grammar (the atoms' conservation).
In the submicroscopic level modeling (apex m), the chemical entities are atoms, molecules or ions. Molecular models (apex V) can be used to visualize the composition and organization of the atoms within the molecule (apex m). The reaction equation can be read at the submicroscopic level (Vm side): “a methane molecule and two oxygen molecules are transformed into a carbon dioxide molecule and two water molecules”. Therefore, during the processing some bonds break and others form to lead to a reorganization of the atoms at the molecular level. The Mm side of the tetrahedron corresponds to a change in the modeling level: in M, chemical formulae represent the substance (a significant number of entities), in m the atomic composition of a molecular or ionic entity.
Thereafter, the quantitative interpretation of the observed changes requires t the experiential data (masses, volumes: apex E) to be linked with the world of theories and models (MVm triplet). For this we must not only know the different variables associated with the “mole” (amount of substance, molar mass, molar volume, Avogadro's number) but also control the different relationships they maintain. It is then that the meaning of stoichiometric numbers needs to be understood, in our example: during the methane combustion reaction, reactants react in the ratio n(O2)/n(CH4) = 2 and the products form in the proportions n(H2O)/n(CO2) = 2.
Research question and methodology
Research questions
The interest in the discursive practices of teachers in the classroom may have multiple objectives. For example, to identify what professional knowledge is implemented by teachers (Cross, 2010), to model the activity for teacher training (Goigoux, 2007; Sensevy, 2007), compare strategies implemented by different teachers to present new information to students (Lorenzo et al., 2010) or to analyze how teaching enables the linking between the experiential field and the world of theories and models (Venturini et al., 2007; Buty and Mortimer, 2008; Aguiar et al., 2010). The aim of our comparative analysis of three teachers' discourse on chemical content is to identify if there are differences or similarities in the way in which teachers connect the experiential field (apex E) with the world of theories and models (MVm triplet).The contextual framework
In Tunisia, during the first year of high school (age 15–16), the chemical reaction is introduced two months after teaching concepts of the world of theories and models: at the submicroscopic level (atom, molecule, ions), macroscopic level (element and compound, mole, molar mass, molar volume) and visualization level (symbolic representations and molecular models). In the official school book (Dhaha et al., 2002), three chapters concern chemical reaction: “concept of chemical reaction” (defined as “a transformation in which chemicals disappear and new ones appear”); “qualitative study of a chemical reaction” (the transformation can be initiated or spontaneous, fast or slow, exothermic or endothermic) and “quantitative study of a chemical reaction.”Observation methodology of teachers' discourse
The three observed teachers are the only teachers who accepted our presence in their classroom. These are ordinary teachers who have undergone four academic years with some teaching practice within a confirmed teacher classroom as their only training. So, the professional knowledge of the Tunisian teachers was gained exclusively from their teaching experience. They have close seniority at this teaching grade in their high school (T1, 12 years; T2, 7 years; T3, 9 years). They teach in three different high schools in Tunis located in similar districts according to their population. Their classes, about 33 students, can be considered of comparable ability levels because of the same success rates for the General Certificate of Education of their high school, relative to the national success rate (about 57%).We observed and recorded the teachers and students speaking during regular classroom situations in May 2009. The analyzed corpus corresponds to audio recording transcripts of these conversations during teaching of the three themes related to chemical reaction proposed in the first year of high school. We have chosen to split up the teaching situations observed with reference to the work of various authors (see example in Tables 1 to 5):
– At the large scale (of the order of one hour or more): the sequence is defined by its thematic unit (here the theme is defined by the title of the schoolbook chapter) and may consist of several sessions, each session corresponding to a continuous time unit of teaching in front of students (official duration one hour) (Robert, 1999; Rogalski, 2003; Tiberghien and Malkoun, 2007).
– At the medium scale (a few minutes): an episode corresponds to an activity that is performed for a specific purpose in the progression (Dumas Carré and Goffard, 1998; Morge, 2001; Mercier and Buty, 2004).
– At the small scale (a few seconds): interventions of teachers and students were transcribed by splitting them into intervention units separated by the mark/(El Hajjami et al., 1999). Some of these intervention units correspond to structured statement introduction, defining and/or linking different objects, events, properties, representations and rules introduced to discuss the chemical reaction. We name them “units of meaning”.
In order to carry out a comparative study of the teacher's discourse, we adopted the point of view according to which for students “The development of understanding is made from small elements of knowledge”(Tiberghien and Malkoun, 2007). These elements of knowledge are contained in units of meaning. As diverse units of meaning can have closely related meanings, to designate these elements we adopted the generic term facets which was introduced by Minstrell (1992) and taken up by (Tiberghien and Malkoun, 2007). For example, the facet “The products name is derived from the name of the reactants” regroups the following units of meaning: “the name comes from sulfur and iron that were transformed”; “it is the iron sulfide that is formed from sulfur and iron”, “as we have the sulfuric acid and ammonia, it is the ammonia chloride (sic).”These facets are similar knowledge or reasoning elements that teachers seem to use in different situations.
The facets have generally been defined after reading the transcripts. The association of units of meaning in the teacher discourses to facets was carried out independently by the two authors. In the case of disagreement, a discussion took place to obtain a consensus. However, some formulations result from an a priori analysis of the techniques that students must be able to implement to solve the tasks listed in the school book.
Analysis of teachers’ discourse
From the whole units of meaning appearing in the three teachers’ discourse, we have defined 124 different facets. The location of these facets in the “expended chemistry triplet” was initially identified: at what apex or side do they correspond? They were then categorized according to the role they perform in the presentation of chemical knowledge: Experiential Field Description (EFD), Experiment Interpretation (EI), Representation of objects and events (R), and Interpretation of Chemical Reaction (ICR). Some examples of facets of each category are given later in the analysis of discourses. The table in the Appendix shows, for each sequence and each teacher, the number of facets of each category appearing in the discourse and their location in the expanded chemistry triplet.Some examples of data collection
In order to illustrate the identification and categorization of facets, we have selected a few episodes illustrating how some chemical knowledge was introduced.| Facet category | Quadruplet location | Teachers (T) and students (S) speaking |
|---|---|---|
| Episode 3 (Sequence 1 – T1) | ||
| 16/T: (suite): so what happened in this reaction? Sorry, what happened in this experiment?/ | ||
| 17/S: total silence in the classroom and then one of the students hesitantly said: change | ||
| EI | In E | 18/T: so there was a change/but change of what?/ |
| 19/S: the same student: color and precipitate | ||
| EI | In E | 20/T: then there is a change/change of?/ |
| 21/S1: solution | ||
| 22/S2: volume | ||
| 23/T: with low voice, which volume?/ | ||
| 24/S2: of matter | ||
| EI | In E | 25/T: (the teacher loses patience and said) of products/there are changes/ |
| EI | EM side | We start from chemicals and we obtain other chemicals/ |
We notice that where the teacher sees a chemicals change, students only see a change in perceptual properties: color change, blue solid formation (students remain in E). It is the same for the different experiments carried out by the three teachers during this sequence.
| Facet category | Quadruplet location | Teachers (T) and students (S) speaking |
|---|---|---|
| Episode 20 (sequence 2 – T2) | ||
| EFD | In E | 57′/T: so since it is white smoke that is a solid/and since we used ammonia and hydrogen chloride/ |
| EFD | In M | |
| EI | EM side | Then what can we get as products?/ |
| 58/S: please madam can you repeat the names | ||
| EFD | In M | 59/T: we took hydrochloric acid, his name is hydrogen chloride, with ammonia/ |
| 60/S: ammonia acid or ammonia chloride | ||
| 61/T: you choose one of the two/ | ||
| 62/S: the acid | ||
| EFD | In M | 63/T: (laugh) here you randomly chose but you're going to know how you can determine the name/it's called ammonium chloride not ammonia ok?/ |
A student, who apparently understood the linguistic method used by the teacher, gives a logical answer: “ammonia chloride or ammonia acid”, with an unjustified preference for the second name. This seems to show the limits of this method.
| Facet category | Quadruplet location | Teachers (T) and students (S) speaking |
|---|---|---|
| Episode 6 (Sequence 3 – T1) | ||
| R | Mm side | So a H2 molecule or mole, a molecule on a submicroscopic scale/ |
| R | Vm side | We say a H2 molecule reacts with a Cl2 molecule to give two HCl molecules/ |
| R | VM side | Now, on a macroscopic level, we say that a mole of molecules of dihydrogen reacts with one mole of molecules of chlorine to give 2 moles of molecules of HCl/ |
The double meaning of the reaction equation is presented without any explicit reference to the reorganization of atoms during the change of reactants into products. Only teacher 2 mentions, following a question from a student, this reorganization in the schematic form of a carbon dioxide molecular model represented by “a black circle surrounded by two red ones” (Table 4).
| Facet category | Quadruplet location | Teachers (T) and students (S) speaking |
|---|---|---|
| Episode 9 (Sequence 3 – T2) | ||
| 52/S: why the 2 red are not related? | ||
| R | In m | 53/T: it is a model, but if you look at the molecule you'll see that the two oxygens are only linked to carbon, and there is no bond between them/ |
| R | Vm side | At the beginning, they are linked, but during the reaction there will be bond breaking/ |
| EI | EM side | You will study the chemical bonds next year/ |
| R | Vm side | That is why you are told that the chemical reaction is a transformation; it is a new arrangement of atoms, right?/ |
| Facet category | Quadruplet location | Teachers (T) and students (S) speaking |
|---|---|---|
| Episode 20 (sequence 3 – T1-extract) | ||
| 106/S: then n = m/M | ||
| EFD | In M | 107/T: but (m) is the mass of methane and not of dioxygen/ |
| (Students are silent. The teacher waits a bit and then said) | ||
| R | Vm side | 108/T: for a methane molecule you have two oxygen molecules, according to the chemical equation/yes or no?/ |
| 109/S: yes | ||
| R | Mm side | 110/T: and if you multiply it by 6.023 × 1023 you get for one mole of methane, 2 moles of oxygen. So it is double/for this we write in a general way that the number of oxygen moles is twice equal to the number of methane moles/ |
| R | Vm side | |
| IRC | Link E with MVm |
Given the numerous studies highlighting the comprehension difficulties encountered by students regarding the meaning of the stoichiometric coefficients and their use to determine the relative amounts of substance in which the reactants react and the products are formed, this simple logical deduction seems difficult to achieve and to generalize.
Analysis of discourses using the expanded chemistry triplet
During the three sequences, 926 expressions of facets have been identified in all units of meaning said by the three teachers. We represent in Fig. 3 the facets percentages relative to the tetrahedron sides and apex formulated by each teacher during the three sequences of chemical reaction teaching.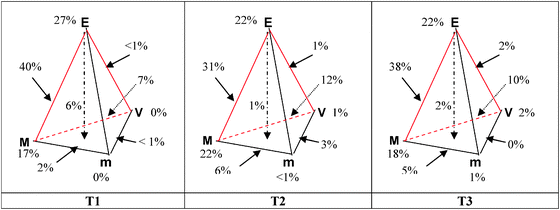 | ||
| Fig. 3 Percentages of facets devoted by each teacher at various levels and their linkage during the three sequences. | ||
A comparison of the tetrahedrons shows:
– That for the three teachers, approximately 90% of the identified facets concern the description, interpretation and representation of objects and events in the experiential field at the macroscopic level (triplet EMV);
– A different emphasis of the three teachers to the world of theories and models (triplet MVm): T2, 44% of facets, T3, 36%, and T1, 26%, with a strong preference for the macroscopic level.
In the EFD facets category, the facets describing the experiment in terms of objects and events of the perceptible world (apex E) are generally formulated during the first two sequences. Among these facets, teacher 1 gives more importance than the other two teachers to those concerning the identification of chemicals at the experiential field level, either by their perceptual properties (color, physical state) or by characteristics tests (magnet, limewater). For the other two teachers, we note a higher proportion of facets relative to the realization of an experiment: “We put in presence/mix two chemicals”; “We initiate/start/we inflame/we approach a flame”, etc.
The experiential field is then, in a three sequence, described in terms of reconstructed world objects at the macroscopic level (apex M). The priority here is given to the facet “nomenclature of chemicals”. The frequent use of this facet appears to be a requirement in relation to a method for determining the names of the products from the names of the reactants: “The composition/the name of products obtained is a function of the composition/the name of reactants/starting chemicals”. Next is the introduction of the reactant and product concepts: “The starting chemicals (disappearing) are called reactants.” “The new formed chemicals (appearing) are called reaction products.” In the third sequence, teacher 1 is the only one to introduce in more detail the notion of excess reactant.
The experiential field is also described by linking the perceptible world with the reconstructed world at the macroscopic level (EM side). During sequences 1 and 2 it is the chemical language specifying the kind of objects or events in the experiential field that is used (units of meaning examples: “There is carbon in the organic matter, in coal, pencil”, “the air contains oxygen”, “the gas that disturbs the limewater is carbon dioxide”). In sequence 3, it is the relations between the experiential field quantity and the variables of the reconstructed world that are introduced: “The chemical amount of substance (nX) is equal to mX/MX.” “To find the mass of a gaseous reactant knowing its volume (or vice versa), we have to calculate its amount of substance; and so on.”
In the EI facets category, the interpretation of experiments by linking the experiential field and the modeling at the macroscopic level (EM side) mainly occurs (T1, 58% T2, 94% and T3, 88% of facets of this category). The connections are mainly performed in three ways:
– First, in terms of change/transformation: “At the end of the experiment we observe the changes in the kinds of chemicals /we get new chemicals; “During a chemical reaction, one or more chemical(s) non present at the outset, form””, for example.
– Then in terms of event in the reconstructed world at the macroscopic level: “It is said that substance X reacts with substance Y to give…” “When the reactants are brought in presence, a reaction occurs/there is formation of …”.
– Finally, according to the official schoolbook definition, in terms of appearance/disappearance: “A chemical reaction is a transformation in which chemicals disappear and new ones appear.”
Note that:
– During the first sequence teacher 1 uses, with a significant proportion (32% of the facets of this category), facets limited to the experiential field (apex E). Such facets are intended to guide, with questions, the attention of his students on the observed changes or the chemicals that appear and disappear: for example, “At the end of the experiment we notice a change/a formation of…”“During the experiment, there are disappearing chemicals, what are they?”“During the experiment, there are appearing chemicals, what are they?”
– During sequence 2, a large proportion of facets linking apex E and M are relative to the different characters of chemical phenomena (about 70% for the three teachers). In this area, the facets relative to the spontaneous and initiated characters are the ones that get back more often.
– Only a few facets made references to the conservation: “During a chemical reaction, nothing is lost, everything turns/there is a change.” “During a chemical reaction there is mass conservation”; and the facets “To form a product containing carbon, we have to start from a chemical that contains carbon” and “products are obtained from reactants”.
The facets representation (FR) mainly concern the symbolic representation of the reaction at the macroscopic level (VM side), first as a chemical word equation. It should be noted that during sequences 1 and 2, teacher 2 gives more importance to the explicitness of the chemical word equation (reaction scheme in Tunisia) writing more rules than the two other teachers: “In the reaction scheme, the involved chemicals are represented by their name (no formula, no special feature).” “The + sign separating the initial chemicals means ‘and’ or ‘reacts with.’” and “The starting chemicals (disappearing) and the ones present at the end/that appear are located on either side of an arrow”, for example. During sequence 3, only teachers 2 and 3 get back to the writing of the chemical word equation.
Teachers 1 and 3 use proportionally more facets devoted to the introduction of the chemical equation at the macroscopic level than teacher 2: “In the chemical equation, chemicals are represented by their formula.” “The coefficients before the formulae are called stoichiometric coefficients.” “The stoichiometric coefficients are used to ‘balance’ the chemical equation” , and so on. On the other hand, teacher 2 emph“asizes the double reading of a reaction equation at the macroscopic scale in terms of moles (VM side: At the macroscopic level, now, we say one mole of dihydrogen molecules reacts with one mole of chlorine molecules to give 2 moles of HCl molecules”) and at the submicroscopic level in terms of molecules (Vm side: “It is said that one molecule of H2reacts with one molecule of Cl2to give two HCl molecules).” In addition, he is the only one that introduced an interpretation of the chemical reaction at the submicroscopic level in terms of the rearrangement of atoms, as a consequence of a student’s question, (Mm side: “In a chemical reaction, bonds between atoms are broken.” “During a chemical reaction we observe a rearrangement of atoms within molecules”) and to relate the symbolic representations level with the submicroscopic level (Vm side: “In the representative formula of such element/compound, there are x atoms A and y atoms B”– facet cited 7 times by teacher 2).
Only teachers 2 and 3 formulate some facets introducing submicroscopic world objects (apex m) and their symbolization (apex V). In m, they concern the atomic structure of a molecule (“In a molecule, the atoms are linked together, for example, two oxygen atoms linked to a carbon atom (CO2) or two oxygen atoms linked together (O2)”). In V, it is the symbolization of atoms (“We represent an atom by a symbol which is its initial capitalized letter of his name, possibly followed by a second lowercase letter in the case of atoms whose names begin with the same letter”), the role of a molecular model (“A molecular model (compact or ball-and-stick) enables the representation of the arrangement of atoms in a molecule”) and the conventions adopted for the construction of molecular models (“In a molecular model, a convention sets the color assigned to atoms”) that are specified.
To interpret the chemical reaction (IRC Facets), the three teachers introduce facets with different priorities during sequence 3:
Teacher 1 seems to have as a first objective the introduction of knowledge allowing problem solving (linking E with the world of theories and models), focusing in this world on the associated variables (amount of substance/mole) and the role played by the stoichiometric coefficients to balance the chemical equation and make calculations: for example, “The reaction equation allows to determine the ratio of the amount of substance along which the reactants react/the products form”; “To determine if the reactants are in stoichiometric proportions we compare the ratio of their stoichiometric coefficients to the ratio of their amount of substance.” He adds to this, facet indicating the procedures to solve problems: “If the ratio nX/nYis greater than the ratio coef. X/coef. Y, reactant X is in excess and reactant Y is the limiting reactant; it is inverse if nX/nY< coef. X/coef. Y”; “To find the amount of substance X that reacts knowing Y we write nX/nY = coef. X/coef. Y = a, then nX = a·nY”; etc.
For teachers 2 and 3, it is the understanding of what represents a chemical equation in terms of conservation of mass and atoms that seems to be the priority of this sequence (VM, Vm and/or Mm sides): “During a chemical reaction the mass conservation is related to the conservation of atoms”; “In a chemical reaction there is conservation of atoms “(facet cited 7 times by teacher 2 and 4 times by teacher 3); “For one same ‘species’, we note that the number of atoms in the reactants is the same as in the products”, and so on.
Discussion
To introduce the chemical reaction, teachers, according to the definition proposed in the official manual, interpret the changes of the observed perceptual properties (apex E) in terms of appearance and disappearance of chemicals (reactants and products), then as a change in the kind of chemicals (EM side).Regarding the description of the experiential field in terms of reconstructed world objects at the macroscopic level (apex M), priorities are given to the introduction of the concepts of reactants and products and to the designation of “chemicals” by their name. Teachers talk about “chemicals” without any precision of their form. Is this name suitable for both a pure solid or gaseous chemical (chemical substance), an element or a compound, as well as a solution containing ions? The concepts of pure chemicals (element or compound), solutions, ions in solution, which were taught earlier in the year, need to be explained in detail at this level.
The analysis of facets identified in the three teachers' discourses allows the perceptible and reconstructed worlds to be linked at the macroscopic level (EM side of the tetrahedron) and is important. But nothing is done to encourage students to look beyond what they perceive to distinguish what is changing and what is conserved in the change. For example, when mixing two solutions containing ions, can we say that the ions disappear during the change?
It is the generalization of specific examples that enables students to understand the connection between the description of a phenomenon and its representation by a chemical word equation (VM side). Only one teacher (T2) attaches importance to the explanation of the rules for reading and writing this first level of the chemical reaction symbolization. To find the product name, a process of determination, usually implicit, from the names of the reactants is implemented by teachers. But this method has limitations, on the one hand the limited chemical knowledge of the students, on the other hand the fact that the exact name of the chemical species that react is not always given by teachers (limestone instead of calcium carbonate, hydrochloric acid instead of hydrogen chloride, for example). The other limitations are the “grammatical” rule that the mere juxtaposition of portions of names should be used.
The introduction of the second level of symbolization of the chemical reaction by its reaction equation seems to be based on evidence. Evidence that students would be able to assign a correct chemical formula to the name of a chemical species (apex V) and evidence regarding the students' understanding of the role played by the stoichiometric coefficients at the macroscopic (VM side) or submicroscopic (Vm side) levels. Only teacher 2 seems to give importance to the procedures allowing this conservation and focus to be established/verified, using representations of “molecular models”, on the double reading of a reaction equation and its significance at the submicroscopic level in terms of the rearrangement of atoms (Mm side). In general, the reference to the submicroscopic modeling level (apex m) and clear and explicit precision of the level change (Mm side) is often absent in the discourses.
Finally, the time devoted to the presentation of the procedures to implement the linking of experiential data (mass, volume) with the world of theories and models (triangle MmS) for the quantitative treatment of the chemical reaction is brief (the resolution of one only exercise). Only teacher 1 presents all the procedures to enable problem solving, though with some shortcomings. The other two teachers just make an incomplete presentation and do not insist on the quantitative treatment of the chemical reaction in the presence of a limiting reactant. It therefore appears that the linking of the experiential state with the world of theories and models for the quantitative treatment of the chemical reaction does not receive much attention.
Conclusion
Observation of the knowledge taught during regular classroom situations shows that the teaching of chemical reactions is mainly devoted to the description, interpretation and representation of experiential field objects and events at the macroscopic level (triplet EVM). The teachers pay little attention to chemical reaction modeling by linking the macroscopic level (reactants, products) with the submicroscopic kind (molecules, ions) of the entity transformed (triangle MVm). Particularly, interest paid to the submicroscopic level to show what is preserved and what changes seems to be very limited. Although there are some differences between the three teachers, we can say that the movement between the experiential field and the three levels of chemical representation is ensured only partially. This can only lead students to encounter the difficulties reported by various authors (e.g.Solsona et al., 2003, De Jong and Van Driel, 2004; Taber, 2009) in reading or writing reaction equations and understanding what they represent both at the macroscopic and the submicroscopic levels. The perception of the meaning of a reaction equation as a tool for visualization of what changes and what is conserved during a chemical change may be foreign to them.In Tunisia, as in most countries, evaluation of the knowledge of chemical reactions acquired is performed through the resolution of quantitative exercises requiring the experiential field data (E) to be linked with the world of theories and models (MVm triangle). It is therefore paradoxical that teachers give limited importance to this relationship and this is the source of many problems for students (see for example: Kousathana and Tsaparlis, 2002; Boujaoude and Barakat, 2003; Schmidt and Jigneus, 2003, De Jong and Van Driel, 2004; Agung and Schwartz, 2007). They seem to think that it is sufficient to simply memorize definitions and rules and apply them carefully, methodically and with common sense to a chemical equation in order to quantify the chemical change?
Implications for teaching
We share the point of view according to which the students' skills depend; in part, on the quality of the teaching they receive (Nahkleh and Mitchell (1993); Mercier and Buty, 2004; Taber, 2012). The teacher's role should be to facilitate the transition from one level of interpretation to another explicitly and progressively (De Vos and Verdonk, 1987; Strang and Shayer, 1993; Barlet and Plouin, 1994; Mey et al., 1994; Fillon, 1997; Taber, 2001, 2009). To help students conceptualize a chemical change and to make sense of the reaction equation, the teacher should encourage them to look beyond what they see, to ask questions, to make proposals and argue, using appropriate scientific terminology. Helping students to be able to differentiate between the different apexes of the “expanded chemistry triplet” then to pass from one to the other would reduce the complexity of understanding the chemical change and its reaction equation.But the teaching received depends not only on the teachers. The knowledge to be taught contained in the official instructions, on which teachers have no control, deserves to be reconsidered. In agreement with several authors, who believe that the concept of substance (or rather chemical species) is a central concept in the teaching of chemical change. Therefore emphasis should be placed, on the definition of chemical reaction, on the transformation of starting substances (chemical species) to give new substances (new chemical species) referring to concepts of pure chemical elements or compounds and ions in solution. To enable the teacher to lead students to perceive what is preserved and what is transformed through the change process, it would be appropriate to focus on the interactions occuring between the chemical species. The world of theories and models will then come to make sense to many key concepts in chemistry and in particular to allow the interpretation of a chemical process such as recombination/rearrangement of atoms present in the reactants when they become products.
Finally, it should be mentioned that the Tunisian teachers have never received any vocational training, especially devoted to the teaching of chemical reactions. As a result, most often their teaching is simply carried out in accordance with the organization of the content of the only official schoolbook. It seems necessary to organize training allowing them to perceive the complexity of the conceptual framework to which belong the concepts of chemical change and its modeling, to reflect on the learning difficulties this may cause for students and to realize the alternative conceptions that students can build themselves.
Facet categories and the number of occurrences for the three sequences and the three teachers
| Facet categories | Seq. 1 | Seq. 2 | Seq. 3 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| T1 | T2 | T3 | T1 | T2 | T3 | T1 | T2 | T3 | |||
| Experiential Field Description (EFD) | In terms of objects and events of the perceptive world (in E) | 32 | 20 | 11 | 26 | 43 | 29 | 1 | 7 | 6 | |
| In terms of objects/data of the reconstructed world at macroscopic level (in M) | 24 | 9 | 11 | 21 | 44 | 22 | 12 | 22 | 12 | ||
| By making a diagram (EV side) | 1 | 2 | 1 | 1 | 3 | 3 | |||||
| By linking the perceptible and reconstructed world at macroscopic level (side EM) | 2 | 6 | 3 | 5 | 10 | 3 | 8 | 4 | 2 | ||
| Subtotal 1 (405: 44%) | 59 | 37 | 26 | 53 | 100 | 57 | 20 | 33 | 20 | ||
| Experiment Interpretation (IE) | At empirical field level (in E) | 21 | 1 | 2 | 9 | 5 | 4 | 0 | 2 | 1 | |
| In terms of events of the reconstructed world at macroscopic level (side EM) | 10 | 1 | 4 | 8 | 8 | 6 | 2 | 2 | 1 | ||
| In terms of change or transformation (side EM) | 19 | 13 | 8 | 2 | 5 | 5 | |||||
| In terms of appearance/disappearance (side EM) | 7 | 1 | 1 | 0 | 2 | 2 | 3 | 1 | 2 | ||
| In terms of conservation (side EM) | 2 | 1 | 0 | 0 | 2 | 1 | 1 | 1 | 0 | ||
| In terms of reaction characteristics (EM) | 0 | 0 | 1 | 52 | 42 | 45 | 0 | 0 | 3 | ||
| Difference with physical transformation (EM) | 6 | 0 | 0 | 0 | 2 | 0 | 1 | 0 | 0 | ||
| Subtotal 2 (318: 34%) | 65 | 17 | 16 | 71 | 66 | 63 | 7 | 6 | 7 | ||
| Representation (R) | Objects at submicroscopic level (in m) | 0 | 3 | 3 | |||||||
| Symbolization of objects at submicroscopic level (in V) | 0 | 5 | 5 | ||||||||
| Of chemical reaction by chemical world equation (VM) | 3 | 13 | 4 | 7 | 11 | 2 | 0 | 5 | 6 | ||
| Of chemical reaction by chemical equation (VM) | 13 | 12 | 13 | ||||||||
| Of chemical reaction in terms of events of the reconstructed world at submicroscopic level (Vm) | 1 | 11 | 0 | ||||||||
| Explicit linking between the two representation level (Mm) | 2 | ||||||||||
| Subtotal 3 (119: 13%) | 3 | 13 | 4 | 7 | 11 | 2 | 16 | 36 | 27 | ||
| Interpretation of chemical reaction (ICR) | In terms of conservation | Mass (EM) | 0 | 11 | 7 | ||||||
| Atoms (Mm) | 5 | 20 | 12 | ||||||||
| In terms of atoms rearrangement (Mm) | 3 | ||||||||||
| In terms of stoichiometry (E-MVm) | 12 | 2 | 4 | ||||||||
| In terms of amount of substance (E-MVm) | 6 | 1 | 1 | ||||||||
| Subtotal 4 (84: 9%) | 23 | 37 | 24 | ||||||||
| Total facets (926) | 127 | 67 | 46 | 131 | 177 | 122 | 67 | 112 | 78 | ||
Acknowledgements
We thank I. Kermen, University d'Artois and Laboratory of Didactic Andre Revuz – University Paris-Diderot, for his participation in fruitful discussions that led to the symbolization of the “expanded chemistry triplet”.References
- Abraham M. R., Williamson V. M. and Westbrook S. L., (1994), A cross age study of the understanding of five chemistry concepts, J. Res. Sci. Teach., 31(2), 147–165.
- Aguiar O., Pazzini Couto F. and Buty C., (2010), Connecting physical phenomena to model: analysis of discourse in a practical physics class, in Çakmakcı G. and Taşar M. F. (ed.), Contemporary science education research: scientific literacy and social aspects of science, Ankara, Turkey: Pegem Akadem, pp. 3–12.
- Agung S. and Schwartz M. S., (2007), Students' understanding of conservation of matter, stoichiometry and balancing equation in Indonesia, Int. J. Sci. Educ., 29(13), 1679–2002.
- Barlet R. and Plouin D., (1994), L'équation bilan en chimie. Un concept intégrateur source de difficultés persistantes, Asteroids, 18, 27–55.
- Barlet R., (1999), L’espace épistémologique et didactique de la chimie, l’actualité chimique, April, 22–33.
- Ben-Zvi R., Eylon B. S. and Silberstein J., (1988), Theories, principles and laws, Educ. Chem., May, 89–92.
- Boujaoude S. and Barakat H., (2003), Students' problem solving strategies in stoichiometry and their relationships to conceptual understanding and learning approaches, Elec. J. Sci. Educ., 7(3), 20.
- Buty C. and Mortimer E. F., (2008), Dialogic/authoritative discourse and modeling in a high school teaching sequence on optics, Int. J. Sci. Educ., 30(12), 1635–1660.
- Chambenois D., Bromont F., Collard E. and Morenas M. C., (2003), Réactions chimiques et réactions des collégiens, Bull. Union Physiciens, 97, 121–134.
- Chappet Pariès M., (2004), Comparaison de pratiques d'enseignement de mathématiques. Relations entre discours des professeurs et des activités potentielles des élèves, Recherches en didactique des mathématiques, 24(2–3), 251–284.
- Cross D., (2010), Action conjointe et connaissances professionnelles de l'enseignant, Éducation et Didactique,4(3), 31–54.
- De Jong O. and Van Driel J., (2004), Exploring the development of student teachers' PCK of the multiple meaning of topics, Int. J. Sci. Math. Educ., 2, 477–491.
- De Vos W. and Verdonk A. H., (1987), A new road to reactions 4, J. Chem. Ed., 64(8), 692–694.
- Dhaha F., Ajili M. T., Kerrou M. and Kilani K., (2002), Sciences Physiques. Première année de l'enseignement secondaire, CNP, Tunis: Ministère de l'Education.
- Dumas Carré A. and Goffard M., (1998), Objectivation des pratiques de tutelle d'un enseignant au cours de séances de résolution de problèmes en physique, in Dumas Carré A. and Weil-Barais A. (ed.), Tutelle et médiation dans l'éducation scientifique, Paris: Peter Lang.
- El Hajjami A., Lahlou F., Benyama S. and Tiberghien A., (1999), Elaboration d'une méthode d'analyse des discours d'enseignants: cas de l'énergie, Didaskalia, 15, 59–86.
- Fillon P., (1997), Des élèves dans un labyrinthe d'obstacles, Asteroids, 25, 113–141.
- Friedel A. W. and Maloney D., (1992), An exploratory, classroom-based investigation of students' difficulties with subscripts in chemical formulas, Sci. Educ., 76(1), 65–78.
- Gilbert J. K. and Treagust D., (2009), Towards a coherent model for macro, submicro and symbolic representations in chemical education, in Gilbert J. K. and Treagust D. (ed.), Multiple Representations in Chemical Education, Models and Modeling in Science Education, New York: Springer Verlag, pp. 1–8.
- Goigoux R., (2007), Un modèle d'analyse de l'activité des enseignants, Éducation et Didactique, 1(3), 47–70.
- Jacob C., (2001), Analysis and synthesis: interdependent operation in chemical language and practice, Hyle, 7(1), 31–50.
- Johnstone A. H., (1982), Macro and micro-chemistry, Sch. Sci. Rev., 64, 377–379.
- Johnstone A. H., (1991), Why is science difficult to learn? Things are seldom like they seem, J. Comput. Assist. Learn., 7, 75–83.
- Johnstone A. H., (2000), Teaching of chemistry – Logical or psychological, Chem. Educ. Res. Pract., 1, 9–15.
- Kermen I. and Méheut M., (2009), Different models used to interpret chemical changes: analysis of a curriculum and its impact on French student's reasoning, Chem. Educ. Res. Pract., 10, 24–34.
- Kousathana M. and Tsaparlis G., (2002), Students' error in solving numerical-equilibrium problems, Chem. Educ. Res. Pract., 3, 5–17.
- Kozma R. B., (2003), The material features of multiple representations and their cognitive and social affordances of science understanding, Learn. Instr., 13(2), 205–226.
- Laszlo P., (1993), La parole des choses ou le langage de la chimie, Paris : Hermann.
- Le Marechal J. F., (1999), Design of chemistry labwork activities aiming basic chemical concepts, Actes of Fourth European Science Education Summer School, Marly le Roi, 1998, pp. 68–80.
- Lorenzo M. G., Farré A. S. and Rossi A. M., (2010), Teachers' discursive practices in a first organic chemistry course, in Çakmakcı G. and Taşar M. F. (ed.), Contemporary science education research: scientific literacy and social aspects of science, Ankara, Turkey: Pegem Akadem, pp. 13–22.
- Martinand J. L., (1995), Introduction à la modélisation, Didactiques des Disciplines Techniques, Cachan 1994–1995: LIREST, pp. 126–138.
- Mercier A. and Buty Y., (2004), Evaluer et comprendre les effets de l'enseignement sur les apprentissages des élèves: problématiques et méthodes en didactique des mathématiques et des sciences, Revue française de pédagogie, 148, 47–59.
- Mey M., Balas A. and Plouin D., (1994), Essai sur la maîtrise de l'équation bilan à l'entrée à l'université, Bull. Union Physiciens, 88(766), 1131–1150.
- Minstrell J., (1992), Facets of students' knowledge and relevant instruction, in Duit R., Goldberg F. and Niedderer H. (ed.), Research in physics learning: theoretical issues and empirical studies, Kiel: IPN, pp. 110–128.
- Morge L., (2001), Caractérisation des phases de conclusion dans l'enseignement scientifique, Didaskalia, 18, 99–120.
- Mzoughi-Khadhraoui I. and Dumon A., (2012), L'appropriation par des élèves tunisiens débutants du langage permettant de représenter la réaction chimique, Recherches en Didactique des Sciences et des Technologies, 6, 89–118.
- Nahkleh M. and Mitchel R., (1993), Concept Learning versus Problem Solving: There is a difference, J. Chem. Educ., 70, 190.
- Onwu G. and Randall E., (2006), Some aspects of students' understanding of a representational model of the particulate nature of matter in chemistry in three different countries, Chem. Educ. Res. Pract., 7(4), 226–239.
- Ramnarain U. and Joseph A., (2012), Learning difficulties experienced by grade 12 South African students in the chemical representation of phenomena, Chem. Educ. Res. Pract., 13, 462–470.
- Robert A., (1999), Pratiques et formation des enseignants, Didaskalia, 15, 123–157.
- Robert A., (2001), Les recherches sur les pratiques des enseignants et les contraintes de l'exercice du métier d'enseignant, Recherches en didactique des mathématiques, 21(12), 57–80.
- Rogalski J., (2003), Y a-t-il un pilote dans la classe ? Une analyse de l'activité enseignante comme gestion d'environnement ouvert, Recherches en didactique des mathématiques, 23(3), 343–388.
- Schmidt H. and Jigneus C., (2003), Students' strategies in solving algorithmic stoichiometry problems, Chem. Educ. Res. Pract., 4, 305–317.
- Sensevy G., (2007), Des catégories pour l'analyse comparée de l'action du professeur: unnessai de mise à l'épreuve, in Sensevy G. and Mercier A. (ed.), Agir ensemble: Eléments de théorisation de l'action conjointe du professeur et des élèves, Rennes: PUR, pp. 25–49.
- Solsona N., Izquierdo M. and de Jong O., (2003), Exploring the development of students' conceptual profiles of chemical change, Int. J. Sci. Educ., 25, 3–12.
- Strang J. and Shayer M., (1993), Enhancing high school students' achievement in chemistry trough a thinking skills approach, Int. J. Sci. Educ., 15(3), 319–337.
- Taber K. S., (2001), Building the structural concepts of chemistry: some considerations from educational research, Chem. Educ. Res. Pract., 2, 123–158.
- Taber K. S., (2009), Learning at the symbolic level, in Gilbert J. K. and Treagust D. (ed.), Multiple Representations in Chemical Education, Models and Modeling in Science Education, New York: Springer Verlag, pp. 75–105.
- Taber K. S., (2012), Vive la Différence? Comparing “Like with Like” in Studies of Learners' Ideas in Diverse Educational Contexts, Educ. Res. Int., 168741.
- Taber K. S., (2013), Revisiting the chemistry triplet: drawing upon the nature of chemical knowledge and the psychology of learning to inform chemistry education, Chem. Educ. Res. Pract., 14(2), 156–168.
- Taber K. S and Bricheno P., (2009), Coordinating Procedural and Conceptual Knowledge to Make Sense of Word Equations: Understanding the complexity of a ‘simple’ completion task at the learner's resolution, Int. J. Sci. Educ., 31, 2011–2055.
- Talanquer V., (2011), Macro, submicro, and symbolic: the many faces of the chemistry “triplet”, Int. J. Sci. Educ., 33, 179–195.
- Tiberghien A., (1994), Modeling as a basis for analysing teaching-learning situations, Learn. Instr., 4, 71–87.
- Tiberghien A. and Malkoun L., (2007), Différenciation des pratiques d'enseignement et acquisition des élèves du point de vue des savoirs, Éducation et Didactique, 1, 29–54.
- Treagust D. F., Chittleborough G. and Mamiala T. L., (2003), The role of submicroscopic and symbolic representations in chemical explanations, Int. J. Sci. Educ., 25(11), 1353–1368.
- Venturini P., Calmettes B., Amade-Escot C. and Terrisse A., (2007), Analyse didactique des pratiques d'enseignement de la physique d'une professeure expérimentée, Asteroids, 45, 211–234.
- Yarroch W. L., (1985), Student understanding of chemical equation balancing, J. Res. Sci. Teach., 22, 449–459.
| This journal is © The Royal Society of Chemistry 2014 |
