Chemistry in context: analysis of thematic chemistry videos available online
Camilla
Christensson
ab and
Jesper
Sjöström
*a
aMalmö University, Faculty of Education and Society, Department of Science-Environment-Society, SE-205 06 Malmö, Sweden. E-mail: jesper.sjostrom@mah.se
bKatedralskolan (an upper secondary school), Stora Södergatan 22, SE-222 23 Lund, Sweden. E-mail: camilla.christensson@utb.lund.se
First published on 25th October 2013
Abstract
United Nations declared 2011 to be the International Year of Chemistry. The Swedish Chemical Society chose twelve themes, one for each month, to highlight the connection of chemistry with everyday life. Examples of themes were fashion, climate change, love, sports, communication, health issues, and food. From the themes various context-based educational materials were produced. One such educational resource, connecting to students' interests, was the Chemistry Calendar. It mainly contains short videos available on YouTube (also in English). The target group for the videos was secondary school. The videos have been analysed using a research-based analysis model consisting of four fields (pure chemistry; applied chemistry; socio-chemistry; nature of chemistry). The video analysis focuses on content and discourses. For example the images of chemistry and the chemist were examined. The analysis shows that the videos have a number of clear messages that are in line with “chemical literacy”, such as chemistry is all around you, chemistry researchers look different, chemical experiments can be of very different nature, chemistry is important for society, and chemistry has historically had some downsides. The Chemistry Calendar videos are unique and could be very useful in context-based chemistry education. However, their weakness regarding critical and reflective aspects must be compensated for in chemistry teaching, for example by highlighting “excluded environmental aspects” and by placing the videos in the contexts of critical citizenship and global sustainability.
Introduction
“The Chemistry Calendar, one video for each month of the IYC-year, shows chemistry as an important part of everyday life. The videos also point to the future by highlighting ongoing research” (Swedish Journal of Chemistry and Biotechnology, 2012)The Chemistry Calendar is an example of a teaching material (available online) where chemistry is placed in context, both regarding everyday life and ongoing research. The material is based on twelve short thematic videos (see further below), which will be analysed in this paper.
Aikenhead (2006) shows in his well-cited book Science Education for Everyday Life substantial evidence-based support for a humanistic perspective in school science. In addition, he shows (in pp. 64–65) that most science teachers (90%) endorse this perspective. However, when it came to implementing it they provided many reasons for not doing so and in practice science teachers “tend to favor abstract decontextualized ‘pure science’” (p. 63). It has been shown that secondary science teachers focus on facts and concepts in their teaching, although the students want to learn about more general issues related to society (Oskarsson, 2011) and everyday life (Broman et al., 2011; Bolte et al., 2013). One reason for the resistance of implementation is the lack of available teaching materials (Aikenhead, 2006).
In many countries, including Sweden, many students consider chemistry unpopular, difficult and abstract, and there has been a decline in the number of students studying chemistry at the university level (Risch, 2010). At the same time, the modern society we live in today requires knowledge in chemistry, either for citizens to be able to actively take a stand on different issues in a democracy, or for scientists to continue to develop our society (Eilks et al., 2013). Chemistry knowledge for – and needed by – all is called “chemical literacy” (Shwartz et al., 2013). In addition to pure chemical knowledge and concepts, it is about understanding “the contribution of chemistry in various contexts”, developing higher-order thinking skills, and having “critical but positive attitudes towards chemistry and its applications” (Shwartz et al., 2013, p. 40).
One of the bases for a humanistic perspective in school science is to build on the interests and experiences of students (Aikenhead, 2006). If chemistry is placed in relevant everyday-life contexts, more students are interested in chemistry and motivated to learn the subject (Eilks et al., 2013). The area of context-based chemistry education has recently been reviewed by King (2012) and Ültay and Çalık (2012). Both the reviews show that this approach improves students' motivation and interest in chemistry.
For some time, chemistry education has faced a number of problems. For instance, more and more chemistry content has been included in the curriculum, as new knowledge has been accumulated, isolated facts have been taught without the students learning how to connect them, and the content has been irrelevant to the students (Gilbert, 2006). Some students learn chemistry by memorizing key concepts and processes by heart, without spending time in achieving meaningful learning. Generally, traditional school chemistry is weakly connected to everyday life, technology, society, chemical research, and history and philosophy of science (Van Berkel, 2005; Van Berkel et al., 2009). Gilbert (2006, p. 960) writes the following about contextualisation:
“A context must provide a coherent structural meaning for something new that is set within a broader perspective. These descriptions are consistent with the function of ‘the use of contexts’ in chemical education: students should be able to provide meaning to the learning of chemistry; they should experience their learning as relevant to some aspect of their lives and be able to construct coherent ‘mental maps’ of the subject.”
United Nations (UN) declared 2011 to be the International Year of Chemistry (IYC). It was seen as being a part of the UN Decade of Education for Sustainable Development (ESD) 2005–2014 and was a joint initiative from the United Nations Educational, Scientific, and Cultural Organization (UNESCO) and the International Union of Pure and Applied Chemistry (IUPAC). The year commemorated the achievements of chemistry and its contributions to the well-being of humankind. The aim was to raise awareness of chemistry to the public and highlight the role of chemistry in solving global problems, and to increase the interest in chemistry among young people. In Sweden, the Swedish Chemical Society led the celebration of IYC, and their vision was that many activities such as lectures, exhibitions, debates in media and especially activities for schools would take place during the year.
The Swedish Chemical Society chose twelve themes, one for each month, to show the connection of chemistry with everyday life. From these monthly themes, various context-based educational materials were produced across Sweden. One such material was the Chemistry Calendar, which mainly contains twelve short videos where the topic of each video follows the monthly themes. They are available on YouTube both in Swedish and English (www.youtube.com/user/chemistrycalendar). The Chemistry Calendar was awarded as Sweden's most innovative and successful activity during the IYC (Swedish Journal of Chemistry and Biotechnology, 2012). A recent study about Swedish upper secondary school chemistry teachers' use of the Chemistry Calendar in their teaching showed that 91% of them found the videos useful or very useful (Christensson, 2012). The teachers found them to be in line with the Swedish chemistry syllabus with regard to the relation between chemistry, everyday life and society.
The Chemistry Calendar videos continue a more than fifty year long tradition of using different types of movies and videos in chemistry education (Pekdag and Le Maréchal, 2010; Blonder et al., 2013). Over the years the movies have developed from longer documentaries to shorter videos that are possible for the teacher to integrate as part of their teaching. Examples of the latter are lab instructions (e.g. calibration or distillation), introduction to chemical analytical equipment, chemical experiments (that are e.g. dangerous and/or expensive to perform), animations, simulations and information of industrial processes of important chemical products (such as ammonia and nitric acid) (Pekdag and Le Maréchal, 2010). Since YouTube – a social network in which users share videos – was launched in 2005, an increasing number of chemistry videos have become available online (Blonder et al., 2013). According to Blonder et al. (2013, p. 270) “[m]any videos serve both teachers and students, thus making learning and teaching easier by making chemistry more concrete”. A recent study on German high school students shows that both girls and boys rank watching films as their second favourite activity in chemistry lessons, after carrying out experiments (Bolte et al., 2013, p. 80).
However, research reports about context-based videos in chemistry education are very few. An exception is a study by Harwood and McMahon (1997). They reported that the use of context-based videos, with some similarities to the Chemistry Calendar videos, positively affected student achievement in high school chemistry. They argued that “educational science videos […] are instructional tools that can be used effectively to bring the often abstract, distant worlds of science into close focus and within the personal meaningful realm of each individual student.” (Harwood and McMahon, 1997, p. 617).
As far as we know this study is the first one analysing chemistry videos from content and discourse perspectives. The term “discourse of chemistry” refers to chemists' common worldviews and values (both explicit and implicit). Often it is useful to describe many chemists' (including many chemistry teachers' and chemistry textbook authors') views of their science and the role of chemistry in society with labels such as reductionism, rationalism and modernism (Sjöström, 2007, 2013).
The aim of this study is to analyse the English versions of the twelve thematic online videos of the Chemistry Calendar. The analysis focuses on content and discourses and is based on an analytical framework, which will be described in more detail in the next section. The following questions were asked:
• What characterizes the communication with the public/students? Which is the image of chemistry portrayed in the videos? Which is the image of the chemist? How is the chemical way of working being described? How is the term “chemicals” being used?
• How is the role of chemistry in society being described? Is the larger cultural milieu in which scientific discoveries and innovations were made being described? Which ethical consequences of chemistry are highlighted in the videos?
• Which aspects of “nature of chemistry” are presented in the videos? How is the balance between the explaining, analysing, synthesizing and problem-solving nature of chemistry?
Background and methods
Development of the analysis model
The analysis model relates to Sjöström's (2013) tetrahedron of chemistry teaching, which is based on Johnstone's (1982, 1993) triangle (also called the triplet relationship), capturing the multidimensionality of pure chemistry. The triangle is complemented with a top symbolising the human element of chemistry teaching (Mahaffy, 2004). The human element includes technological, historical and philosophical components, as well as societal issues and focuses on students' learning. Sjöström argues that the tetrahedron can be subdivided into three levels: applied chemistry (Level 1); socio-cultural context (Level 2); and a critical-philosophical approach (Level 3) (see Fig. 1).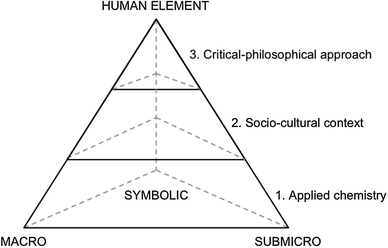 | ||
| Fig. 1 The tetrahedron of chemistry teaching as described by Sjöström (2013). | ||
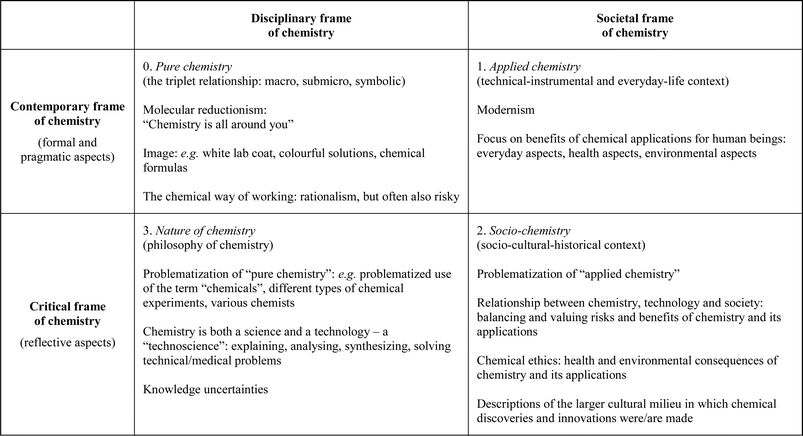
Based on Sjöström's (2013) tetrahedron of chemistry teaching, we have developed the analysis model shown in Fig. 2. It consists of four fields, with contemporary and critical frames of chemistry on one axis and disciplinary and societal frames of chemistry on the other axis.
Traditional school chemistry teaching focuses on formal aspects (the upper-left field in Fig. 2: contemporary and disciplinary – triangle base or Level 0 in Fig. 1) and includes at best also pragmatic and often trivial everyday-life aspects. Such aspects are part of the upper-right field in Fig. 2: contemporary and societal (Level 1 in Fig. 1).
An alternative to traditional, contemporary, school chemistry is “critical chemistry”, or Bildung-oriented chemistry education, as Sjöström (2013) also calls it. Reflective aspects are important and this approach also includes – in addition to content knowledge in chemistry – knowledge about chemistry, both about the nature of chemistry and about its role in society. This is in line with Hodson (2008, p. 23) who emphasizes learning about science, by which he means “developing an understanding of the nature and methods of science, an appreciation of its history and development, and an awareness of the often complex interactions among science, technology, society and environment.” Critical chemistry is about problematization, understanding uncertainties, and balancing the benefits and risks of chemistry. It needs “meta-perspectives” and a subject-critical distance.
We have subdivided critical chemistry into “socio-chemistry” (the lower-right field in Fig. 2: critical and societal – Level 2 in Fig. 1) and “nature of chemistry” (the lower-left field in Fig. 2: critical and disciplinary – Level 3 in Fig. 1) as shown in Fig. 2. Reflective aspects are common for these two fields.
The different levels in Fig. 1 and fields in Fig. 2 are related to Van Berkel's (2005) three general aims in most chemistry curricula: Fundamental Chemistry (FC), Chemistry, Technology, and Society (CTS), and Knowledge Development in Chemistry (KDC), respectively. The orientation of FC is normally disciplinary, but it can also be based on an everyday life orientation (Level 0–1). CTS is related to Level 2 and KDC to Level 3.
Below, the four fields in Fig. 2 are described in more detail. We will refer to different previous studies and other sources, but the analysis model is especially based on the tetrahedron model of chemistry teaching (described above) and previous tables by Sjöström (2007, 2013). However, an analysis model with four fields is new for this study and furthermore the model has been finely-adjusted as a result of the video analysis described in this paper. For example the previously published tables did not include the white lab coat as a typical attribute of contemporary chemistry. In other words, the final model (as shown in Fig. 2) is a mix of an analytical tool based on previous research and also – to some extent – a result of this study. We believe that the analysis model developed and used here is of potential use also in other forthcoming studies in chemistry education research, for example for textbook analysis and teaching studies.
It is common that chemists view their science as “the central, useful and creative science” (Breslow, 1997). Such a view is typical for contemporary chemistry (fields 0 and 1). Chemistry is seen as central because of its position between physics and biology. It is generally regarded as more useful than both the other mentioned sciences, e.g. due to its own industry (Sjöström, 2007), and also because of its importance in technology as well as medicine. It is creative for example in the meaning that new molecules, with unique properties, can be created.
Within contemporary chemistry there is a tension between pure research on one hand (field 0) and applied research on the other hand (field 1) and this tension has historical roots – “[t]he chemist is both a ‘craftman and a philosopher’”, as Kovac (2001) has written. Actually, the tension is indicated in the name of IUPAC, the International Union of Pure and Applied Chemistry.
As already described above, reflective aspects are common for both fields 2 and 3. The societal frame of chemistry is in focus in field 2, whereas the disciplinary frame is in focus in field 3.
Analysis of video messages
The Chemistry Calendar was developed as a joint project between Molecular Frontiers, Chalmers University of Technology, University of Gothenburg, and Universeum, all located in Gothenburg, Sweden. Together with the film company Untamed Science they launched one video per month during the International Year of Chemistry in 2011. All the videos are between 5 and 7 minutes in length. Table 1 describes the themes of the context-based videos.| Monthly theme | Descriptiona |
|---|---|
| a The quotations are taken from: www.youtube.com/user/chemistrycalendar (visited 2 August 2013). | |
|
January
Art and culture |
“History of a color.” |
|
February
Fashion |
“What is Gore-Tex?” |
|
March
Climate and energy |
“What is the greenhouse effect? And how does it relate to global warming?” |
|
April
Industry |
“From tree to paper – how does it work? And what is really the difference between toilet paper and paper towels?” |
|
May
Love |
“What happens in our bodies when we fall in love? Which molecules are involved in our feelings? […W]e explore the chemistry of love!” |
|
June
Water and air |
“Water is a very interesting molecule made up of only one oxygen atom and two hydrogen atoms. Even if it seems simple, all life on Earth is dependent on it for survival. […W]e explore some of the chemical properties that make water so unique.” |
|
July
Sustainability |
“Sustainable Development and chemicals? It may sound like these two don't go hand in hand but could we really live without chemicals? […W]e learn that chemists have an important role in the work towards a sustainable future.” |
|
August
Sports |
“When we are physically active our body will burn the chemically stored energy we get from the food we eat. But what happens if we don't do any exercise at all? […W]e do a little chemistry experiment to compare our energy expenditure between rest and hard training.” |
|
September
Communication |
“[…W]e investigate why the name Silicon Valley is associated with a lot of our new technology. Why would one of the elements in the Periodic Table give name to one of the centers for technological research?” |
|
October
Health |
“There is of course usually more to good health than taking medicines. But sometimes we have to. […W]e investigate how antibiotics work.” |
|
November
Food |
“There are many similarities between cooking and a chemical experiment. We mix ingredients to get a desired end result. But an understanding of chemistry can also help us explain many other things around food. […W]e take a look at some of the chemical reactions taking place while cooking.” |
|
December
History of chemistry and Alfred Nobel |
“Chemistry has been a part of our lives for much longer that we have even called it chemistry. […W]e sum up the year by taking a quick journey through the history of chemistry and take a closer look at Alfred Nobel and the Nobel Prizes.” |
The target group for the videos was secondary school. The producers wanted to influence this age group, and show them that chemistry is interesting and involves many different kinds of jobs in the future. Louise Fornander at the film company Untamed Science writes:
“We wanted to show different aspects of chemistry in all twelve videos (periodic table, history of chemistry, chemical bonds etc.), we also wanted to show research from Chalmers and University of Gothenburg and that the videos should start with an exciting question for the target group and, the most difficult of all. It should be possible to film it. There is an incredible amount of exciting chemistry, everywhere, but a lot of it is difficult to make a video of.” (e-mail 9 September 2012, translated from Swedish by us)
The twelve videos are quite different in character, and thus give a very broad picture of what chemistry is all about. They have a high pace, and show many different aspects of chemistry. They usually have a main story, which is supplemented with explanations and interviews with chemists. According to a note in the Swedish Journal of Chemistry and Biotechnology (2012) “[t]he videos appeal to children in all ages – and many adults!”. Per Thorén at Molecular Frontiers, who initiated the project, said: “We combine scientific content with humor, adventure and action. We want to inspire the audience to learn more.”
With each video comes teaching material with facts and simple experiments, and also lab videos demonstrating some of the experiments. However, this material is not included in the analysis in this paper. The analysis of the twelve thematic videos was done by watching the videos several times. When watching, interesting things and quotations were written down. The content in the videos was compared with the analysis model shown in Fig. 2 by independent analyses by both authors. Differences were discussed and final markings negotiated.
Results of video analysis
Below, the results of the video analysis are presented and discussed. The order follows the fields (0–3) in Fig. 2 introduced in the methods part. As will be clear, the subdivision into the four fields is not always easy and should be seen more as a way to analyse the material, than an absolute truth. Most of the results are summarised in Table 2, which is also subdivided into the four analytical fields/frames. The selected parameters in Table 2 follow Fig. 2. However, Fig. 2 has a higher level of abstraction, while Table 2 is more concrete. Some complex parameters, such as various use of the term “chemicals”, is not included in Table 2, but instead discussed qualitatively and extensively in the text.| January | February | March | April | May | June | July | August | September | October | November | December | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0. Pure chemistry | ||||||||||||
| Chemical formulae and/or symbols | X | X | X | X | X | X | X | X | X | X | X | |
| White lab coat | X | X | X | X | X | X | X | X | X | |||
| Colourful solutions | X | X | X | X | X | |||||||
| Risky experiments | X | X | ||||||||||
| 1. Applied chemistry | ||||||||||||
| Modernism | X | X | X | X | X | X | X | X | ||||
| Benefits only | X | X | X | X | X | X | X | X | ||||
| Everyday aspects | X | X | X | X | X | |||||||
| Health aspects | X | X | X | X | X | |||||||
| Environmental aspects | X | X | X | X | X | |||||||
| 2. Socio-chemistry | ||||||||||||
| Historical context | X | X | X | |||||||||
| Risks and benefits/chemical ethics | X | X | X | |||||||||
| Larger cultural milieu | ||||||||||||
| 3. Nature of chemistry | ||||||||||||
| Explaining | X | X | X | X | X | X | X | |||||
| Analysing | X | X | X | |||||||||
| Synthesizing | X | X | X | X | ||||||||
| Solving technical/medical problems | X | X | X | X | X | X | X | X | X | |||
| Knowledge uncertainties | ||||||||||||
0. Pure chemistry
Basic chemistry aspects are highlighted in most of the videos, for example molecular structures (e.g. January, March, April), intermolecular bonds (June), and reaction formulae (August, November). In the February video, we learn that a polymer is a large molecule composed of small building blocks called monomers. The polymers can have very different properties depending on which monomers they are composed of and how these are attached to each other. In the January video, the characteristics of dyes are described and how a small change in the molecular structure can have a large impact on its macro level properties, in this case its colour.As indicated by the examples above, the submicro and symbolic levels are in focus in several of the videos. It is especially clear in the June video when Louise Fornander points to the ocean and says: “There is no question why our planet is called the blue planet. About 70% of its surface is covered by this pretty simple molecule H2O.” Furthermore, the end message in all the videos – “chemistry is all around you” – indicates a molecular reductionistic view of our world.
In line with this, in the July video Jonas Stenström says: “We wouldn't be able to live completely without chemicals” and Louise says: “Everything is a chemical. We are chemicals. The air we breathe is made up of chemicals. Water is a chemical.”
The term “chemicals” is also mentioned in some of the other videos. The May video presents three “chemicals” that are involved in love: adrenaline, dopamine and serotonin – the so called “love chemicals”. In the April video, it is told that different “chemicals” are added to the paper pulp to give the paper different properties and strengths. This last use of the term “chemicals” is probably the one that is most close to a common sense use of the term. However, the broader use of the term – where water is also regarded as a chemical – is in line with the end message in all the videos: “chemistry is all around you”.
Many examples are taken from the subdiscipline environmental chemistry. Some of the videos focus on commonly found elements and compounds on Earth. In the June video, the unique properties of water are described and in the September video, we learn that silicon is the second most abundant element on Earth, and that much of it is bound in the mineral quartz. The March and June videos treat the climate changes. The March video presents the greenhouse gases carbon dioxide (CO2), water vapour (H2O) and methane (CH4), and explains the chemical mechanisms behind global warming. The June video is about the findings of atmospheric chemists that particles in the air – like dust and pollutants – have an effect on cloud formation and therefore on the entire climate system of the earth. In the July video, we learn about persistent chemicals.
There are also a lot of examples taken from the subdiscipline biochemistry. Some of these are fundamental and others are more applied, but all of them are presented here together. The examples from biochemistry can be divided into the following five areas:
(1) The body's energy metabolism. In the August video, it is described how large molecules are broken down to smaller ones, such as glucose, which are transported to the muscles. The transportation of oxygen from the lungs to the muscles is also described.
(2) Cells and their chemical reactions. In the October video, biochemical reactions in the human body are presented. The difference between mammalian cells and bacterial cells is shown. We learn that proteins can act as building blocks and catalysing agents, such as enzymes. Protein synthesis from DNA, via RNA to protein is described.
(3) Neurotransmitters. In the May video, the chemistry of love is presented as signals that are transferred between nerve cells to receptors, which act like locks and keys making sure that only the right nerve signal is passed on.
(4) Chemistry in the kitchen. In the November video, yeast, a living fungus, is used to make the dough rise. It is described how the yeast uses the energy in sugar to live and multiply and that the waste products water and carbon dioxide are produced. The latter gas is collected in a glove put on the top of a flask with the reaction mixture. The Maillard reaction behind many of the specific colour, smell and flavour of foods is described as the reaction between the amino acids in proteins and the carbonyl group in glucose. Oxidation is demonstrated with an apple that is cut, and eventually turns brown because of a reaction with oxygen forming a brown melanin pigment. Nutritional uptake and bioavailability are studied.
(5) Chemistry of plants and animals. In the January video, we learn that the dye indigo comes from a plant, and in the September video, we learn that pheromones and bioluminescence are used for communication between animals.
Generally the chemical researcher is portrayed in a white lab coat. Such a typical chemist is present in nine of the videos. For example, in the May video, when talking about scientists, a man wearing a white lab coat and safety goggles is shown. In the July video there is a discussion of sustainable development. Then Louise says the following, when wearing white lab coat, safety goggles and gloves: “Now, let's add chemicals to this equation… Ye, the word does have a pretty bad ring to it, doesn't it? Well, I'm a chemist and I still want to work to help the planet”. However, in the different videos we meet various chemists such as a biochemist (October), analytical chemist (June), bioanalytical chemist (May), biopolymer chemist (February), materials chemist (September), atmospheric chemist (March and June), marine chemist (June), industrial chemist (April), physical chemist (December), food science researcher (November), and an environmental science chemist (July). Not all of them are wearing white lab coats.
Another typical picture of chemistry is shown in the July video. When synthesis methods are mentioned, a flask with a red solution in a fume cupboard is displayed in the background. Actually, colourful solutions are shown in five of the videos.
That chemical experiments could be risky is emphasized in the November video. We learn that if one ingredient is changed, it could end up with a disaster. At the same time, a typical chemist in the lab is adding a chemical to a beaker, and there is an explosion and a lot of smoke is generated. The November video ends with the typical warning: “Don't eat in the lab!”
1. Applied chemistry
Pragmatic aspects of chemistry are clearly highlighted in all the videos, except the December video. They can be subdivided into the following three groups: (1) everyday aspects (in five videos), (2) health aspects (in five videos), and (3) environmental aspects (in five videos), respectively. Examples are given below for each group.(1) Everyday aspects. Materials in our everyday life are clearly presented in three of the videos. Examples include different kinds of polymers such as nylon in early parachutes and women stockings (February), Gore-Tex in ski wear (February), and modified cellulose in food (April), and also silicon in transistors (September). Cellulose is described as long and rigid molecules that can be found in the cell walls of tree cells. They are glued together with lignin. Different paper strengths are explained with this theory in the April video.
(2) Health aspects. Especially the October video is about a healthy life, i.e. eating right, doing physical activity and trying to stress less. In the same video we also learn about the mechanism of action of various antibiotics. There are also health aspects in other videos; food preservation is mentioned in the November video and we learn about pharmaceutical treatment of asthma, cardiac arrest, Parkinson's disease and depression in the May video. In the July video, which mainly focuses on sustainability issues, an optimistic message about the possibilities of chemical research is given: “Chemical research is […] providing us with lifesaving medicines and clean water to needed parts of the world.”
(3) Environmental aspects. In the November video, we learn about biotechnology and that yeast cells can be used to produce e.g. plastics. Future biodegradable products that can be recycled, such as cellulose-based diapers and cellulose combined with biodegradable corn to produce a material with plastic-like properties, are mentioned in the April video. The message in the March video is that chemistry can make us limit the amount of greenhouse gases with new techniques such as artificial photosynthesis, and using algae to produce biofuels. Jonas says: “But can chemistry also help us in the future by limiting the amount of greenhouse gases we produce? Well, of course it can!”
There are signs of developmental optimism in eight of the videos. Another term for this is modernism (Sjöström, 2007). In the February video, Jonas tells us that “nowadays new chemical research is resulting in new innovations”. In the July video new improved materials that can be recycled, better ways to get energy and more environmentally friendly pesticides are mentioned, and in the August video new improved materials in sports equipment are mentioned. In the September video, it is pointed out that chemists are behind innovations such as semiconductors, touch screens, and LCD-technology when Jonas says: “Without an understanding of chemistry and how we can use the properties of the chemical elements none of this would have been possible today!” The following quote by Louise from the February video indicates that everybody does not associate these kinds of inventions with chemistry: “Did you know that the inventor of Gore-Tex was a chemist? It's true!”
2. Socio-chemistry
Historical context is especially highlighted in three of the videos, the January, July and December videos. The latter video is about the history of chemistry, although it mainly deals with the research behind some of the chemistry-related Nobel prizes. We learn that the prize symbolises a historically important discovery. The first prize was awarded in 1901, so it is mainly discoveries during the 20th century that have been rewarded. Some Nobel prizes in chemistry, as well as physics and medicine, are presented in the video: synthesis of colours (1905), citric acid cycle (1953), transistor (1956), DNA structure (1962), ozone (1995), dopamine (2000), transcription (2006), and ribosome (2009).Some chemical history before the 20th century is also described in the December video. Examples of early chemistry that is mentioned are use of paints in cave paintings, cooking and preservation of food, use of metals and alloys, ways to harvest energy such as petroleum, discovery of the atom and description of the elements. Pictures of historical chemists and the textbook Alchemica (published in 1597) are shown. “You could almost say that the history of chemistry dates back to long before we even called it chemistry.” However, just like Vesterinen et al. (2013) concluded in a study about nature of science aspects in Nordic upper secondary school textbooks, we conclude that descriptions of the larger cultural milieu in which chemical discoveries and innovations were made are missing in the videos.
Application-oriented chemical history is being described or mentioned in some videos. The January video is about historic and current methods to produce dyes for fabrics. The old method of extracting the blue dye from the indigo plant is compared with the new method where the same molecule is synthesized. Nylon, one of the first synthetic polymers, is mentioned in the February video.
Historical perspectives on experiments are included in the January and December videos. In the January video the story of the dye indigo is told. Indigo is insoluble in water at neutral pH. The solution should have a high pH for the dye to be soluble. Historically, this was accomplished by adding alkaline urine. In the December video, photos of century-old chemical laboratories are shown. In the same video Louise says:
“It's important to understand that the knowledge we have at any given time in history is never the work of only one person. It's the result of years of observations, and experiments passed on and built upon through generations.”
Risks versus benefits are highlighted in three of the videos, the July, October and December videos. In the July video, the dark history of long-term effects of pesticides in the 1900s is highlighted. Problems with antibiotic resistance are discussed in the October video, and the dark side of dynamite is described in the December video. Dynamite was invented by Alfred Nobel, the Swedish chemist who willed his fortune to the Nobel prizes.
Especially two of the videos highlight environmental effects related to industrial chemistry. It is the March video, which is about global warming, and the July video, which is about product life cycles. In the beginning of the July video Louise says:
“We stand before a potential problem; what's going to happen to our planet in the future? We've obviously made some bad cause in the past and honestly a few of these are a result of chemicals that have turned out to do more damage than good. So, with this track record from the past, should chemistry be left out of the whole sustainable development plan for the future?”
Of course the answer is that chemistry is very important in the work for sustainable development, but we think it is good that the dark environmental side of chemicals and chemical technology is also highlighted in the Chemistry Calendar. However, this is clearly done in only one of the videos, the July video.
Recently, this journal published a themed issue on chemistry education and its role in the field of ESD (Eilks and Rauch, 2012). For example a perspective paper by Burmeister et al. (2012) reflects on the meaning of ESD pedagogy in relation to chemistry education. Educational models such as adopting green chemistry principles in lab work, using sustainability strategies as content, and using controversial issues to drive chemistry education are discussed.
In another recently published paper, Vilches and Gil-Pérez (2013) state that sustainability issues are practically absent in many high school and university chemistry curricula all over the world. They argue for chemistry education focusing on possible solutions for a sustainable future and interdisciplinarity. The growing subdiscipline green chemistry focuses on solutions instead of problems (Sjöström, 2006). In the July video, green chemistry is described as a way to produce chemicals that is less detrimental to the environment. Such an approach builds on non-toxic and renewable substances and would reduce risks. The environmental chemist in the video says that when working with green chemistry you have to ask yourself: “What is going to happen to the chemical or the material after use?” She further explains that you have to take the responsibility for the full life cycle of the chemical and think about whether it is recyclable or biodegradable.
As shown above, some chemical consequences for the environment are described in the July video. However, the other eleven videos do not describe (often evident and well-known) environmental drawbacks of the chemical applications that the videos are about. We can call this “excluded environmental aspects”. For example, in the March video, nothing is mentioned about the problems with the energy requirements of the society. They talk about the current search for new energy sources, but not about the net emissions of carbon dioxide as an important cause for the enhanced greenhouse effect. In the January video, nothing is mentioned about the drawbacks of dyes, such as heavy metals and azo dyes. In the February video, neither a general concern regarding plastics nor any drawbacks of Gore-Tex, such as the effects of fluorinated compounds on the environment (Sjöström, 2013), are mentioned. In the August video, Jonas is shown waxing skis, but nothing is mentioned about the possible environmental risks with ski waxes. In the September video, nothing is mentioned about the drawbacks of new metals or electronic scrap, in the October video nothing is mentioned about pharmaceuticals as potential pollutants, and in the November video nothing is mentioned about remains of pesticides in food, or a more general selection of food from an environmental perspective.
3. Nature of chemistry
Talanquer (2013b) states that “chemistry should be […] conceived as a hybrid of academic and industrial endeavors”. This tension between science and technology is highlighted in some of the videos, for example in the March video where Jonas says: “So chemistry plays a role in pretty much every step of the global warming process, from explaining its source to finding new improved solutions for the future.” In other words the role of chemistry is both to explain and to find technical solutions. In the December video, it is the former aim that is emphasised. Jonas says: “We must understand that not everyone can receive a Nobel Prize. There are many more great discoveries and advancements being made every year to help us understand the world we live in a little bit better.”Other aims of chemistry – such as analysing and synthesizing – are present in much fewer of the videos. Chemical analysis is present in the June video where water analysis is mentioned, in the August video where urine is analysed, and in the March video where ice cores are analysed to study how the concentration of carbon dioxide in the atmosphere has changed over time.
Chemical synthesis is indicated in four of the videos. The difference between natural and synthetic substances is highlighted in the February and July videos. The February video is about polymers; wool and silk are given as examples of natural polymers, and plastics and nylon are given as examples of synthetic polymers. In the July video, which is about sustainability, Jonas says the following about the difference between natural and synthetic chemicals:
“Now you probably say most of those things [water, air, our body] are natural chemicals. What we need to get rid of are the unnatural chemicals. Well, this is true in a sense. But, it turns out that many of the chemicals that we have invented are actually, literally life savers.”
Another aspect of the nature of chemistry is the chemical experiment. In addition to the traditional image of the chemist in a white lab coat doing risky experiments, different aspects of chemical experiments are presented in some of the videos. In the November video chemical experiments are compared with cooking: “Pretty much every aspect of cooking is like a chemical experiment, from storing our ingredients, to mixing them in the right proportions, and heating and cooling our mixtures to specific temperatures.”
In the March and August videos, entirely different types of experiments are presented. In the March video, chemical analyses are done on samples of glacier ice at various depths and in the August video, the chemical experiment is about clinical nutrition. The subjects are to exercise as much as possible for one period and then compare with a period of almost complete sedentary. Prior to the experiment Louise says: “We are on our way to the clinical nutrition department here at the university, to do a little ‘chemistry experiment’. We want to find out how our body's use of energy is related to the amount of exercise we do.”
In the February and November videos, the technical aspects of experiments are highlighted. In the February video, synthesis of nylon is shown and the function of Gore-Tex is demonstrated. In the November video, a sampling robot and an artificial stomach are shown.
Knowledge uncertainties in the interpretation of results are not mentioned at all in any of the videos.
Concluding discussion
In this study we have used a new research-based analysis model (Fig. 2) to analyse the English versions of twelve thematic and context-based online videos of the Chemistry Calendar. As already mentioned we think that the model is of potential use also in other forthcoming studies in chemistry education research.When comparing the messages in the twelve videos with the analysis model (Fig. 2), it can be concluded that both pure chemistry and all the different human elements – applied chemistry, socio-chemistry and nature of chemistry – are represented in the video material. However, it is mainly pragmatic aspects (field 1) that are highlighted. Especially the Chemistry Calendar “shows chemistry as an important part of everyday life. The videos also point to the future by highlighting ongoing research” (Swedish Journal of Chemistry and Biotechnology, 2012). Different types of ongoing chemical research are presented, but the messages could have been problematized further.
Below we respond briefly to the questions asked in the introduction:
What characterizes the communication with the public/students? Which is the image of chemistry portrayed in the videos? Which is the image of the chemist? How is the chemical way of working being described? How is the term “chemical” being used?
Some basic chemistry, but mostly applied chemistry and ongoing research, is presented in the videos. There is a tendency of molecular reductionism. In addition to the traditional image of the chemist in a white lab coat doing risky experiments different types of chemists and chemical experiments are presented in the videos. Furthermore, the message “chemistry is all around you” is an attempt to give a wider perspective of chemistry than the usual image. Likewise, the term “chemicals” is used in various ways.
How is the role of chemistry in society being described? Is the larger cultural milieu in which scientific discoveries and innovations were made being described? Which ethical consequences of chemistry are highlighted in the videos?
The videos reflect modernism, where problems created by science can be solved with even more science and technology. They mainly present beneficial aspects. They do, however, present some downsides of chemistry as well, but only in three of the videos risk-benefit issues are raised. A broader and problematized socio-perspective is missing. Given IYC being part of the UN Decade of ESD 2005–2014, it is surprising that the perspective of sustainability does not permeate the videos. Vilches and Gil-Pérez (2013, p. 1869) state that “chemical education is an ethically laden activity that can and must incorporate sustainability as an essential dimension”.
Which aspects of “nature of chemistry” are presented in the videos? How is the balance between the explaining, analysing, synthesizing and problem-solving nature of chemistry?
The “nature of chemistry” aspects in the videos are focused on explaining and solving problems. Much less analysing or synthesizing is present. Among the various chemists included in the videos, none is an organic chemist. This is remarkable considering that many chemists synthesize new substances (Schummer, 1997; Hargittai, 2003). Furthermore, nothing is mentioned about knowledge uncertainties in the interpretation of results.
A conclusion by Kouns (2010) about upper secondary school chemistry teaching is also applicable to the Chemistry Calendar videos. Some years ago she made an extensive empirical study in which she observed a large number of chemistry lessons in an upper secondary school science class. She concluded that the studied chemistry teaching included formal and pragmatic aspects (compare with fields 0 and 1 in Fig. 2), but not reflective aspects (compare with fields 2 and 3 in Fig. 2). Kouns wrote (p. 95):
“The reflexive domain in which the subject knowledge is questioned and examined from a wider societal perspective is not represented in the material. Connections from chemistry to everyday life and society are instead made in the approach to a content area by how chemistry is applied.” (translated from Swedish by Maria Kouns)
We conclude that reflective aspects are represented in the Chemistry Calendar videos, but formal and pragmatic aspects dominate. However, our analysis also shows that the videos have a number of clear messages that are in line with “chemical literacy” (Shwartz et al., 2013): chemistry is all around you, chemistry researchers look different, chemical experiments can be of very different nature, chemistry is important for the society, and chemistry has historically had some downsides.
At the same time, we believe that reflective aspects could have been more prominent in the videos. For instance, the downsides of chemistry and the socio-cultural-historical context around chemical innovations could have been highlighted more. However, many students consider chemistry unpopular, difficult and abstract (Risch, 2010) and the public rank the chemical industry below the overall average of all industrial sectors (Cefic, 2010). The positive attitude in the videos might be an attempt to counteract these negative attitudes.
Concerning Gilbert's (2006) discussion on achieving meaningful learning through “chemistry in context” we conclude that the Chemistry Calendar videos are connected to everyday life, technology, society, chemical research, and history of chemistry. The videos are contextualising chemistry by providing “a coherent structural meaning for something new that is set within a broader perspective” (Gilbert, 2006, p. 960). Most of the videos can be expected to be relevant and meaningful for the secondary school students. However, they do not fully fulfil the most developed of four models of context evaluated by Gilbert. In the fourth model “the social dimension of a context is essential” (p. 969).
Could the videos have been done differently? Well, in our opinion the concept is good and the length of the videos is suitable for the purpose to influence students in secondary school and to show them that chemistry is interesting and involves many different kinds of jobs in the future. Thus, not much could be added to the videos without deleting something else. However, the nature of chemistry in field 3 could have been developed more in for example the December video. In addition to describing the story behind the Nobel prizes, the research processes (including knowledge uncertainties) of some of the examples presented in the previous videos could have been elaborated.
Furthermore, synthesis and analysis, which are very central in the chemical practice, could have been more emphasized. Actually, chemical analysis is the area in chemistry that upper secondary school students find most difficult (Broman et al., 2011). In fact, more detailed information about analytical methods was missing in the videos. They did show that some chemical analyses were carried out, but never mentioned which methods were used. This could easily have been supplemented by mentioning methods such as spectrophotometry, HPLC or mass spectroscopy. Then the teachers could have further developed the theory of the analytical methods after watching the video in the classroom. Although the students might have still perceived the analytical methods to be complicated, they would hopefully have found them easier to understand if they had seen a meaningful use that interested them.
As stated in this paper the thematic context-based online videos of the Chemistry Calendar are unique and potentially very useful. They absolutely make chemistry more concrete, thus making teaching and learning of chemistry easier, as Blonder et al. (2013) claim for chemistry videos in general. However, the critical frames of both nature of chemistry and socio-chemistry could have been more pronounced in the Chemistry Calendar videos. This drawback can be obstructed by using additional teaching materials and by – even more pronounced than is already done – placing the chemistry videos in the contexts of critical citizenship and global sustainability (Eilks and Rauch, 2012; Zoller, 2012; Sjöström, 2013). For example the videos have to be complemented with what we have here called “excluded environmental aspects”. We agree with Fischer (2012, p. 179) that
“Sustainability offers a context for learning chemistry that is rich in possibilities that connect with our students – their interests, their priorities, and the challenges they will face as they enter society as adults.”
References
- Aikenhead G. S., (2006), Science education for everyday life – evidence-based practice, New York: Teacher College Press.
- Blonder R., Jonatan M., Bar-Dov Z., Benny N., Rap S. and Sakhini S., (2013), Can you tube it? Providing chemistry teachers with technological tools and enhancing their self-efficacy beliefs, Chem. Educ. Res. Pract., 14, 269–285.
- Bolte C., Streller S. and Hofstein A., (2013), How to motivate students and raise their interest in chemistry education, in Eilks I. and Hofstein A. (ed.), Teaching Chemistry – A Studybook, Rotterdam: Sense Publishers, pp. 67–95.
- Breslow R., (1997), Chemistry today and tomorrow: the central, useful, and creative science, Washington, DC: American Chemical Society.
- Broman K., Ekborg M. and Johnels D., (2011), Chemistry in crisis? Perspectives on teaching and learning chemistry in Swedish upper secondary schools, NorDiNa: Nord. Stud. Sci. Educ., 7, 43–53.
- Burmeister M., Rauch F. and Eilks I., (2012), Education for Sustainable Development (ESD) and Chemistry Education. Chem. Educ. Res. Pract., 13, 59–68.
- Cefic, (2010), Facts and Figures. 2010: The European Chemical Industry in a Worldwide Perspective.
- Chamizo J. A., (2013), Technochemistry: one of the chemists' ways of knowing, Found. Chem., 15, 157–170.
- Christensson C., (2012), The usability of the “International Year of Chemistry 2011” in the chemistry education in the upper secondary school, Thesis for the Degree of Master of Arts in Education, Malmö University, Faculty of Education and Society, Malmö (in Swedish).
- Eilks I. and Rauch F., (2012), Sustainable development and green chemistry in chemistry education, Chem. Educ. Res. Pract., 13, 57–58.
- Eilks I., Rauch F., Ralle B. and Hofstein A., (2013), How to allocate the chemistry curriculum between science and society, in Eilks I. and Hofstein A. (ed.), Teaching Chemistry – A Studybook, Rotterdam: Sense Publishers, pp. 1–36.
- Fischer M. A., (2012), Chemistry and the challenge of sustainability, J. Chem. Educ., 89, 179–180.
- Gilbert J. K., (2006), On the nature of “context” in chemical education, Int. J. Sci. Educ., 28, 957–976.
- Hargittai I., (2003), Candid science III – more conversations with famous chemists, London: Imperial College Press.
- Harwood W. S. and McMahon M. M., (1997), Effects of integrated video media on student achievement and attitudes in high school chemistry, J. Res. Sci. Teach., 34(6), 617–631.
- Hodson D., (2008), Towards scientific literacy – a teachers' guide to the history, philosophy and sociology of science, Rotterdam: Sense Publishers.
- Johnstone A., (1982), Macro- and micro-chemistry, Sch. Sci. Rev., 64, 377–379.
- Johnstone A. H., (1993), The development of chemistry teaching – a changing response to changing demand, J. Chem. Educ., 70, 701–705.
- King D., (2012), New perspectives on context-based chemistry education: using a dialectical sociocultural approach to view teaching and learning, Stud. Sci. Educ., 48, 51–87.
- Kouns M., (2010), Inga IG i Kemi A! En språkdidaktisk studie av en kemilärares undervisningsstrategier i en gymnasieklass med elever med svenska som andraspråk, (Malmö Studies in Educational Sciences, Licentiate Dissertation Series 2010:15), Malmö University, Faculty of Education and Society, Malmö (in Swedish).
- Kovac J., (2001), Gifts and commodities in chemistry, HYLE: Int. J. Philos. Chem., 7(2), 141–153.
- Lehn J. M., (1995), Supermolecular chemistry concepts and perspectives, Weinheim: VCH.
- Mahaffy P., (2004), The future shape of chemistry education, Chem. Educ. Res. Pract., 5, 229–245.
- Oskarsson M., (2011), Viktigt – men inget för mig. Ungdomars identitetsbygge och attityd till naturvetenskap, (Doctoral thesis, Studies in Science and Technology Education, No. 50), Linköping University, Norrköping (in Swedish).
- Pekdag B. and Le Maréchal J.-F., (2010), Movies in chemistry education, Asia-Pacific Forum on Science Learning and Teaching, 11(1), article 15.
- Risch B. (ed.), (2010), Teaching chemistry around the world, Münster: Waxmann.
- Schummer J., (1997), Challenging standard distinctions between science and technology: the case of preparative chemistry, HYLE: Int. J. Philos. Chem., 3, 81–94.
- Shwartz Y., Dori Y. J. and Treagust D. F., (2013), How to outline objectives for chemistry education and how to assess them, in Eilks I. and Hofstein A. (ed.), Teaching Chemistry – A Studybook, Rotterdam: Sense Publishers, pp. 37–65.
- Sjöström J., (2006), Green chemistry in perspective – models for GC activities and GC policy and knowledge areas, Green Chem., 8, 130–137.
- Sjöström J., (2007), The discourse of chemistry (and beyond), HYLE: Int. J. Philos. Chem., 13, 83–97.
- Sjöström J., (2013), Towards Bildung-oriented chemistry education, Sci. & Educ., 22(7), 1873–1890.
- Swedish Journal of Chemistry and Biotechnology, (2012), Videos about Gore-Tex, love and the greenhouse effect awarded as Sweden's best IYC activity, Kemivärlden Biotech med Kemisk Tidskrift, no. 3/2012, p. 67.
- Talanquer V., (2013a), Chemistry education: ten facets to shape us, J. Chem. Educ., 90, 832–838.
- Talanquer V., (2013b), School chemistry: the need for transgression, Sci. & Educ., 22(7), 1757–1773.
- Ültay N. and Çalık M., (2012), A thematic review of studies into the effectiveness of context-based chemistry curricula, J. Sci. Educ. Technol., 21, 686–701.
- Van Berkel B., (2005), The structure of current school chemistry. A quest for conditions for escape, PhD Thesis, Utrech University.
- Van Berkel B., Pilot A. and Bulte A., (2009), Micro-macro thinking in chemical education: Why and how to escape, in Gilbert J. and Treagust D. (ed.), Multiple Representations in Chemical Education, Springer Verlag.
- Vesterinen V.-M., Aksela M. and Lavonen J., (2013), Quantitative analysis of representations of nature of science in Nordic upper secondary school textbooks using framework of analysis based on philosophy of chemistry, Sci. & Educ., 22(7), 1839–1855.
- Vesterinen V.-M., Aksela M. and Sundberg M., (2009), Nature of chemistry in the national frame curricula for upper secondary education in Finland, Norway and Sweden, NorDiNa: Nord. Stud. Sci. Educ., 5, 200–212.
- Vilches A. and Gil-Pérez D., (2013), Creating a sustainable future: some philosophical and educational considerations for chemistry teaching, Sci. & Educ., 22(7), 1857–1872.
- Zoller U., (2012), Science education for global sustainability: What is necessary for teaching, learning, and assessment strategies? J. Chem. Educ., 89, 297–300.
| This journal is © The Royal Society of Chemistry 2014 |