Thinking about practical work in chemistry: teachers' considerations of selected practices for the macroscopic experience
Brian
Lewthwaite
James Cook University, Townsville 4811, Australia. E-mail: brian.lewthwaite@jcu.edu.au
First published on 16th October 2013
Abstract
This study explores teachers’ thinking about practical work, especially in regards to the types of practical work they privilege in their teaching of chemistry to support students in their learning. It seeks to investigate the view that practical work, especially the type of practical work selected, is unthinkingly and uncritically selected by chemistry teachers. The study is conducted at the end of a five-year professional development initiative associated with the implementation of a new curriculum chemistry initiative for Grade 11 and 12 advocating for a ‘tetrahedral’ orientation to the teaching of chemistry emphasizing the ‘practical’ experience on the ‘macroscopic’ level as one of four essential dimensions of the chemistry learning experience. Responses indicate that teachers’ thinking is informed by pragmatic, philosophical and psychological considerations; the latter largely informed by an understanding of the importance of ‘multi-level’ chemistry experiences encouraged by the ‘tetrahedral’ model.
Introduction
Images of practice in chemistry classrooms are commonly represented by student and teacher engagement in various forms of practical work. These common representations are indicative of the accuracy of the assertion by Millar (2002) that practical work is a normalised [chemistry education] practice and the ‘natural’ and ‘right’ thing to do. As Toplis (2012) asserts, practical work has become part of the ‘tradition’ of the chemistry classroom. For many teachers of chemistry, practical work in its various forms is seen as the basic modus operandi for their teaching (Abrahams and Reiss, 2012). Wellington (1998) suggests that for many teachers practical work is carried out [in chemistry classrooms] [only] because chemistry is a practical subject. Wellington's comment resonates with Hodson's suggestion that teachers of science “unthinkingly see hands-on practical work as the universal panacea, the educational solution to all learning problems” (Hodson, 1990, p. 33). Hodson's criticism suggests that teachers may give little consideration to why they engage students in and use practical work because they are subject to the ‘powerful, myth-taking’ that suggests that they need to engage students in practical work simply because chemistry is a practical subject. As Osborne (1998) notes, the doing of practical work rests on a set of assumptions that need further examination, especially the inherent ‘value’ of practical work especially if it is used uncritically as ‘tradition’ and ‘convenience’. The study described here explores teachers’ thinking about practical work, especially in regards to the types of practical work they privilege in their teaching of chemistry to support students in their learning. It seeks to validate or dispel the view that practical work and, especially the type of practical work selected, is unthinkingly and uncritically selected. The study is conducted at the end of a five-year professional development initiative associated with the implementation of a new curriculum initiative advocating for a ‘tetrahedral’ orientation to the teaching of chemistry (Mahaffy, 2006) emphasizing the ‘practical’ experience as one of four essential dimensions of the chemistry experience – the ‘macroscopic’, which is characterised by tangible, evidential experiences.Defining practical work
The term practical work in the province of Manitoba, Canada, where this study is situated, is an umbrella term often used to describe a variety of first-hand experiences students might have in the learning of chemistry (Manitoba Education Citizenship and Youth, 2006, 2007). This is similar to Millar et al. (1998) who take a broad view of practical work as “those teaching and learning activities in science which involve students at some point in handling or observing real objects or materials (or direct representations of these)” (p. 36). Practical work contrasts with student engagement with virtual objects and materials such as those obtained from a DVD, on-line simulation, or a text-based account of the phenomena (Millar, 2010). Similar to that described in the international literature (for example, Gott and Duggan, 2007), practical work may include a variety of ‘types’ such as demonstrations, teacher-directed experiments and teacher- or student-directed investigations. The types of practical work used in chemistry classrooms have been categorised historically, according to various authors (e.g. Eggleston et al., 1975 ; Hacker, 1984; Woolnough and Allsop, 1985; Kirschner and Meester, 1988; Hegarty-Hazel, 1990), based upon (1) the reason for the practical work being undertaken; (2) the nature of the student–teacher interaction; (3) whether the student or teacher directs the process and (4) the emphasis placed on process skills.For example, within the Manitoba context (MECY, 2006, 2007) demonstrations are typically teacher-directed and manipulated and performed with minimal student–student interaction and moderate teacher–student interaction and limited attention to process skill development. In contrast, experiments are typically student performed but orchestrated by the teacher and are performed with high levels of student–student interaction and minimal student–teacher interaction. Although pedagogical practices for performing demonstrations and experiments such as Discrepant Events (Piaget, 1971) and the Predict–Observe–Explain framework (White and Gunstone, 1992) encourage student cognitive engagement, both demonstrations and experiments are typically characterized as having a pre-determined sequence, encourage students to be passive participants and recipients of information, and lead to teacher and textbook explanations for observed phenomena (Vhurmuku, 2011). Experiments and investigations, on the other hand, are typically student performed and promote significant student–student interaction and reduced teacher–student interaction. The difference between experiments and investigations is in the degree to which the student directs the process. Within the Manitoba context, in experiments the procedures for process are prescribed whereas in investigations the procedures of process are not. Experiments are typically ‘recipe’ or ‘cook-book’ tasks ( Kind et al., 2011 ) used to elucidate or verify a specific chemistry idea whereas investigations are used as a vehicle to explore chemical phenomena through inquiry. Within the Manitoba context experiments are synonymous with the terms laboratory exercise (‘mini-lab’ or ‘lab’ in short) and, less commonly termed, closed investigation. In Manitoba the term investigation implies openness in the practical work process (MECY, 2006, 2007). Although in both experiments and investigations students collect their own data; interpret results and draw their own conclusions (Dudu and Vhurumuku, 2011), their primary distinction is in the nature of the student direction in the formulation of the researchable question and process to be pursued. As stated by Hegarty-Hazel (1990), investigations imply that there is a degree of latitude given to learners by teachers to ask or frame questions and design and conduct investigations. In both experiments and investigations students collect their own data; interpret results and draw their own conclusions. The process skills employed in investigations are seen to be characteristic of the full spectrum of skills employed by professional scientists in authentic scientific inquiry whereas in experiments the skills used are often isolated and limited.
Practical work in chemistry in the context of Johnstone's triplet and Mahaffy's tetrahedron
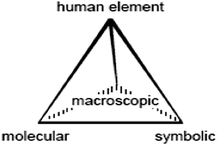
Considerable attention has been given in the past two decades in the chemistry education literature to Alex Johnstone’s (1982, 1991) reference to the chemistry ‘triplet’. Keith Taber’s (2013) recent overview of the triplet and its ontology clarifies the three ‘domains’ or ‘levels’ of chemical knowledge – the macroscopic, the submicroscopic and the symbolic – and their relationship and importance in fostering student learning in chemistry. The central idea in Johnstone’s (1991) assertion was that chemistry learning was made difficult because learning required students to be involved in ‘multilevel thought’ (Johnstone, 1982, 1991). As an example, in the teaching of a conceptually difficult concept such as strong and weak acids (e.g., hydrochloric acid and acetic acid respectively), students might be (1) provided with macroscopic, first-hand experiences through a demonstration by observing the difference in the pH and conductivity of equimolar solutions of hydrochloric and acetic acid; (2) shown visual representations of what is happening at a molecular level when a strong and weak acid dissociate in water to produce different concentrations of hydronium ions through computer-based software and discuss as a class to explain why these differences in conductivity and pH exist; and (3) working symbolically in calculating or being provided with numerical dissociation constants based upon chemical formulae for each acid's dissociation process. As Taber asserts, “Johnstone's triangle has become highly influential in chemistry education and has been adopted and adapted in a variety of ways that demonstrate its utility in the field” (Taber, 2013, p. 1). It is noteworthy for this study that Johnstone's reference to the macroscopic infers students experiencing sensory, tangible and visible experiences which, as Taber suggests, are “descriptive and functional; that is, referring to the seeing and handling of materials leading to descriptions of the physical and chemical properties in terms of density, flammability, colour and so on” (Taber, 2013, p. 2). It is not surprising that Gilbert and Treagust (2008) refer to the macroscopic as ‘phenomenological’ as the macroscopic experience “seeks to represent [chemical] phenomena with the senses” (p. 4).More recently, Peter Mahaffy (2006) suggests that the triplet model omits an essential dimension, the human element. In response to this omission, he encourages chemistry educators to move beyond the triangular planar (which he asserts focuses chemistry education solely on conceptual understanding and content acquisition) to incorporate a further dimension of investigative experience and communication, thus changing the model to a tetrahedron as illustrated in Fig. 1 below. As Mahaffy (2006) states “this rehybridization emphasizes [the] need to situate chemical concepts, symbolic representations, and chemical substances and processes in the authentic contexts of the human beings who create substances, the cultures that use them, and the students who try to understand them”. Mahaffy describes the need to develop public understanding and trust through the exploration of contemporary applications of chemistry and the social and environmental issues associated with chemical production and use. Overall, his advocacy for the inclusion of the human element rests in a supposition that engagement with, learning and communication in and appreciation of chemistry may be hampered by an insufficient integration of the human element into the content of chemistry and a three mode pedagogical framework. This ‘tetrahedral’ approach not only includes the explicit learning of chemistry, but also includes the learning about chemistry as it is dealt with in society ( Burmesiter et al., 2012 ). Further it ensures the processes of the learning of chemistry are made explicit, especially in ensuring that teachers give consideration to how one might approach the teaching of chemistry that ensures students active engagement in and learning of chemistry phenomena. As Taber asserts, dealing with the ‘complexity’ of chemistry requires teachers to ‘slow the pace’ to provide sufficient opportunities through a range of contexts that include macroscopic, submicroscopic and symbolic representation (Taber, 2013, p. 11).
Despite the more recent emphasis placed upon the ‘triplet’ and ‘tetrahedron’ as pedagogical frameworks in teaching chemistry, what is apparently absent from the literature is consideration of the means by which students might engage in macroscopic experiences; that is, what types of practical work – demonstrations, experiments and investigations – might provide the most meaningful macroscopic experiences for students to support student engagement with the other dimensions of the triplet or tetrahedron? As well, what reasons do teachers provide for justifying their choice of practical work provided in making these connections explicit? The study described herewith explores these questions.
Context of the study
This study is located in Manitoba, Canada where the chemistry curriculum for Grade 11 and 12 (Manitoba Education Citizenship and Youth (MECY), 2006, 2007 respectively) explicitly emphasize a tetrahedral orientation as a pedagogical framework for the teaching of chemistry (Mahaffy, 2006). Mahaffy's model, as previously mentioned, an extension of the chemistry ‘triplet’ often espoused in the chemistry education literature (Gilbert, 2005; Gilbert and Treagust, 2008; Taber, 2013) is believed by the Manitoba curriculum development team (of which the author is a primary writer) to be the first chemistry curriculum internationally to be underpinned by the ‘tetrahedral’ model. For this reason, this research project reported here, under the umbrella of a much larger chemistry education professional development project (Lewthwaite, 2008; Lewthwaite and Wiebe, 2011, 2012), was seen to be an important contribution to the chemistry education community, especially in seeing the relationship between chemistry education theory and classroom practice.The provincial curricula were not developed in response to Mahaffy's suppositions, but, instead, evolved out of the curriculum development teams’ understanding of evidence-based appropriate pedagogy for experiencing and fostering learning in science (Osborne and Wittrock, 1985) and, especially, chemistry through explicit reference to the multi-dimensional modes of representation and participation (as examples, Gabel, 1999; Bunce and Gabel, 2002; Gilbert, 2005; Taber, 2010, 2013). This tetrahedral orientation is evidenced consistently throughout the Grade 11 and 12 chemistry curriculum. As an example, in the example cited previously about strong and weak acids, students are expected to examine the differences between strong and weak acids at the experiential level (macroscopic) and seek to understand these differences in properties through their molecular dissociation differences (microscopic) and how this difference is represented through chemical equations and quantitative data (symbolic) and influences biochemical processes such as blood pH, equilibrium and stomach digestion in the human body (human element) (MECY, 2007).
As might be expected, the new Manitoba chemistry curricula with its advocacy for a tetrahedral orientation brings with it an orientation to chemistry teaching and learning that is unlikely to be consistent with current teaching practice. As suggested by Johnstone (1991) and Gilbert and Treagust (2008), most chemistry teachers and, consequently their students, focus primarily on the abstract teaching, thinking and communicating level in the written symbolic form, and, thereby, any effort to bring about reform-based changes to chemistry teaching and learning practice must be accompanied by significant support. As Hoffmann and Laszlo (2001) assert, this shift requires teachers and students to engage in a ‘language’ or communication of chemistry beyond the written symbolic level.
As research asserts, a new curriculum is rarely accompanied by teacher change unless accompanied by significant and strategic support (Fullan, 1992). In response to this challenge, the University of Manitoba's Centre for Research, Youth, Science Teaching and Learning (CRYSTAL) in 2007 embarked on a five-year research and development project to support the improvement of teaching and learning of chemistry in accordance with the intent of the new curriculum and its tetrahedral orientation. The project endeavoured to monitor its success based upon how teachers were teaching in accordance with the tetrahedral orientation of the curriculum and, correspondingly, students responded to and were influenced by this approach to teaching. The study described here ensues from a research inquiry pursued in 2012, at the end of the five-year study.
The professional development initiative
The extended project involved over five years three groups of chemistry teachers from different geographical regions of Manitoba, Canada for a total of 74 chemistry teachers. During the course of a year, teachers from each cohort attended five professional development days focusing on the topics of either the Grade 11 or Grade 12 curriculum. Thus, most teachers, by this study's stage, had participated in approximately 120 hours of face-to-face professional development. The sessions typically involved the identification of specific learning outcomes that required a teaching orientation unlikely to be consistent with current teaching practice. Teachers in attendance and facilitators participated in tangible teaching examples (for example, demonstrations, laboratory experiments and investigations, practical applications, computer simulations) that addressed these outcomes in a manner consistent with the curriculum's tetrahedral orientation. As an example, in the example of strong and weak acids cited earlier, four hours in one session were placed on how teachers could use demonstrations, experiments and investigations to represent the chemical and physical characteristics of strong and weak acids. As well, computer-based simulations were used to illustrate visually how strong acids in contrast to weak acids dissociate to produce a higher concentration of hydronium ions and, consequently, influence the macroscopic properties associated with a higher conductivity and lower pH of the resulting solution. Accordingly, various kinaesthetic models, manipulative software and web-based simulations were critiqued in terms of their clarity in making the connections between the macroscopic, molecular and symbolic levels.Further, practical examples of the reason behind the role and importance of strong and weak acids were considered in the context of the human body, including the role of hydrochloric acid, a strong acid, in stomach digestion and the homeostatic role of weak acids in the maintenance of blood pH Sessions were seen as an opportunity to collaboratively assist teachers and facilitators in becoming more familiar and comfortable with the pedagogy associated with the three vertices of the tetrahedron that they are least accustomed to, in particular the human element, molecular and macroscopic. It is estimated that these three dimensions combined for the majority of the focus of the sessions and, correspondingly, the more than 200 resources developed by the project leader and teachers. Because they are regarded as less orthodox teaching strategies, special emphasis was placed on effective pedagogy to make explicit the connections among the four dimensions. As examples, the use of computer-based visualizations, historical accounts of chemistry ideas, and practical applications of chemistry, were commonly examined alongside engaging macroscopic experiences such as experiments, demonstrations and investigations. What is noteworthy for this study was that no explicit reference was made over the five-year program as to what type of practical work would be most suitable for presenting macroscopic experiences. The facilitators and writers of the new curriculum expected teachers to make their own decision about what type of practical work to use assuming, in contrast to what Hodson (1990) suggests, that teachers would thinkingly make these choices based upon what would be best support students in their learning.
Capturing teacher practices during the professional development
The instrument used to gauge teacher development over the five years in this project was the Chemistry Teacher Inventory (CTI), an instrument developed specifically for this project within the context of the Manitoba curriculum and its' tetrahedral orientation. Its development and content is based upon what students primarily, and the literature and teachers involved in this project suggested influence student learning in chemistry. The development of the instrument is described elsewhere (Lewthwaite and Wiebe, 2011) but for this study Table 1 below presents five examples of the 33 items in total contained within the four dimensions of the tetrahedron pedagogical orientation as well as one further dimension pertaining to general pedagogy. The items on the CTI are referred to as low-inference behaviours (Murray, 1999) meaning that they are easily identified through observation, in this study's case by the teachers themselves, the observing researchers and the students that are experiencing them.| Dimension | Item example |
|---|---|
| Macroscopic | I perform chemistry demonstration |
| Sub-microscopic | I use computer simulations to represent what is happening molecularly |
| Symbolic | Students perform calculations |
| Human element | We talk about the historical development of chemical ideas |
| General pedagogy | Students copy or take notes as chemical ideas are presented or discussed |
It is noteworthy for this study that the CTI contains only three items specific to the macroscopic level. These include (1) i perform chemistry demonstrations; (2) students carry out experiments (mini-labs, set labs); and (3) students carry out investigations. Although other forms of practical work could possibly be included in the CTI (e.g., circuit of experiments, field work investigations), these were not included because they did not feature in the preliminary interviews that informed the development of the CTI in 2007.
Prior to the commencement of the five-year initiative and, subsequently, over the duration of the study, teachers annually completed the CTI. As well, at least one teaching class was observed annually in which teaching behaviours were monitored according to the 33 items on the CTI. Further, post-teaching interviews were conducted to discuss each teacher's practice specific to the tetrahedral intent of the curriculum and professional development. As well, telephone conversations were conducted with those not observed to elicit their perceptions of their progress in teaching. Finally, a student version of the CTI (Lewthwaite and Wiebe, 2011), also containing 33 items, was used in which students documented the frequency of use of the low-inference behaviours they were experiencing in their classroom. As an example, an item on the CTI is: “i perform chemical demonstrations”. On the student form of the instrument this item is worded, “The teacher in this classroom performs chemical demonstrations.” This variety of methods was used as a means of triangulation to provide some confirmation of the trends evident in the data specific to the low-inference behaviours teachers were practicing. As documented in another study (Lewthwaite and Wiebe, 2011), there was little variation in teachers' perceptions of frequency of use of these low-inference behaviours and students' and researchers' observations of such teaching behaviours.
Table 2 above presents data on teacher's perceptions of the frequency of the three practices used in the classroom that pertain to the macroscopic level – demonstrations, experiments and investigations. In the table the average ‘rank of use’ of each practice is provided in comparison to the other 33 items on the CTI. As well, the mean use on a 1 (never used) to 5 (always used) Likert-type scale are provided. As well, the ranked frequency of each practice across the five years is listed. The data indicate that experiment use changed from a rank of 10th to 5th over the five years; demonstration use changed from a rank of 24th to 19th and investigations changed from a rank of 27th to 31st. What prompted this inquiry was the statistically significant difference in the use of the demonstrations, experiments and investigations. Despite the equal emphasis provided to each type of practical work in the professional development and the curriculum, why were teachers rarely engaging their students in investigations? Further, why were teachers increasingly using experiments and, to a lesser extent, demonstrations and decreasingly using investigations? These questions emanating from these data promoted this inquiry. That is, what reasons do teachers provide for their choice of practical work type provided to their students?
![[x with combining overline]](https://www.rsc.org/images/entities/i_char_0078_0305.gif) ) (1 (never)-5 (always)) for the 33-item Chemistry Teaching Inventory (CTI) over the five years of the professional development
) (1 (never)-5 (always)) for the 33-item Chemistry Teaching Inventory (CTI) over the five years of the professional development
| Item | 2007 | 2008 | 2009 | 2010 | 2011 |
|---|---|---|---|---|---|
| I perform chemistry demonstrations |
24 (![[x with combining overline]](https://www.rsc.org/images/entities/i_char_0078_0305.gif) = 2.9) = 2.9)
|
24 (![[x with combining overline]](https://www.rsc.org/images/entities/i_char_0078_0305.gif) = 3.3) = 3.3)
|
18 (![[x with combining overline]](https://www.rsc.org/images/entities/i_char_0078_0305.gif) = 3.0) = 3.0)
|
19 (![[x with combining overline]](https://www.rsc.org/images/entities/i_char_0078_0305.gif) = 2.9) = 2.9)
|
19 (![[x with combining overline]](https://www.rsc.org/images/entities/i_char_0078_0305.gif) = 2.9) = 2.9)
|
| Students carry out experiments (mini-labs, set labs) |
10 (![[x with combining overline]](https://www.rsc.org/images/entities/i_char_0078_0305.gif) = 3.8) = 3.8)
|
10 (![[x with combining overline]](https://www.rsc.org/images/entities/i_char_0078_0305.gif) = 3.9) = 3.9)
|
7 (![[x with combining overline]](https://www.rsc.org/images/entities/i_char_0078_0305.gif) = 3.9) = 3.9)
|
5 (![[x with combining overline]](https://www.rsc.org/images/entities/i_char_0078_0305.gif) = 4.1) = 4.1)
|
5 (![[x with combining overline]](https://www.rsc.org/images/entities/i_char_0078_0305.gif) = 4.1) = 4.1)
|
| Students carry out investigations |
27 (![[x with combining overline]](https://www.rsc.org/images/entities/i_char_0078_0305.gif) = 2.3) = 2.3)
|
31 (![[x with combining overline]](https://www.rsc.org/images/entities/i_char_0078_0305.gif) = 2.3) = 2.3)
|
32 (![[x with combining overline]](https://www.rsc.org/images/entities/i_char_0078_0305.gif) = 2.2) = 2.2)
|
31 (![[x with combining overline]](https://www.rsc.org/images/entities/i_char_0078_0305.gif) = 1.3) = 1.3)
|
31 (![[x with combining overline]](https://www.rsc.org/images/entities/i_char_0078_0305.gif) = 1.2) = 1.2)
|
Methods
This inquiry was pursued in 2012 once the five-year professional development initiative had concluded. All 72 teachers participating in the professional development were emailed, a means of communication commonly used during the initiative. Teachers were asked three questions:(1) On a scale of 1 (Never) to 5 (Always), how often do your students experience the following three types of practical work (a) demonstrations; (b) investigations and (c) experiments (labs, mini-labs)?
(2) Provide detailed reasoning as to why as a chemistry teacher you would (a) perform a demonstration, (b) have students do an experiment, or (3) carry out an investigation? and,
(3) Over the professional development program explain your reasons for doing the following types of practical work more or less frequently (a) performing a demonstration; (b) having students do an experiment; and (c) carry out an investigation.
In all, 61 of the participating teachers responded. The answer to question 1 was determined through descriptive statistical analysis. The results for question 2 and 3 were processed by the author and a research assistant. Each independently coded and identified emergent major and minor themes from the comments provided by teachers for their reasons for carrying out the three types of practical work. In most cases teachers provided multiple reasons for their use of each type of practical work.
What informs teachers' selection of type of practical work?
In all, teachers ranked their use of the three types of practical work similar to the overall trends evidenced in Table 2 across the five years of the initiative. Investigations were rarely used (mean score 1.9); demonstrations were used some of the time (mean score 3.0); and experiments were used most of the time (mean score 4.1).The reasons for selecting a type of practical were coded under several broad categories that emerged from the analysis of data. These included comments made pertaining to: (1) time implications; (2) resource implications; (3) management implications; (4) safety implications; (5) learning and engagement, and (6) the authenticity of the practical work type in regards to scientific inquiry and the nature of science. Points (1), (2), (3) and (4) were aggregated under the broad category pragmatic reasons, keeping (5) under the broad category of psychological reasons and (6) under the broad category of philosophical reasons. In Table 3 examples of comments that pertain to each of these three categories are presented as well as the frequency of the reason provided. It is noteworthy that the reasons provided are distributed across the three broad categories suggesting that teachers' thinking is informed by the three categories of thought, albeit to varying degrees.
| Type of practical work | Resource and management implications (pragmatic reasons) | Authenticity as scientific process (philosophical reasons) | Beliefs about utility in promoting learning of chemistry (psychological reasons) |
|---|---|---|---|
| Teacher demonstrations |
• Low demand on resources (9)
• Useful when equipment and supplies are limited and costs are of concern (9) • Easy to manage and organize (18) • Limit risk for students through a teacher-directed demonstration (17) • Time efficient (9) • Valuable as a classroom management strategy (6) • Addresses concerns associated with student manipulative and procedural skills (9) |
• Limited authenticity unless demonstration prompts questions or ideas that lead to further demonstration or investigation (6)
• Students are primarily observers rather than active investigators. Tend to be teacher directed and limits the process being influenced by students (20) |
• Allows for practical skills to be modelled prior to students proceeding onto experiments or investigations (14)
• Allows for easy transition to explaining, especially at the molecular level, what has been observed during the observation (11). If not, • Tend to lead to explanations that students have trouble understanding (unless consideration is given to how to explain it to foster conceptual understanding) (7) • Engage students cognitively (7) • Teacher can focus students' observation on critical aspects central to the demonstration focus (21) • Limits students' development of manipulative and procedural skills (6) |
| Prescriptive experiments |
• Moderate demand on resources (9)
• Required materials easily organised beforehand (7) • Moderate difficulty in managing experiments (14) • Moderate time and risk demand (14) • Can be interrupted to clarify some procedural aspect or explain some aspect (7) |
• Limited authenticity unless experiment prompts questions or ideas that lead to investigation (9)
• Students are manipulators of equipment and observers but not active investigators because of the prescriptive nature of the experiment (13) |
• Elucidates key ideas because often done to verify a chemistry concept (14)
• Can be structured so the sequence of the experiment has students engaged in ‘multi-level’ thought (24) • Promotes development of procedural and manipulative skills (21) • Assumes students will come to understanding of the phenomena through the process (3) • Students can be more focused on the manipulation of equipment and completion of the task rather than actively engaged in understanding the purpose of the process (7) • Critical aspects to be observed may be overlooked (15) |
| Investigations |
• High resource demand (7)
• Challenging to execute as a teacher (18) • Can cause teacher to become a ‘technician’ in serving student requirements and organization (4) • High management demand, especially in variable time completion for groups (9) • High time demand because students direct the process (29) • Higher risk (11) • Expensive to resource (9) • Concerns with student skill level in investigating (9) |
• High authenticity – captures the nature of chemistry in promoting chemistry as a process of inquiry. Students are active investigators because of the non-prescriptive nature of the investigation (11) |
• Promotes the development of all investigative skills from planning through to carrying out, analysing and communicating findings (9)
• Requires students to be active inquirers (21) • Problematic if students have limited investigative skills or motivation for self-directed inquiry (11) • Students can be preoccupied with the technical part of the carrying out rather than focusing on the macroscopic experience (34) • Difficult to direct students’ ‘multi-level’ thought (16) |
In comparing the frequency of the reasons provided in Table 3, teachers reasons for demonstrations are most commonly informed by pragmatic reasons (80 comments), in contrast to psychological reasons (66 comments), and philosophical reasons (26 comments). In experiments, teachers draw primarily from psychological reasons (84 comments) in contrast to pragmatic reasons (41 comments) and philosophical reasons (22 comments). In investigations, teachers' comments again predominantly pertain to pragmatic reasons (96 comments) in contrast to psychological reasons (91 comments) and philosophical reasons (11 comments).
Teachers' thinking about why they privileged or used sparingly a type of practical work is presented in the section that follows and is summarised under each type of practical work in Table 3. In each section reference to the themes that emerged in each category are documented and examples of commentary associated with the themes are provided.
Decisions about demonstrations
A. Pragmatic reasons for demonstrations
Teachers' reasons for using demonstrations corresponded closely to the existing commentary on this type of practical work (for example, Millar, 2002). Comments (80/172) suggested that demonstrations were primarily used for pragmatic reasons. The major themes emerging within the pragmatic comments were ease of execution (18) and reducing risk (17). Minor themes emerging included their economy of use in terms of time (9), expenditure (9), and resource availability (9); their value as a management strategy (6) and their value in addressing concerns associated with students' manipulative and procedural skills (9).Some comments are listed below noting that the examples indicate a variety of pragmatic reasons within each of the comments. Using three examples, the responses by which the responses were coded are listed.
For all of the topics, we were exposed to many examples of demonstrations, closed investigations and open investigations. Some practical activities are best done by demonstration. They are easily performed in comparison to students having to carry out a similar task. They require less time to perform and clean up and don't gobble-up the budget. Some demonstrations are quite risky so in the end I tend to perform demonstrations for these reasons.
In this response, reasons associated with ease of execution, economy of time, economy of expenditure, and safety were identified.
The resourcing is an issue, especially with multiple classes. I recall my first year of teaching and how much silver nitrate I used and the stains on desks and students' hands. I couldn't control how much they used and how careful they were. It [The cost] was an eye opener when I ordered the following year. Now I'll demonstrate [both to reduce costs and prevent staining].
In this example, reasons associated with management, economy of cost and safety are evidenced.
Many of the procedures are rather complicated so you need to be demonstrating. They are a great way to start a class – just gathering their attention – and ensuring they understand what is required. If we went to an experiment [at the start of the class], it's just more problematic in terms of management [because of their inadequate skill base].
Finally, in this comment reasons are identified associated with the role of demonstrations in addressing concerns with students' procedural and manipulative skill deficiency and for engaging students cognitively.
B. Psychological reasons for demonstrations
Although the comments made predominantly pertained to pragmatic considerations, comments (66/172) also pertained to psychological reasons, that is, reasons associated with influencing learning. The major themes for performing demonstrations in influencing learning included modelling of practical skills prior to students proceeding on to experiments or investigations (14), focusing attention on the demonstrator and what the demonstrator seeks to demonstrate (21), and how demonstrations allow for easy transition to explanations, especially at the molecular level (11). Three comments are presented to elucidate this thinking, especially in teachers' thinking associated with linking the macroscopic experience to conceptual understanding of chemistry phenomena.Even though the reaction of alkali [metals] is something we are not encouraged to do [with our students] I still see the need to demonstrate the difference in reactivity [between lithium, sodium and potassium]. They can't work with them directly but seeing the reactivity is so important [and explaining later why this is]. At one time [early in my career] they would have done it in a lab [experiment/closed investigation] but now it's just best to demonstrate.
When I perform a demonstration it is easy to transition from the macroscopic [to the other tetrahedron dimensions]. I can have a simulation ready to go [for the sub-microscopic] and once students have made the observation, making clear the understanding of why it has occurred is easy. If it was a lab (experiment) I wouldn't be able to control the learning process because they would be all over the place [different steps in the experiment sequence].
In a demonstration you can get students to focus on a particular detail that becomes the focus for the learning. I am not convinced that they would otherwise. It's like that in experiments they can do it and miss seeing the point [in their observations].
A few teachers (7) voiced their concerns about the utility of the demonstration because of the abstract nature of the explanations that often followed the demonstration.
There are some really good demonstrations. But I have become a lot more aware of what comes after. Often the explanation that follows is just too abstract. Would I still do the demo? Yes. But it's what comes after I'm more concerned with.
Apparent in these responses is an awareness of the role the macroscopic experience can have and should have in assisting students in their learning. As Taber asserts (2013), the demonstration allows the students' learning to be scaffolded with specific attention to ‘cues’ that are essential in making connections between the macroscopic level and the formal theoretical levels at the submicroscopic and symbolic levels.
Associated with this psychological category was reference from teachers (7) of the role of the demonstration in terms of engaging students cognitively.
Students look forward to demonstrations. But over time I have become much more sensitive to how they should be used. I just used to use them as novelty but now when I do use them they have a purpose. I seem to focus on manipulating the demonstration to make them think of what outcome there might be if I change something. I wait for the answers. They know now that if there's a demonstration I'll be asking them to think about what's going on.
They get that if there's a demo, I'm likely trying to confuse them. If I have them thinking about it, then they'll be in the right from of mind to learn.
Again, in both responses is teacher awareness of the role the macroscopic experience can have and should have in assisting students in their learning. Creating engagement and disequilibration (Piaget, 1971) as an antecedent for learning become the reason for the macroscopic experience through demonstration.
Finally, some teachers (6) mentioned that the development of procedural and manipulative skills is limited through demonstrations.
I often think if I am doing a demonstration I would be better off doing a lab. If I think they can develop some skills I'll consider doing a lab instead. They have their use but really just to demonstrate an idea – to get a point across.
C. Philosophical reasons for demonstrations
A final broad theme arising from respondents (26/172) pertained to the authenticity of demonstrations in the larger consideration of the scientific inquiry process. Several (20) mentioned that students during demonstrations are primarily cast in the role of observers rather than active investigators.Demonstrations are fine to get them engaged or to set the tone for further learning, but they are a bit contrived. Where in science [outside of the teaching of science] is there demonstrating? I'd much rather have students engaged in their own learning rather than me just entertaining them.
I don't like demonstrations simply because I want students to direct the process. I find when I am in the role of demonstrator, it becomes too teacher-directed and that's not what I encourage in my teaching.
Although there was perceived inadequacy of demonstrations for promoting scientific inquiry and student engagement as investigators, there was awareness by some (6) that the demonstration could provide the foundation for investigation if the demonstration was structured in such a manner to allow such opportunity.
I'll use them to get students thinking [during the demonstration] and then it provides the foundation for something more [like an experiment or investigation]. I model how to do something and then leave it hanging so they now can explore something themselves. I like that reason for demonstrations but other than that it simplifies what the scientific [inquiry] experience is really like.
Although this final comment gave indication that teachers' thinking is also influenced by philosophical reasons associated with their view of the nature of scientific inquiry, this reasoning is considerably less evident than psychological and, especially, pragmatic reasons.
Explanations for experiments
Similar to the themes outlined in the previous section, teachers' thinking about why they had their students participate in experiments gave evidence of pragmatic, psychological and philosophical considerations.A. Psychological reasons for experiments
The most frequent reason (84/147) for engaging students in experiments (closed-investigations, mini-labs) were associated with learning and were categorised as psychological reasons. These reasons were distributed across five main ideas. Two main ideas pertained to the significance of experiments in supporting development of conceptual understanding. These included that experiments helped to elucidate key ideas (14) and can be structured to engage students in multi-level thinking (24). Teachers often made reference to the importance of experimental work as a foundational macroscopic experience for developing conceptual understanding at the more theoretical sub-microscopic and symbolic levels. Several made explicit reference to teaching chemistry with a multi-dimensional orientation in which the macroscopic experience in experiments was, as Taber suggests (2013), an essential cue in making connections between the macroscopic level and the formal theoretical levels at the submicroscopic and symbolic levels during the experiment.The important reference in the professional development was on structuring lessons around the tetrahedron. The labs I structure and we were exposed to always have reference to these [four] dimensions and experiments provide them with than foundational experience that they can use as a group to then go on to consider the other dimensions. We might start with a human element context idea, but then we'll branch into other aspects. They are challenged to think on these levels [during the experiment] and I think it just gives it all better meaning in a real time efficient manner. I might need to stop the lab and interject to get a point across but overall, they seem to navigate [the dimensions] quite easily.
They know that in the experiment they can't just muck around with things. The [macroscopic part of the experiment] is just part of the whole. In the past [many] students would pack up their books [and minds] when the [macroscopic] experimental work ended, but now it's all a part of a whole and the macroscopic does not consume the whole time. It's just part of the learning.
Students know the lab experience is just a part of the package. I emphasise that. They get that. But, it has to be made clear – all the time. The lab will not just be about the macro[scopic]. They'll have to consider the other [dimensions] as well. They'll be required to think on each level.
Further, many (21) comments pertained to the utility of experiments for supporting learning of manipulative and procedural skills deemed necessary, especially for further study.
I give them every opportunity to develop their competency in skills and processes they'll need at university. If I feel they have mastered a particular skill, I'll extend it further by adjusting the experiment.
Several made comment of concerns that they had with experiments, even though they indicated preference for this type of practical work. Several teachers (15) were aware of the caution to assume that students would come to understanding of the phenomena through the process or that critical elements of the experiment may be missed.
We are regularly doing labs, but it is with caution. I can't assume they learn just by the doing [as they are working through the lab]. I don't leave the learning to chance.
Demonstrations have the advantage you can focus on a specific observation. In experiments you just have less control of what they should be observing.
Further, seven teachers mentioned students can be more focused on the manipulation of equipment and procedural skills and task completion rather than being actively engaged in understanding the conceptual purpose of the experiment.
Too often the time is spent just working on the task – what they need to do procedurally – and fail to give attention to the focus of the learning. I often remind them not to get side-tracked by just doing it.
B. Pragmatic reasons for experiments
Many of the comments (41/147) made pragmatic comments associated with economy of resources and time as well as management considerations.Experiments need not be long. I prefer mini-labs where the focus is clear. It gives them that concrete experience that then supports their learning of the more abstract ideas.
If I have a choice, it will always be an experiment [over an investigation and demonstration]. They just have lots of merits. If you're setting up for a demo, you might as well set up for a lab. The time requirements aren't greatly different in terms of preparing, especially if you're mixing chemicals. They are easy to manage. We're all on the same page.
As well, teachers made reference to the ease of execution of experiments, especially relative to investigations.
They [are] much easier to enact in comparison to investigations. Just the gear and the guidance you need to provide in investigations. There just is more control of the process but again it's not as controlled as a demo[nstration].
You can interrupt labs to clarify a point of view [to the class as a whole]. Just to ensure they make the connection between the macro and what causes the macro. They have to understand that what they see happening is caused by something at the macro level. I interrupt their experimental work just to ensure they make these connections.
C. Philosophical reasons for experiments
Teachers (22/167) also made reference to experiments based on philosophical authenticity of experiments, especially in terms of the investigative process that might be encouraged if experiments were constructed to allow open-endedness.It's what science is inclined to be like. It might be a bit contrived but you are asking them to make connections. They are observing, recording, manipulating but also thinking about why things are happening. I try to ensure that whatever experiment it is they just don't go through the motions.
I always anticipate that there should be questions coming through as they do the labs. If they aren't asking about possibilities [variable adjustments] I think they are being too mechanical. Experiments provide that possibility [for extension and transition into investigation].
In all, teachers' comments indicated psychological, pragmatic, and philosophical considerations for experimenting. Prominent within the comments was that experiments were an efficient and effective means of exposing students to the macroscopic experience needed to be a component of the ‘multi-level’ learning experience. Because of the structured nature of experiments, teachers perceived this ‘multi-level’ experience was not difficult to execute and, consequently, privileged experiments over demonstrations and investigations.
Insights into investigations
Similar to the themes outlined in the previous sections, teachers' thinking about why they tended to encourage (or more commonly discourage) investigations as a practical work type gave evidence of pragmatic, psychological and philosophical considerations.A. Psychological reasons for experiments
Teachers reasoning for the use of investigations (91/198) were again primarily associated with psychological reasons.There is really nothing better than seeing students engage with an investigation and then begin to reason as to why something might happen or something does happen. If something doesn't work out as they expect, it is great to see them consider why this might be.
When we do experiments, it tends to just confirm what we have already been exposed to. Investigations just seem to open up more opportunities for them to think deeply and consider options rather than being bound by what is expected.
In making reference to investigations and despite the positive comments previously made, teachers' comments were typically critical of their merit. Although teachers recognized the merit of investigating in terms of being the type of practical work most similar to scientific inquiry (34/198) their questioning of their merit was challenged according to pragmatic and psychological reasons.
I realize investigating is at the heart of scientific inquiry, but they are not time efficient. I think the effort and time that goes into investigating does not warrant their use. I might do one a year, but that's only for assessment purposes. They tend to know what is expected [as a result of the investigation] and typically aren't that motivated to carry it out. It's a lot of time, a lot of equipment. They're all at different stages, some done earlier than others. Not worth it.
Investigations are arguably as close as we can get to the way scientists work. But I don't give them much room in chemistry. I do in Grade 9 and 10, but not in chemistry. We're driven more by conceptual understanding rather than the doing, so they just don't fit in as well with the overall focus [priority].
A dominant message (34/198) in the reasons for not choosing investigations was teachers concern about students being focused on the procedural aspects of the macroscopic dimension (for example observing, manipulating, and recording).
When they carry out an investigation their thinking is primarily procedural. That's where they invest their time. Expecting them to then consider why things are happening in the investigation is another layer of thinking. That's why I am more apt to do experiments. They invest time on the procedural aspects and then can get down to the more abstract ideas as to why things have occurred. If there's too much going on, it becomes unnecessarily complicated.
As well, teachers raised concern (16/198) about how it is difficult to direct students “multi-level’ thought during investigations.
In experiments, they can be easily structured to get students to move between the levels. The lab will be structured to make them move from macro to micro to symbolic to contextual. Investigations aren't that efficient. They get caught up for too long on the macro and even then it really isn't macro – just planning and organizing – then the class is finished. I can see merit but not with the structure of the way we're encouraging students to engage with chemistry – too much emphasis is just on one level.
B. Pragmatic reasons for investigations
Many concerns associated with the use of investigations were primarily pragmatic (96/198). Corresponding to the comments made about demonstrations teachers made comments about the relative difficulty of time (29), execution (18), risk (11), expenditure (9), and resource availability (7); management (9); and concerns associated with students' manipulative and procedural skills (9).I know their value, but that has to be placed against the tremendous time demand they place unless they just alter a simple variable after a lab [experiment]. I find them much more difficult to manage. You end up being like a technician just addressing the equipment issues.
Valuable, yes. There are increased risks and I have to monitor what they explore. I don't get the sense that they are as valuable for the learning [of ideas]. Maybe they become more confident skill-wise but, overall, I use them sparely [sic].
C. Philosophical reasons for investigations
Despite the criticisms associated with investigations, it is not surprised that teachers (11/198) saw the merits in students investigating, especially in terms of the authenticity of the experience they provided.When we do the Rates of Reaction unit, they plan and carry out a rates investigation. Students don't always respond as well to the open-endedness but they do appreciate that investigating is likely as close to the heart of chemistry as you can get.
In all, teachers' reasons for the use of investigations (albeit in our study's case mainly the relative lack of use) were largely a ‘heterogeneous’ view informed by, primarily, pragmatic and psychological reasons. Although investigations were seen to be philosophically more synonymous with authentic science, teachers were not convinced of their overall benefit because of their perceived limitations due to pragmatic and psychological reasons. At the heart of the psychological reasoning was that investigations could not ensure that students were focused on the conceptual focus of the investigation primarily because students were preoccupied procedurally.
Teachers' thinking about practical work – a summary
This study has focused on examining teachers' thinking in regards to the macroscopic experiences they provide their students. It has explored teachers' thinking about practical work, especially in regards to the types of practical work they privilege in their teaching of chemistry to support students in their learning. Specifically it has focused on understanding why teachers involved in this five-year project frequently used experiments and, to a lesser degree, demonstrations, but rarely engaged their students in investigations.The various themes identified through this inquiry were represented in Table 3. In the table, the dominant themes – pragmatic, psychological, philosophical – are listed as headings and give examples of teachers' considerations under these themes, albeit that some of these considerations may not be evidenced in the quotations listed in this article. Although some of these inclusions' accuracy might be challenged by the chemistry education community, the comments do illustrate the thinking of teachers associated with the selection. The table is a valuable representation of three cohorts of chemistry teachers' collective thinking and likely provides a foundation for thoughtful consideration for current and future chemistry educators, especially in giving critical consideration to assumed practices that “unthinkingly see hands-on practical work as the universal panacea, the educational solution to all learning problems” (Hodson, 1990, p. 33). It is the author's understanding that no such representation exists, especially in being grounded in teachers' thinking about practical work within the context of chemistry.
Teachers comments suggest that of the three forms of practical work illustrated in the study and the professional development, experiments are the ‘preferred’ type of practical work. The tendency towards increased use of experiments, as opposed to demonstrations and especially investigations, is based upon a composite of mainly psychological and pragmatic reasons, as well as, to a lesser extent, philosophical matters. Overall, the number, breadth and informed nature of the responses suggest that teachers are critical and have moved beyond practical work as ‘tradition’ and ‘convenience’ (Millar, 2002). The study described challenges the view that practical work and, especially the type of practical work selected, is unthinkingly selected. In contrast, teachers deliberate over unstated assumptions and values about each type of practical work, despite any explicit attempt in the professional development to prioritize one type of practical work over another. Such reasoned thinking indicated by deliberation and consideration is the essence of thinking critically (Ennis, 2002).
Although it cannot be claimed that teachers’ thinking is a product of their involvement in a sustained and focused professional development initiative that is premised upon a chemistry curriculum advocating for a tetrahedral or triplet learning experience, it is evident that teachers’ thinking is, overall, informed significantly by this construct. As identified, the tetrahedral construct surfaced in the justification for the majority of comments pertaining to reasons for selected use. Thus, when teachers are selecting a type of practical work, their awareness of the ‘triplet’ or ‘tetrahedron’ is evident as a theoretical foundation in informing their selection. It was important to the author that the overall message of the professional development initiative was demonstrated in teachers’ thinking, especially in terms of how students needed to be seeing the practical work experience as a part of the multi-level thinking being encouraged by the curriculum and its tetrahedral orientation in an effort to assist students in learning. Although Johnstone cautions chemistry educators to be considerate of asking students to think about very different types of things at once, teachers repeatedly showed evidence of how Mahaffy’s tetrahedral pedagogical framework (2006) and, especially, Johnstone's triplet (1991) is informing their practice. Constantly evidenced in the teachers' responses was the need for the practical work experience to be seen as a foundational macroscopic experience that communicated with the submicroscopic and symbolic theoretical levels and, to a lesser extent, the human element dimension. Teachers comments show their awareness of Taber’s assertion (2013) that the macroscopic experience allows the students' learning to be scaffolded with specific attention to ‘cues’ that are essential in making connections between the macroscopic level and the formal theoretical levels at the submicroscopic and symbolic levels.
Further, attention is drawn to Mahaffy's (2006) comment that chemistry educators need to move beyond the ‘triplet’ that he asserts focuses chemistry education solely on conceptual understanding and content acquisition. It would appear that teachers in this study have moved beyond the triplet to the tetrahedral but, still, place their emphasis on content acquisition and conceptual understanding. The role of practical work and the choice of practical work is predominantly influenced by teachers’ thinking about what type of practical experience – whether it be a demonstration, experiment or investigation – most efficiently and effectively provides for the multi-dimensional exposure proposed to influence chemistry learning. The tetrahedron may provide students with a broader chemistry education experience by exploring the ‘human element’, but the overall imperative of the tetrahedral or triplet experience remains primarily focused on conceptual learning rather than the development of scientific inquiry and investigative competency, a tension commonly acknowledged in science education (Gott and Duggan, 2007).
It is important to reiterate that the study is conducted at the end of a five-year professional development initiative associated with the implementation of a new curriculum initiative advocating for a ‘tetrahedral’ orientation to the teaching of chemistry (Mahaffy, 2006) emphasizing the ‘practical’ experience as one of four essential dimensions of the chemistry experience – that being the ‘macroscopic’, tangible, evidential experience – provided for students to foster student learning. The author believes that the informed stance that the thinking articulated is unlikely to be consistent with chemistry teachers who have had little exposure to the ‘triplet’ or ‘tetrahedron’ and the’ multilevel thought’ these models encourage, thus asserting that if teachers fail to be exposed to the nature of chemistry (Johnstone, 2000) which “exists in three forms … where [n]o form is superior to another, but each one complements the other.” It is apparent from this study that the very nature of chemistry is an imperative construct for informing pre-service and in-service chemistry teacher education, but that prolonged exposure to the ‘triplet’ or ‘tetrahedron’ is likely necessary to bring about a critical awareness for informing chemistry pedagogy in general and practical work specifically. The implications of this are significant. If teachers are going to critically think about what type of practical work they privilege in the macroscopic, then a pedagogical framework for informing such decisions is necessary. Teachers' reasoning in this study would suggest that the ‘triplet’ and ‘tetrahedron’ provide the framework for, as Ennis (2002) suggests, such reasoned consideration.
Further, although this study does not include any reference to how students responded to their teachers’ teaching and their selected practical work types, the outcomes of the larger professional development initiative suggest that the diversification of teaching practice advocated by the tetrahedral or triplet approach requires substantial changes to both teachers’ chemistry ‘teachering’ and chemistry students ‘studenting’ (Mason and McFeetors, 2007). Although students commonly experience practical work in chemistry, the integration of the macroscopic experience into the ‘triplet’ and ‘tetrahedron’ advocates for a chemistry learning experience that challenges the orthodoxy of students being predominantly focused on the most abstract theoretical level, the symbolic. This becomes particularly contentious when students are not only thinking on each of these levels but also communicating and, more significantly, being assessed on all four levels showing they can “jump freely from level to level in a series of mental [and communication] gymnastics” (Johnstone, 1982; Johnstone, 1991, 2000).
References
- Abrahams I. and Reiss M. J., (2012), Practical work: its effectiveness in primary and secondary schools in England, J. Res. Sci. Teach., 49(8), 1035–1055 .
- Bunce D. and Gabel D., (2002), Differential effects on the achievement of males and females of teaching the particulate nature of chemistry, J. Res. Sci. Teach., 39(10), 911–927 .
- Burmesiter M., Rauch F. and Eilks I., (2012), Education for sustainable development (ESD) and chemistry education, Chem. Educ. Res. Prac., 13, 59–68 .
- Dudu W. and Vhurmuku E., (2011), Teachers' practices of inquiry when teaching investigations: a case study, J. Sci. Teach. Educ., 23, 579–600 .
- Eggleston J. F., Galton M. J. and Jones M., (1975), A Science Teaching Observation Schedule Schools Council Research Series, London: Macmillan .
- Ennis R., (2002), A Super-Streamlined Conception of Critical Thinking, http://faculty.education.illinois.edu/rhennis/SSConcCTApr3.html, retrieved August 18, 2013 .
- Fullan M., (1992), Successful school improvement, Buckingham: Open University Press .
- Gabel D., (1999), Improving teaching and learning through chemistry education research, J. Chem. Educ., 76, 548–553 .
- Gilbert J. K., (2005), Visualization: a metacognitive skill in science and science education, in Gilbert J. K., (ed.), Visualization in science education, Dordrecht: Springer, pp. 9–27 .
- Gilbert J. K. and Treagust D. F., (2008), Reforming the teaching and learning of the macro/submicro/symbolic representational relationship in chemical education, in Ralle B. and Eilks I. (ed.), Promoting successful science learning – The worth of science education research, Aachen: Shaker, pp. 99–110 .
- Gott R. and Duggan S., (2007), A framework for practical work in science and scientific literacy through argumentation, Res. Sci. Technol. Educ., 25(3), 271–291 .
- Hacker R. G., (1984), A typology of approaches to science teaching in schools, Eur. J. Sci. Educ., 6, 153–167 .
- Hegarty-Hazel E., (1990), Tertiary science classrooms, in E. Hegarty-Hazel (ed.), The student laboratory and the science curriculum, London: Routledge, pp. 357–382 .
- Hodson D., (1990), A critical look at practical work in school science, Sch. Sci. Rev., 70(256), 33–40 .
- Hoffmann R. and Laszlo P., (1991), Representation in chemistry, Angew. Chem., Int. Ed. Engl., 30, 12–24 .
- Johnstone A. H., (1982), Macro- and micro-chemistry, Sch. Sci. Rev., 64(227), 377–379 .
- Johnstone A. H., (1991), Why is chemistry difficult to learn? Things are seldom what they see, J. Comput. Assist. Lear., 7, 701–710 .
- Johnstone A. H., (2000), Teaching of chemistry – logical or psychological? Chem. Educ. Res. Pract., 1(1), 9–15 .
- Kind P. M., Kind V., Hofstein A. and Wilson J., (2011), Peer argumentation in the schools science laboratory – exploring effects of task features, Int. J. Sci. Educ., 33(18), 2527–2558 .
- Kirschner P. and Meester M., (1988), Laboratory approaches, High. Educ., 17, 81–98 .
- Lewthwaite, B. E., (2008), Towards treating chemistry teacher candidates as human, Res. Sci. Educ., 38(3) 343–363 .
- Lewthwaite B. E. and Wiebe R., (2011), Fostering teacher development towards a tetrahedral orientation in the teaching of chemistry, Res. Sci. Educ., 40(11), 667–689 .
- Lewthwaite B. E. and Wiebe R., (2012), Fostering the development of chemistry teacher candidates: a bioecological approach, Canadian Journal of Mathematics, Science and Technology Education, 12(1), pp. 36–61 .
- Mahaffy P., (2006), Moving chemistry education into the 3D: A tetrahedral metaphor for understanding chemistry, J. Chem. Educ., 83(1), 49–55 .
- Manitoba Education Citizenship and Youth (2006), Grade 11 Chemistry: A framework for implementation, Winnipeg: Manitoba Education, Training and Youth .
- Manitoba Education Citizenship and Youth (2007), Grade 12 Chemistry: A Framework for Implementation, Winnipeg: Manitoba Education, Training and Youth .
- Mason R. T. and McFeetors P. J., (2007), Student trajectories in high school mathematics: Issues of choice, support, and identity-making, Canadian Journal of Science, Mathematics and Technology Education, 7(4), 291–316 .
- Millar R., (2002), Thinking about practical work, in Amos S. and Booka R., (ed.), Aspects of teaching secondary science: perspectives on practice, London: Routledge .
- Millar R., (2010), Analysing practical science activities, Hatfield: Association for Science Education .
- Millar R., Le Maréchal J.-F. and Tiberghien A., (1998), A map of the variety of labwork. Labwork in Science Education (LSE) Project, Working paper 1, European Commission TSER ProgrammeProject PL95-2005.
- Millar R., Tiberghien A. and Le Marechal J.-F., (2002), Teaching and learning in the science laboratory, in Psillos D. and Niedderer H. (ed.), Dordrecht: Kluwer Academic Publishers, pp. 9–20 (science technology education library) .
- Murray H. G., (1999), Low-inference teaching behaviors and college teaching effectiveness: recent developments and controversies, in Smart J. C. (ed.), Higher education: handbook of theory and research, New York: Agathon Press, vol. 15, pp. 323–345 .
- Osborne J., (1998), Science education without a laboratory? in Wellington J. (ed.), Practical work in school science. Which way now? London: Routledge, pp. 156–171 .
- Osborne R. J. and Wittrock M. C., (1985), The generative learning model and its implications for science learning, Stud. Sci. Educ., 12, 59–87 .
- Piaget J., (1971), Psychology and epistemology: towards a theory of knowledge, Middlesex, UK: Penguin .
- Taber K. S., (2010), Straw men and false dichotomies: overcoming philosophical confusion in chemistry education, J. Chem. Educ., 87(5), 552–558 .
- Taber K. S., (2013), Revisiting the chemistry triplet: drawing upon the nature of chemical knowledge and the psychology of learning to inform chemistry education, Chem. Educ. Res. Pract., 14(2), 156–168 .
- Toplis R., (2012), Students' views about secondary school science lessons: the role of practical work, Res. Sci. Educ., 42(3), 531–549 .
- Vhurmuku E., (2011), High school chemistry students' scientific epistemologies and perceptions of laboratory inquiry, Chem. Educ. Res. Pract., 12, 47–56 .
- Wellington J., (1998), Practical work in science. Time for a reappraisal, in Wellington J. (ed.), Practical work in science: Which way now? London: Routledge, pp. 3–15 .
- White R. and Gunstone R., (1992), Probing understanding, London: Falmer Press .
- Woolnough B. and Allsop T., (1985), Practical work in science, Cambridge: Cambridge University Press .
| This journal is © The Royal Society of Chemistry 2014 |