Associating animations with concrete models to enhance students' comprehension of different visual representations in organic chemistry
Sulaiman M.
Al-Balushi
*a and
Sheikha H.
Al-Hajri
b
aCurriculum and Instruction Department, College of Education, Sultan Qaboos University, P.O. Box 93, P.C. 123 SQU, Muscat, Sultanate of Oman. E-mail: sbalushi@squ.edu.om; Tel: +968 99453799
bMinistry of Education, Educational Directoriate of Sharquiah North, Ibra, Sultanate of Oman. E-mail: sheika.alhajri@yahoo.com
First published on 7th November 2013
Abstract
The purpose of the current study is to explore the impact of associating animations with concrete models on eleventh-grade students' comprehension of different visual representations in organic chemistry. The study used a post-test control group quasi-experimental design. The experimental group (N = 28) used concrete models, submicroscopic animations of molecules and chemical reactions to study an organic chemistry unit in their textbook. On the other hand, the control group (N = 22) used concrete models only. To assess students' comprehension of different visual representations in organic chemistry, a test called the Organic Chemistry Visualisation Test (OCVT) was designed and administered at the end of the study. The results indicated that the experimental group significantly outperformed the control group. Students in the experimental group, with the help of animations, were able to view multiple representations simultaneously, rotate molecules and inspect them from different angles, and comprehend the characteristics of organic molecules such as connectivity, chirality, bond angle, stereochemistry and the spatial arrangement of atoms within molecules. It was observed that the students in the experimental group were excited to navigate between different types of representations of the same molecule and to check whether they predicted its configuration (e.g. three-dimensional, two-dimensional or Fischer Projection models) correctly. Also, students were able to view more complex molecules using animations rather than concrete models. Limitations of the study and implications for teaching, learning, and further research are discussed.
Introduction
Comprehending chemical phenomena is based on two pillars: “how molecules are made” and “what molecules do” (Mammino, 2008, p. 155). Chemists develop the talent to mentally visualise molecules and their transformations (Kozma and Russell, 2005). These internal representations are expressed externally in different forms such as equations, concrete models, drawings, graphs, maps, tables, analogies, computerised animations and simulations (Harrison and Treagust, 2000; Kozma and Russell, 2005; Mammino, 2008). These external representations help students visualise what chemists visualise, understand the text, pay attention to key concepts, become familiarized with invisible submicroscopic entities, generate proper mental models that facilitate the desired learning of chemistry, communicate scientifically and translate among the three levels of chemistry: macroscopic, symbolic and submicroscopic (Johnstone, 1993; Harrison and Treagust, 2000; Kozma and Russell, 2005; Mammino, 2008).Generally speaking, there are three main factors that determine students' comprehension and interpretation of external representations. These are students' reasoning ability, their understanding of the concept under study, and the representational mode (Schonborn and Anderson, 2009, 2010). More specifically, researchers (e.g.Gilbert, 2005, 2008; Kozma and Russell, 2005; Schonborn and Anderson, 2010) in chemistry education and its related branches (i.e. organic chemistry, biochemistry, etc.) have identified a set of cognitive skills needed for students to make use of external representations and eventually become visually literate. Examples of these skills are:
– making sense of the symbolic information in the representation;
– using representations to explain phenomena, make predictions, and solve problems;
– spatially manipulating representations;
– incorporating internal representations (mental models) to produce an appropriate external representation;
– explaining why a given representation is more appropriate than others for a certain phenomenon;
– horizontally translating across multiple representations of a phenomenon;
– vertically translating between different levels of representations;
– being able to evaluate the limitations of a given representation.
Chemistry textbooks illustrate chemical molecules, which are three-dimensional entities, in two-dimensional forms. The two-dimensional representations serve as approximations of the three-dimensional entities and phenomena and can consequently create distorted mental models and hamper desirable learning (Stieff et al., 2005). School students rarely get the opportunity to examine these molecules in three-dimensional forms, which is an essential element for understanding chemical phenomena at the submicroscopic level (Wu et al., 2001). Research shows that superficial experience with the submicroscopic level leads students to miss the opportunity to comprehend the interrelationships among the macroscopic, submicroscopic and symbolic levels (Gabel, 1993; Johnstone, 1993; Treagust et al., 2003; Cheng and Gilbert, 2009; Rosenthala and Sanger, 2012), examine molecules through a chemist's eyes (Bucat and Mocerino, 2009) and visualise molecular transformations and interactions (Kozma and Russell, 2005). They also become unable to link the visual and conceptual information of representations (Wu et al., 2001) and to differentiate between models and reality (Cokelez and Dumon, 2005). Consequently, they remain at the macroscopic (sensory) level and being unable to understand the language of chemistry, which relies on its three levels (Johnstone, 1993, 2006; Wu et al., 2001). Not being able to think of chemical phenomena at all three levels simultaneously is the source of many misconceptions in chemistry (Johnstone, 2000). Studies have revealed that students enter first-year college chemistry courses with a clear lack of ability to use and construct proper mental models of chemical entities and phenomena (Mammino, 2008). Research on twelfth-grade Omani students reveals that they struggle with test items that require an inspection of submicroscopic visual representations of chemical entities and phenomena (Al-Balushi et al., 2012).
Researchers have suggested different teaching strategies to overcome this epistemological problem and make students familiar with the three levels of chemistry, especially the submicroscopic level. Examples include using analogies (Harrison and Treagust, 2000), submicroscopic representations (Al-Balushi, 2012), multiple levels of representations (Treagust and Chandrasegaran, 2009), computer simulations and animations (Kozma and Russell, 2005), computer-based visualisation tools (Wu et al., 2001) and manipulation of 3D concrete models (Copolo and Hounshell, 1995).
The current study focuses on organic chemistry, which is a molecular science that studies complex inter- and intra-molecular three-dimensional spatial relationships. Since learning and problem solving in molecular sciences such as chemistry and biology require these three-dimensional spatial relationships to be perceived, understood and manipulated, visualisation tools have become important instructional strategies for these sciences (Stieff et al., 2005). Another feature of organic chemistry is that it relies on multiple submicroscopic representations. For instance, Fischer Projection models highlight the connectivity between the atoms. On the other hand, the ball-and-stick models stress the three-dimensional relationships (Stieff et al., 2005). Therefore, teaching organic chemistry is unsuccessful without an amalgamation of different types of visual representation (Treagust et al., 2004; Stieff et al., 2005). Although organic chemistry is traditionally considered to be difficult for novices, by visually representing simple submicroscopic ideas related to the number of bonds that each type of atom in organic compounds can form, students can go a long way through hydrocarbons, alcohols, aldehydes, ketones, esters, carbohydrates, fats, proteins and plastics (Johnstone, 2000).
The current study combines both 3D concrete models (physical/hand-held models) and Internet-available tools that illustrate 3D models for organic molecules and help students construct them. The rationale behind focusing on 3D representations is that the world of molecules is three-dimensional (Habraken, 1996). Some organic compounds are relatively large. Therefore, they require a higher level of spatial inspection than small compounds. Students need, for instance, to take into consideration their connectivity, chirality, orbital shape, bond angle and bond length. Additionally, students need an adequate comprehension of the concept of stereochemistry, through which they manage to understand three-dimensional spatial arrangements. This structural understanding, therefore, helps students to comprehend and predict chemical reactivity and the physical properties of chemical compounds (Stieff et al., 2005).
Research shows that 3D concrete models such as ball-and-stick models and space-filling models and their animated electronic versions, by which students can inspect each molecule from different angles, facilitate students' visualisations of these chemical characteristics (Wu et al., 2001; Wu and Shah, 2004). However, using concrete models might generate a misconception that bonds are “sticks” connecting “solid” atoms (Harrison and Treagust, 2000). This is because extensive use of certain models make the students conceptualize them as ‘fact’ (Chittleborough and Treagust, 2007). In addition, concrete models alone are limited (Stieff et al., 2005) in terms of modelling complex molecules, providing a variety of colours and sizes, modelling dynamic interactions, and being able to illustrate special forms of representations such as Fischer Projection models. Therefore, students fail to visualize that chemical processes are dynamic interactions between sub-microscopic particles. They become unable to construct appropriate mental models (Garnett et al., 1998). These limitations are rectified by the utilisation of technological tools. These tools help students translate one type of representation to another, visualise the interactions during chemical reactions, predict the products (Stieff et al., 2005), and consequently generate a coherent understanding of chemical phenomena (Michalchik et al., 2008). Dori and Barak (2001) found that combining concrete and virtual models helped high school students to gain a better understating of organic chemistry concepts such as isomerism and functional groups. Also, they developed an ability to mentally navigate across different levels of chemistry. They were also able to successfully translate 2D representations of molecules to 3D representations and vice versa.
The ability to translate from one type of representation to another is considered a fundamental metavisual capability (Gilbert, 2005). According to Kozma and Russell's classification of representational competence levels, this skill falls under the fourth level (out of five), which is called the semantic use of formal representations (Kozma and Russell, 2005).
In the current study, animations refer to dynamic computerised representations of the behaviours and interactions of submicroscopic entities such as atoms and molecules during chemical reactions. They also refer to the rotation of three-dimensional representations of organic molecules in order to inspect them from different angles. Some types of Internet-based animations allow users to manually rotate molecules. This feature permits a high level of three-dimensional inspection that allows the spatial arrangements of atoms within molecules to be visualised from different sides. Research shows a significant impact of dynamic animations on students' understanding of chemical concepts at the submicroscopic level (Williamson and Abraham, 1995; Yang et al., 2003; Kozma and Russell, 2005). Researchers attribute this positive impact of animations to the construction of dynamic mental images by students for targeted phenomena (Williamson and Abraham, 1995). Visualising dynamic interactions between scientific entities mentally is considered an important aspect of students' successful achievement in science (Shepard, 1988; Mathewson, 1999; Yair et al., 2003; Al-Balushi, 2009; Stieff et al., 2010; Al-Balushi and Coll, 2013). Additionally, visual media tools have a positive impact on students' attitudes towards learning chemistry and on their required study skills as well as their social interaction and development (Turkoguz, 2012).
The design of the current study compared alternate 3D representations, namely concrete versus concrete and electronic representations. This design will enable determination of how the superiority of 3D representations could be further enhanced. The rationale for this is based on the findings of previous research: (1) multiple representations impose large cognitive demands (Chiu and Wu, 2009); (2) students sometimes become confused when presented with more than one explanation for a phenomenon under study (Harrison and Treagust, 2006; Al-Balushi, 2013); and (3) students might not be able to notice the underlying abstract themes that regulate multiple representations of a given phenomenon (Gericke and Hagberg, 2007) or see how they are related (Davidowitz and Chittleborough, 2009). Therefore, we, as researchers, need to decide what combinations of representations work best. Previous research has explored the effectiveness of different combinations. For instance, Mayer and Anderson (1991) investigated the effectiveness of different combinations of text and animations: text-with-animations, text-before-animations, text-without-animations, animations-without-text and no training (control). They found that students who received a combination of animation and textual information together outperformed other groups in problem solving skills and scientific explanations. Another example is a study by Dechsri et al. (1997), which found that using a chemistry laboratory manual with pictures and diagrams was better for enhancing college students' achievement and psychomotor skills than using a laboratory manual without pictures or diagrams.
In addition, using concrete models in both study groups is what differentiates the current study from most previous studies. In other words, the control was not too traditional with no visual tools at all. Students in this group used concrete models that were three-dimensional – a high level of spatial representation. By doing so, the current study was able to examine the effectiveness of concrete models as they stood alone rather than being associated with animations.
The current study takes advantage of available concrete kits and technological tools with the purpose of examining their impact on students' visualisation of chemical entities and phenomena. The rationale behind implementing these available resources is that not all local schools in Oman and countries with similar or lower economic status are able to provide their students with commercial chemical model-building software. Thus, research in chemical education should direct some of its effort to informing chemistry teachers in these countries about effective instructional strategies that utilise affordable resources.
Purpose of study
The purpose of the current study is to examine the impact of associating animations with concrete models on eleventh-grade students' comprehension of different visual representations in organic chemistry.Participants
The participants of the current study were 50 female eleventh-grade students studying in two classes at a public school in the Al-Sherqyah North region in the Sultanate of Oman. This school was purposely selected for the following reasons:(1) The availability of a computer laboratory in the school.
(2) The willingness of the school administration to host the study and collaborate with the researchers.
(3) In some other schools, the number of students who were studying chemistry in grade 11 was small and could not be divided into two groups. The presence of two classes studying chemistry in this school made it possible for one group to serve as an experimental group and another as a control group.
The two classes were randomly assigned into an experimental group (N = 28) and a control group (N = 22).
The school system in Oman is composed of two phases: the basic education phase and the post-basic education phase. The basic education phase has two cycles: cycle one that covers grades 1–4 and cycle two that covers grades 5–10. Cycle one schools are mixed-gender schools taught by female teachers. Females and males study in different schools in grades 5–12.
Research design
The design of the study is a post-test control group quasi-experimental design. Since the post-test used in the study was a content-based test, we decided not to use it as a pre-test. The questions in the test were based on the concepts presented in the organic chemistry unit taught in both groups of the study. Students had no previous experience with organic chemistry concepts before the study. Therefore, we thought that using the test as a pre-test was not the right way to assess the equivalency between the two groups. An alternative was the post-test only design, which becomes a suitable research design when introducing a new subject (Campbell and Stanley, 1966; Moore, 1983). In a post-test-only design, one possible threat to the internal validity is the interaction between the treatment effect and history, i.e. events other than the treatment that occur during the course of study before administration of the post-test. Also, this design does not allow the researcher to check the initial equivalency of the study groups (Campbell and Stanley, 1966; Fisher and Foreit, 2002; Kirk, 2013). Therefore, we decided to analyse the students' pre-chemistry achievement score from the semester before to test the equivalency between the two groups. This methodological research decision of not to use identical pre- and post-tests and to use a proxy variable as the best estimate of students' prior performance is called “proxy pre-test design” in research methodology literature (Moore, 1983; Trochim and Donnelly, 2007; Carbonell, 2012). When the proxy variable is students' pre-achievement, the research design is known as “achieved proxy pre-test design” (Trochim and Donnelly, 2007).The experimental group studied the organic chemistry unit in the eleventh-grade chemistry textbook using concrete models and animations. The control group studied the same unit using concrete models only. The concrete models were balls and sticks.
The Organic Chemistry unit was the last unit that students studied in grade eleven. This unit covered two chapters: (1) Organic Compounds – Hydrocarbons and (2) Derivatives of Hydrocarbons. The study was conducted when students had completed three out of the four units included in the chemistry curriculum for this grade level. These three units were:
“Elements: Trends and Intermolecular Forces”, which covered two chapters: (1) Periodic Trends in Atomic Properties and (2) Molecular Shapes and Intermolecular Forces.
“Matters: Solutions, Acids and Bases”, which covered two chapters: (1) Solutions and (2) Acids and Bases.
“Stoichiometry”, which covered two chapters: (1) Chemical Equations and Calculations and (2) Limiting Reagent for Chemical Reactions and Titrations.
Students were exposed to 3D shapes (presented in 2D illustrations in the textbook) when they studied the “Molecular Shapes and Intermolecular Forces” chapter. No real 3D hands-on experience with concrete models or animations was used. This was the only chapter that used 3D representations. The study lasted for eight weeks with a total of 15 lessons that were taught in 33 class periods, each of which was 45 minutes long. At the end of the study, an organic chemistry achievement test was administered to both groups. Both groups were taught by the same chemistry teacher, who was trained by the second researcher on the use of the teacher's manual for both groups. The training was done in the form of a three-day workshop, six hours per day. During the course of study, the second researcher attended all lessons in both groups of study. The purpose of her attendance was to assure that the teacher implemented the treatment according to the teacher's manual, help in solving the technical problems with computers and the Internet and making sure that the use of the concrete models in both groups was identical. She would also jot down some observations.
The study was conducted during the second semester of the school year. This allowed the use of students' results in semester one as a measure of achievement in chemistry to be used as a covariate in the analysis of covariance (ANCOVA). Students' achievement in the first semester was divided into three major parts: midterm (15%), classroom participation and homework (10%), laboratory (15%), and final exam (60%). The final exam had two parts: written and practical. The practical part was conducted in the laboratory one week before the written part. During the practical part, students were asked to conduct an experiment. Teachers used a lab observation form to grade students.
Instructional materials
Teacher's manual
A teacher's manual was designed for the experimental group. The manual was designed around the organic chemistry unit in the student's eleventh-grade chemistry textbook, which covers the following main topics: alkanes, alkenes, alkynes, functional groups, alcohols, aldehydes, ketones, carboxylic acids, esters, amines, polymers and isomers. The main factors that informed the design of the teacher's manual were (1) the organic chemistry content in the target textbook unit, (2) the learning outcomes and types of assessment prescribed by the national curriculum for this unit, (3) specific learning outcomes designed to target different aspects of students' understanding of organic chemistry at the submicroscopic level, (4) manipulation and visualisation at the submicroscopic level of matter, (5) literature-informed cognitive skills important for nurturing students' visualisation at the submicroscopic level in chemistry, and (6) the availability of an appropriate animation for the topics found in the unit.The manual consisted of an introduction, the theoretical framework for chemical models and modelling, the manual's objectives, instructions to the teacher, the unit plan, a brief guide on how to use the animations, a brief guide on how to use the modelling kits, the lesson plans, the worksheets and the list of references.
Collectively, the worksheets asked students to: identify molecules based on their 3D, 2D Fischer Projection or symbolic representations, translate one type of representation to another, build 3D models, identify types of bonds between atoms within organic molecules, complete chemical equations, predict the final molecules of a series of reactions and explain a phenomenon. Animations and modelling kits were incorporated with some of these tasks based on the nature of the lesson. Appendix I illustrates sample tasks required from students in the worksheets.
Another teacher's manual was produced for the control group. It was the same manual designed for the experiment group except that it did not have the animations and Internet-based activities. Instead, students did the same activities using the concrete models only.
Three-dimensional kits
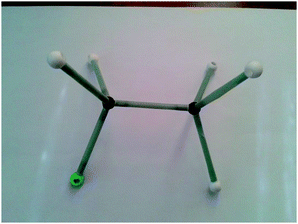
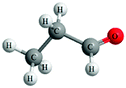
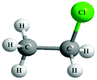
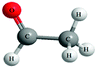
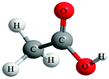
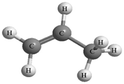
Both the experimental and control groups were provided with 14 boxes of molecular model kits. These were balls and sticks used to construct concrete models for organic molecules. Fig. 1 illustrates an example of the concrete models used in the current study. This example represents chloroethane. The black balls represent carbon atoms, the white balls represent hydrogen atoms and the green ball represents the chlorine atom. Students in both groups used concrete models to build 3D physical models of organic molecules, manipulate them to view their spatial orientations and produce another physical model as a mirror image to the one previously built. Also, students used paper and pencil to translate these 3D models to other forms of representations such as 2D models, Fischer Projections or symbolic representations.Electronic materials: the animations
Fifty-two different animations were used in the current study. Two important conditions were applied during the process of selecting the animations: (1) their scientific accuracy and (2) the appropriateness of their complexity level to that of eleventh-grade students. Also, we selected the animations for their potential to allow students to: (1) translate among different levels and types of representations, and (2) visualise and change the spatial orientation of the molecules.These animations allowed students to view the molecules in both 3D and 2D. Students could choose to visualise the organic molecule using wire-frame models, ball-and-stick models, and space-filling models. For some molecules, Fischer Projection models were also available. This allowed them to translate among different types of representations. On the other hand, this automatic translation among different representations was not an option when using concrete models only. In the absence of animations, students in the control group needed to rely on their cognitive skills such as imagination and spatial ability to perform these processes when required. They also relied, on many occasions, on using the paper and pencil method to draw the molecules. This provided a good opportunity for the students in this group to practise translating the concrete 3D models into 2D models on paper and vice versa. Constructing such models is an expressive technique to construct a mental model (Stieff et al., 2005). In addition, some animations allowed students in the experimental group to rotate and visually inspect some of the organic molecules under study. In the control group, students rotated concrete models to inspect the molecules from different angles. This was not an option for complex molecules, which the students were not able to represent using balls and sticks.
The computer laboratory
Due to limited availability, we were unable to secure a computer laboratory that accommodated all students in the experimental group. We managed to reserve a computer laboratory with 14 computers. The students had to work on the computers in pairs. They were allowed to discuss the given tasks together. However, each student had her own worksheet to work on. They may have worked together on the given task, but they needed to respond individually to the given questions on the worksheets. This would give them the opportunity to cognitively process the tasks independently in their own minds, in the same manner that was required in the post-test exam. This setting is similar to that in studies done on cooperative learning, where students work together when conducting classroom activities while they have to respond individually to the post-test (e.g.Acar and Tarhan, 2007, 2008; Kırıka and Boz, 2012).Review of instructional materials
A panel of experts was asked to review the teacher's manual and the electronic materials. The experts included five science education professors, three chemistry professors, three curriculum specialists working at the Ministry of Education, five chemistry supervisors at the Ministry of Education and four experienced chemistry teachers. We asked them to check the scientific accuracy of the animations and whether their complexity level was appropriate for the cognitive level of eleventh-grade students. In addition, we asked them to assess the suitability of each animation for the content topic. Also, we asked them to evaluate the whole collection for its potential to help students translate among different types and levels of representations and spatially manipulate orientations of the molecules. Examples of these translation processes are:– Constructing a 3D concrete model for a molecule from a 2D drawing (and vice versa)
– Producing a Fischer Projection model from a 3D concrete model or any other 2D drawings
– Writing the molecular formula for a molecule given in 3 or 2D form
– Drawing a 2D sketch for a 3D model of a molecule after rotating it to a certain degree
– Constructing a 3D mirror copy of a molecule given in 3D form
This review and validation process resulted in adding an instructions section to the teacher's manual, modifying some lesson plans and replacing some complex and higher-level animations with simpler ones. According to the literature (Rosenthala and Sanger, 2012), this last point was important. Overly complex submicroscopic animations cause students to misinterpret chemical phenomena. Also, the reviewers checked whether the selected animations were scientifically accurate and provided sufficient information to guide students to construct scientifically accepted understanding.
The instrument
To measure the impact of the treatment on students' comprehension of different visual representations in organic chemistry in both groups in the study, an instrument was designed called the Organic Chemistry Visualisation Test (OCVT). We decided not to use any existing visualisation test for the following reason. The focus of the study was students' comprehension, which is closely linked to the content of the organic unit taught in the targeted grade level (in this case, grade eleven). Thus, the test should be content-based. It was therefore difficult to find an existing test that is well aligned to the content and topics of the organic chemistry unit undertaken.This content-based visualisation test covered the topics discussed in the organic chemistry unit in the students' eleventh-grade chemistry textbook. It consisted of 24 items: 18 multiple-choice items, three items requesting drawn structural formulae, two about naming molecules, and one requiring writing a synthesis equation. The maximum score was 30. The test covered all the topics discussed in the organic chemistry unit under study. Although the OCVT was a visualisation test, its items were generated from the content covered in the unit under study. There were 11 items (46%) covering the topics discussed in the first chapter “Organic compounds – hydrocarbons” and 13 items (54%) covering the topics discussed in the second chapter “Derivatives of hydrocarbons”. These percentages were determined based on the length of each chapter and the number of topics covered in each. Thus, the OCVT was designed to have a balanced coverage of the content discussed in the unit under study and to test different visualisation skills. Examples of these skills included: examining one or more visual representations of an organic molecule in order to identify the type of molecule(s), predicting the outcome of a reaction, arranging molecules according to their boiling or melting points, naming a molecule, identifying the types of chemical bonds, identifying the type of functional group, writing an equation for a reaction or drawing the structural formulae for different molecules.
Appendix II illustrates sample items from the test. These sample items help to demonstrate how the OCVT items were at the intersection between the content and skill dimensions. For instance, to solve item no. 3, students needed to inspect three given representations (visualisation skill) and decide which had the highest boiling point and which had the lowest (content knowledge). To solve item no. 4, students needed to have knowledge about how HBr reacts with alkenes and the ability to visualise what the arrangement of atoms should be like in the produced molecule. The question that combines items 13–17 requires students to analyse the verbal statements in the items, relate them to the content knowledge they had when they studied the organic unit, and choose the correct molecule that suits each item. Once they chose a molecule, they needed to translate among different representations, back and forth, until they found the correct molecule from the given ones.
The OCVT was reviewed by a panel of experts. These included four science education professors, one evaluation and measurement professor, three chemistry professors, three chemistry supervisors working at the Ministry of Education and five experienced chemistry teachers. The panel was asked to comment on the scientific accuracy of the items, the linguistic clarity, the appropriateness of the items' cognitive level to the cognitive level of the eleventh-grade students, the scoring of the test and the overall layout of the test. This validation process resulted in rephrasing some items, replacing items with new ones and raising the cognitive level of some items.
The OCVT was piloted on 30 female students from a different school than the one that hosted the study. This piloting process was used to estimate the reliability of the test, determine the time required to do the test and check the readability of the items. The estimated time was 90 minutes and the Cronbach's alpha reliability coefficient was 0.78.
Results
The purpose of the current study was to examine the impact of associating animations with concrete models on eleventh-grade students' comprehension of different visual representations in organic chemistry. We illustrate in this section how both study groups scored in the Organic Chemistry Visualisation Test (OCVT). Also, we present the results of the ANCOVA statistical analysis, which compared the performance of both groups on the OCVT when their pre-achievement was taken as a covariance. Table 1 displays the means and standard deviations of the study's two groups on the OCVT. Table 2 displays the results of the analysis of covariance (ANCOVA). The results indicated that there was a statistically significant difference between the two study groups (F = 28.08, p < 0.01). The experimental group, which used animations and concrete models, outperformed the control group, which used concrete models only.| Group | N | Pre-achievementa (max 100) | Post-test OCVTb (max 30) | ||||
|---|---|---|---|---|---|---|---|
| M | SD | Range | M | SD | Range | ||
| a Pre-achievement score (100 points) = midterm (15 points) + classroom participation and homework (10 points) + laboratory (15 points) + final exam (60 points). b The maximum score on the OCVT is 30. | |||||||
| Control | 22 | 73.55 | 9.42 | 35.00 | 13.23 | 4.20 | 15.50 |
| Experimental | 28 | 78.61 | 9.02 | 36.00 | 20.16 | 5.41 | 20.50 |
| Group | SSM | df | MS | F | p |
|---|---|---|---|---|---|
| Intercept | 161.62 | 1 | 161.62 | 17.18 | 0.001 |
| Pre-achievement | 717.84 | 1 | 717.84 | 76.32 | 0.001 |
| Group | 264.07 | 1 | 264.07 | 28.08 | 0.001 |
| Error | 442.05 | 47 | 9.41 |
Based on the observations made by the second author during her attendance with both groups of study, we noticed that the students in the experimental group were excited to use the animations to navigate between different types of representations of the same molecules. They were eager to see whether their predictions of various models of molecules (e.g. three-dimensional, two-dimensional or Fischer Projection models) were correct. The most interesting models that students liked the most were the ball-and-stick models. They remarked that these models allowed them to count the number of atoms and types of bonds easily. They also commented that space-filling models helped them to compare the sizes of atoms and the shapes of their electron clouds. Additionally, they enjoyed rotating molecules and inspecting them from different angles. Compared to concrete models, animations were more successful for illustrating the visual representations of more complex molecules such as aromatic compounds, carboxylic acids, esters and amino acids. Concrete models were limited to small molecules.
Understanding organic chemistry reactions also became easier with the help of animations that incorporated the three levels of chemistry: macroscopic, submicroscopic and symbolic. For instance, the researchers observed that it was easier for students in the experimental group to discover the reasons behind the high boiling points of carboxylic acids compared to other hydrocarbons. The animation used in this lesson illustrated clearly how hydrogen bonds form and how molecules are bonded together. On the other hand, even with the help of blackboard sketches, students in the control group struggled with this process. Additionally, the concept of hybridisation became clear with the help of animations. Consequently, students successfully managed to visualise why atoms in some organic molecules assume certain spatial arrangements that affect the shapes of these molecules.
Discussion
The results of the current study indicated that animations of submicroscopic organic molecules and reactions enhanced the utilization of concrete models in the teaching of organic chemistry. The concrete models did not have the same effect when used alone with no animations. Apparently, animations helped participants cope with the visualisation skills [specified below] required to comprehend the spatial aspects of organic molecules. Studies have shown that there is a moderate correlation between chemistry achievement and spatial ability (Wu et al., 2001). More specifically, some of the animations used in the study and some of the Internet links provided for the participants permitted them to view multiple representations simultaneously; they could also rotate organic molecules manually and view them from different perspectives. This also promoted their ability to translate from one type of representation to another, especially from 2D to 3D representations and vice versa.In addition, animations played the role of visual external representations that provided learners with the opportunity to visually inspect different elements within the undertaken molecules. Therefore, connectivity, chirality, bond angle, stereochemistry and the spatial arrangement of atoms within molecules became easier to visualise with the aid of the animations. Instead of trying to mentally visualise (i.e. imagine) these properties, the presence of animations reduced the mental load, the abstractness of these properties and the chances of producing an inaccurate image of the undertaken molecules. These results are consistent with previous research which found that animations aid students in improving their incomplete mental models by constructing dynamic mental images of chemical phenomena (Wu and Shah, 2004; Kozma and Russell, 2005), reducing the cognitive load by making information explicitly available to students (Wu and Shah, 2004; Chiu and Wu, 2009), facilitating imagining abstract phenomena (Chiu and Wu, 2009) and reducing the formation of common misconceptions related to the subject matter (Kozma and Russell, 2005). Also, animated dynamic submicroscopic representations of organic reactions facilitated participants' visualisation of the chemical reactivity of the molecules and their prediction of the end products. The significant impact of dynamic animations on students' comprehension of organic chemistry (Williamson and Abraham, 1995; Wu et al., 2001; Yang et al., 2003; Kozma and Russell, 2005) and other chemical concepts (Ardac and Akaygun, 2005; Stern et al., 2008; Urhahne et al., 2008) has been incorporated in previous studies.
Although concrete models help students practise some of these cognitive skills, we believe that using animations accelerates the process of acquiring these skills and makes learning more efficient. The findings are in accord with the findings of previous studies (Dori and Barak, 2001; Stieff et al., 2005).
In summary, we believe that associating animations with concrete models helped students in the experimental group practise the following cognitive skills that researchers (e.g.Gilbert, 2005, 2008; Kozma and Russell, 2005; Schonborn and Anderson, 2010) believe are essential for students of chemistry in making use of external representations:
– Decode symbolic information (e.g. decode the red sphere depicting an oxygen atom).
– Make predictions (e.g. predict the structural formula of the molecule that results from the reaction of an alkyne and HCl).
– Explain phenomena (e.g. explain why alkanes are insoluble in water).
– Solve problems (e.g. solve the problem of how to produce a given molecule from another given one by outlining the intermediate reactions).
– Manipulate models spatially (e.g. manipulate a 3D concrete model of ethane to change it to ethene).
– Justify a choice of a representation for a given phenomenon (e.g. justify why a 3D animated representation is better than the 2D sketched representation to understand the difference between the solubility of alcohols and alkanes in water).
– Study and produce multiple representations for a given phenomenon (e.g. produce a 3D concrete model, molecular formula, structural formula and Fischer Projection model for 3-methylhexane).
– Consider different levels of representations (e.g. study the macroscopic, symbolic and different submicroscopic representations of ethanol).
– Determine the limitations of different representations (e.g. determine the limitations of using the molecular formula only to study different chemical properties of hexane).
Conclusions and recommendations
The findings of the current study demonstrate that authentic, reliable and well-designed submicroscopic animations available on the Web could serve as an effective instructional tool for enhancing chemistry learning. Schools with limited resources could take advantage of these resources. Another implication of these findings is that relying on instructional tools such as concrete models and textbook representations alone may not be very helpful to a new generation of learners. Learners nowadays are surrounded by different types of visual technologies (Stieff et al., 2005) such as smart phones and animated games. Teachers need to use more than traditional techniques to get the attention of these learners. Teachers should utilise new technologies to facilitate their students' penetration into the submicroscopic level of chemistry, the world of atoms and molecules. Previous research reached a similar conclusion that students should be provided with the opportunity to interact with and manipulate both concrete and technology-based models (Wu et al., 2001).The items of the OCVT instrument used in the current study were visual items that asked participants to examine or produce different types of submicroscopic representations (Appendix II). The successful performance of the participants in the experimental group in these visual-based submicroscopic items, compared to the control group, promises to remedy students' failure to deal with similar test items, as reported in a recent study on 786 twelfth-grade Omani students (Al-Balushi et al., 2012). Therefore, Omani chemistry teachers and textbook writers should incorporate more modelling instructions and provide students with more opportunities to manipulate different types of submicroscopic representations, especially animated and concrete 3D models.
The mental visualisation of organic molecules and processes is highly spatial in nature (Stieff et al., 2005). The results of the current study reveal a significant positive impact of associating animations with concrete models on students' comprehension of different visual representations in organic chemistry. Further research might explore the impact of such a combination of concrete and electronic representations on students' spatial ability. Another interesting research idea would be to explore the effectiveness of providing students with a modelling experience using an electronic means that allows them to construct organic compounds by themselves. This research could be done by comparing three groups: (1) concrete models only, (2) animations and concrete models, and (3) modelling, animations and concrete models. Different dependent variables might be examined, such as chemistry achievement, spatial thinking, and the ability to translate between the three levels of chemistry: macroscopic, symbolic and submicroscopic.
The results of our study revealed a novelty effect. Students in the experimental groups were excited to use the computer, view the animations, and manipulate the 3D molecules. The control groups did not have this opportunity. This observation highlights the need for an additional study that controls this effect. One practical suggestion would be a comparison between an experimental group that works with both concrete models and animations at the submicroscopic level and a control group that uses concrete models and also uses the computer to view chemical phenomena at the macroscopic level of matter. This proposed research design controls the novelty effect by making sure that the excitement that is generated from using computers is controlled for in both groups. The novelty effect of increased participant engagement, enthusiasm or attention because they are doing something different or new has been controlled for in some previous studies in different fields. An example is reported by Keller et al. (2005). Also, studies by Kırıka and Boz (2012) and Ayyıldıza and Tarhan (2013) represent examples of acknowledging that novelty effects resulting from implementing new approaches with students pose an external threat to these studies.
There is another effect that might undermine the generalizability of the results of the current study. That is the expectancy effect. The literature expects that a combination of representations should be more effective (e.g.Chiu and Wu, 2009; Davidowitz and Chittleborough, 2009; Treagust and Chandrasegaran, 2009). However, as explained in the Introduction section above, the design of the current study goes beyond that when it compares different combinations of 3D representations: concrete models only vs. concrete and animations. Thus, we consider the current study to be a contribution to our knowledge in this regard. Further studies should consider examining other combinations of external representations.
Resources
The readers could find useful animations for organic chemistry topics in different Internet websites. Examples are:http://www.yteach.co.uk
http://www.learnerstv.com
Appendix I
Sample tasks required from students on the worksheets1. Write the scientific names for the following molecules using the IUPAC system:
2. Watch animation no. 4 and draw the shown representations as stated below for 3-bromo-butane:
3. Watch animation no. 5 and answer the following questions:
a. Illustrate different stages of this reaction using chemical equations:
b. What is the type of reaction between chlorine and fluorine?
c. Watch animation no. 6 and explain why alkanes are insoluble in water:
Appendix II
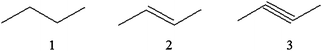
Sample items from the Organic Chemistry Visualisation Test (OCVT)Item no. 3
Study the following compounds:The order of these compounds in terms of their boiling point is:
A. 1 > 2 > 3
B. 3 > 2 > 1
C. 2 > 3 > 1
D. 1 > 3 > 2
Item no. 4
The compoundreacts with HBr in the presence of a catalyst. What is the main product of this reaction?
A.
B.
C.
D.
Item no. 8
The compound with the highest boiling point is:A.
B.
C.
D.
Item no. 9
The monomer that is used in addition polymerisation is:A.
B.
C.
D.
Items 13–17
Study the matrix below and answer the questions underneath it:13. The compound that is used to produce ethanol in the fermentation process is no. ____.
14. The compound that is produced from the hydrogenation of cyclohexane is no. ____.
15. The two compounds that are position isomers are no. ____ and no. ____.
16. The compound that is produced from the addition of water to 2-methylbutane is no. ____.
17. The compounds that are oxidised when they react with an acidic solution of potassium dichromate are no. ____, no. ___, no. ____ and no. ____.
References
- Acar B. and Tarhan L., (2007), Effect of cooperative learning strategies on students' understanding of concepts in electrochemistry, Int. J. Sci. Math. Educ., 5(4), 349–373.
- Acar B. and Tarhan L., (2008), Effects of cooperative learning on students' understanding of metallic bonding, Res. Sci. Educ., 38(4), 401–420.
- Al-Balushi S. M., (2009), Factors influencing pre-service science teachers' imagination at the microscopic level in chemistry, Int. J. Sci. Math. Educ., 7(6), 1089–1110, DOI: 1010.1007/s10763-10009-19155-10761.
- Al-Balushi S. M., (2012), The effect of macroscopic and submicroscopic pictorial representations on pre-service science teachers' explanations, Int. J. Acad. Res., Part B, 4(6), 10–14, DOI: 10.7813/2075-4124.2012/7814-7816/B.7812.
- Al-Balushi S. M., (2013), The relationship between learners' distrust of scientific models, their spatial ability, and the vividness of their mental images, Int. J. Sci. Math. Educ., 11(3), 707–732, DOI: 10.1007/s10763-10012-19360-10761.
- Al-Balushi S. M. and Coll R., (2013), Exploring verbal, visual and schematic learners' static and dynamic mental images of scientific species and processes in relation to their spatial ability, Int. J. Sci. Educ., 35(3), 460–489, DOI: 10.1080/09500693.2012.760210.
- Al-Balushi S. M., Ambusaidi A., Al-Shuaili A. and Taylor N., (2012), Omani twelfth grade students' most common misconceptions in chemistry, Sci. Educ. Int., 23(3), 221–240.
- Ardac D. and Akaygun S., (2005), Using static and dynamic visuals to represent chemical change at molecular level, Int. J. Sci. Educ., 27(11), 1269–1298.
- Ayyıldıza Y. and Tarhan L., (2013), Case study applications in chemistry lesson: gases, liquids, and solids, Chem. Educ. Res. Pract., 14(4), 408–420, DOI: 10.1039/c3rp20152j.
- Bucat B. and Mocerino M., (2009), Learning at the sub-micro level: structural representations, in Gilbert J. and Treagust D. F. (ed.), Multiple representations in chemical education, models and modeling in science education, Dordrecht, The Netherlands: Springer, vol. 4, pp. 11–30.
- Campbell D. and Stanley J., (1966), Experimental and quasi-experimental designs for research, Boston: Houghton Mifflin Company.
- Carbonell P., (2012), Quasi and experimental research designs, Interdiscip. Rev. Res. Dev., 1(1), 5–11.
- Cheng M. and Gilbert J., (2009), Towards a better utilization of diagrams in research into the use of representative levels in chemical education, in Gilbert J. and Treagust D. F. (ed.), Multiple representations in chemical education, models and modeling in science education, Dordrecht, The Netherlands: Springer, vol. 4, pp. 55–73.
- Chittleborough G. and Treagust D. F., (2007), The modelling ability of non-major chemistry students and their understanding of the sub-microscopic level, Chem. Educ. Res. Pract., 8(3), 274–292.
- Chiu M.-H. and Wu H., (2009), The roles of multimedia in the teaching and learning of the triplet relationship in chemistry, in Gilbert J. and Treagust D. F. (ed.), Multiple representations in chemical education, models and modeling in science education, Dordrecht, The Netherlands: Springer, vol. 4, pp. 251–283.
- Cokelez A. and Dumon A., (2005), Atom and molecule: upper secondary school French students' representations in long-term memory, Chem. Educ. Res. Pract., 6(3), 119–135.
- Copolo C. F. and Hounshell P. B., (1995), Using three-dimensional models to teach molecular structures in high school chemistry, J. Sci. Educ. Technol., 4(4), 295–305.
- Davidowitz B. and Chittleborough G., (2009), Linking the macroscopic and sub-microscopic levels: diagrams, in Gilbert J. and Treagust D. F. (ed.), Multiple Representation in Chemical Education, Dordrecht, The Netherlands: Springer, vol. 4, pp. 169–191.
- Dechsri P., Jones L. and Heikkinen H., (1997), Effect of a laboratory manual design incorporating visual information-processing aids on student learning and attitudes, J. Res. Sci. Teach., 34(9), 891–904.
- Dori Y. J. and Barak M., (2001), Virtual and physical molecular modeling: fostering model perception and spatial understanding, Educ. Technol. Soc., 4(1), 61–72.
- Fisher A. and Foreit J., (2002), Designing HIV/AIDS intervention studies: an operations research handbook, Washington, DC: Population Council.
- Gabel D. L., (1993), Use of the particle nature of matter in developing conceptual understanding, J. Chem. Educ., 70(3), 193–194.
- Garnett P., Oliver R. and Hackling M., (1998), Designing interactive multimedia materials to support concept development in beginning chemistry classes, in Chan T.-W., Collins A. and Lin J. (ed.), Global Education on the Net: Proceedings of the Sixth International Conference on Computers in Education, Beijing/Heidelberg: China Higher Education Press/Springer Verlag, vol. 1, pp. 297–304.
- Gericke N. M. and Hagberg M., (2007), Definition of historical models of gene function and their relation to students' understanding of genetics, Sci. Educ., 16, 849–881.
- Gilbert J., (2005), Visualization: a metacognitive skill in science and science education, in Gilbert J. K. (ed.), Visualization in science education, Dordrecht, the Netherlands: Springer, pp. 9–27.
- Gilbert J., (2008), Visualization: an emergent field of practice and enquiry in science education, in Gilbert J., Reiner M. and Nakhleh M. (ed.), Visualization: theory and practice in science education, Dordrecht: Springer, pp. 3–24.
- Habraken C., (1996), Perceptions of chemistry: why is the common perception of chemistry, the most visual of sciences, so distorted? J. Sci. Educ. Technol., 5(3), 193–201.
- Harrison A. G. and Treagust D. F., (2000), A typology of school science models, Int. J. Sci. Educ., 22(9), 1011–1026.
- Harrison A. G. and Treagust D. F., (2006), Teaching and learning with analogies, in Aubusson P., Harrison A. G. and Ritchie S. (ed.), Metaphor and analogy in science education, Netherlands: Springer, pp. 11–24.
- Johnstone A. H., (1993), The development of chemistry teaching, J. Chem. Educ., 70(9), 701–705.
- Johnstone A. H., (2000), Teaching of chemistry – logical or psychological? Chem. Educ. Res. Pract., 1(1), 9–15.
- Johnstone A. H., (2006), Chemical education research in Glasgow in perspective, Chem. Educ. Res. Pract., 7(2), 49–63.
- Keller J., Deimann M. and Liu Z., (2005, October), Effects of integrated motivational and volitional tactics on study habits, attitudes, and performance, Paper presented at the Annual Meeting of the Association for Educational Communications and Technology, Orlando, Florida.
- Kirk R., (2013), Experimental design: procedures for the behavioral sciences, 4th edn, Thousand Oaks, CA: Sage.
- Kırıka O. and Boz Y., (2012), Cooperative learning instruction for conceptual change in the concepts of chemical kinetics, Chem. Educ. Res. Pract., 13, 221–236.
- Kozma R. and Russell J., (2005), Students becoming chemists: developing representational competence, in Gilbert J. K. (ed.), Visualization in science education, Dordrecht, the Netherlands: Springer, vol. 1, pp. 121–146.
- Mammino L., (2008), Teaching chemistry with and without external representations in professional environments with limited resources, in Gilbert J., Reiner M. and Nakhleh M. (ed.), Visualization: theory and practice in science education, Dordrecht, the Netherlands: Springer, vol. 3, pp. 155–185.
- Mathewson J. H., (1999), Visual-spatial thinking: an aspect of science overlooked by educators, Sci. Educ., 83, 33–54.
- Mayer R. and Anderson R., (1991), Animations need narrations: an experimental test of a dual-coding hypothesis, J. Educ. Psychol., 83, 484–490.
- Michalchik V., Rosenquist A., Kozma R., Kreikemeier P. and Schank P., (2008), Representational resources for constructing shared understandings in the high school chemistry classroom, in Gilbert J., Reiner M. and Nakhleh M. (ed.), Visualization: theory and practice in science education, Dordrecht, the Netherlands: Springer, vol. 3, pp. 233–282.
- Moore G., (1983), An empirical test of design patterns for children's environments, in Joiner D. (ed.), People and the physical environment research. Wellington, New Zealand: Victoria University of Wellington.
- Rosenthala D. and Sanger M., (2012), Student misinterpretations and misconceptions based on their explanations of two computer animations of varying complexity depicting the same oxidation–reduction reaction, Chem. Educ. Res. Pract., 13, 471–483.
- Schonborn K. and Anderson T., (2009), A model of factors determining students' ability to interpret external representations in biochemistry, Int. J. Sci. Educ., 31(2), 193–232.
- Schonborn K. and Anderson T., (2010), Bridging the educational research-teaching practice gap: foundations for assessing and developing biochemistry students' visual literacy. Biochem. Mol. Biol. Educ., 38(5), 347–354.
- Shepard R., (1988), The imagination of the scientist, in Egan K. and Nadaner D. (ed.), Imagination and education, New York, NY: Teachers College Press, pp. 153–185.
- Stern L., Barenea N. and Shauli S., (2008), The effect of a computerized simulation on middle school students' understanding of the kinetic molecular theory, J. Sci. Educ. Technol., 17(4), 305–315.
- Stieff M., Bateman R. and Uttal D., (2005), Teaching and learning with three-dimensional representations, in Gilbert J. K. (ed.), Visualization in science education, Dordrecht, the Netherlands: Springer, vol. 1, pp. 93–120.
- Stieff M., Hegarty M. and Dixon B., (2010), Alternative strategies for spatial reasoning with diagrams, in Goel A. K., Jamnik M. and Narayanan N. H. (ed.), Diagrams 2010, Berlin Heidelberg: Springer-Verlag.
- Treagust D. F. and Chandrasegaran A., (2009), The efficacy of an alternative instructional programme designed to enhance secondary students' competence in the triplet relationship, in Gilbert J. and Treagust D. F. (ed.), Multiple representations in chemical education, models and modeling in science education, Dordrecht, The Netherlands: Springer, vol. 4, pp. 151–168.
- Treagust D. F., Chittleborough G. D. and Mamiala T. L., (2003), The role of submicroscopic and symbolic representations in chemical explanations, Int. J. Sci. Educ., 25(11), 1353–1368.
- Treagust D. F., Chittleborough G. D. and Mamiala T. L., (2004), Students' understanding of the descriptive and predictive nature of teaching models in organic chemistry, Res. Sci. Educ., 34, 1–20.
- Trochim W. and Donnelly J. P., (2007), The Research Methods Knowledge Base, 3rd edn, Mason, OH: Thomson Publishing.
- Turkoguz S., (2012), Learn to teach chemistry using visual media tools, Chem. Educ. Res. Pract., 13, 401–409.
- Urhahne D., Nick S. and Schanze S., (2008), The effect of three-dimensional simulations on the understanding of chemical structures and their properties, Res. Sci. Educ., 39(4), 495–513.
- Williamson V. M. and Abraham M. R., (1995), The effects of computer animation on the particulate mental models of college chemistry students, J. Res. Sci. Teach., 32(5), 521–534.
- Wu H. and Shah P., (2004), Exploring visuospatial thinking in chemistry learning, Sci. Educ., 88, 465–492.
- Wu H., Krajcik J. S. and Soloway E., (2001), Promoting conceptual understanding of chemical representations: students' use of a visualization tool in the classroom, J. Res. Sci. Teach., 38(7), 821–842.
- Yair Y., Schur Y. and Mintz R., (2003), A “thinking journey” to the planets using scientific visualization technologies: implications to astronomy education, J. Sci. Educ. Technol., 12(1), 43–49.
- Yang E., Andre T., Greenbowe T. J. and Tibell L., (2003), Spatial ability and the impact of visualization/animation on learning electrochemistry, Int. J. Sci. Educ., 25(3), 329–349.
| This journal is © The Royal Society of Chemistry 2014 |