Fluorogenic displacement biosensors for PSA detection using antibody-functionalised quantum dot nanoparticles†
Nunzianda Frascione*a,
James Goocha,
Vincenzo Abbateb and
Barbara Daniela
aDepartment of Forensic and Analytical Science, King's College London, Franklin Wilkins Building, 150 Stamford Street, London, SE1 9NH, UK. E-mail: Nunzianda.Frascione@kcl.ac.uk; Tel: +44 (0)2078484978
bInstitute of Pharmaceutical Science, King's College London, Franklin Wilkins Building, 150 Stamford Street, London, SE1 9NH, UK
First published on 18th December 2014
Abstract
Novel quantum dot conjugated immunosensors are presented for the accurate identification of seminal fluid in forensic casework. The production of a fluorescence signal upon the displacement of moderately bound quencher-labelled peptide analogues by Prostate Specific Antigen (PSA) overcomes many of the practical disadvantages associated with traditional lateral flow cartridge testing.
Accurately identifying the presence of human semen is an integral part of forensic sexual assault casework, providing both investigative information and possible association between a suspect and an offence via DNA profiling. Testing typically consists of a two-stage process in which potential fluid deposits are presumptively indicated or excluded at the scene of a crime prior to a secondary laboratory-based confirmation required for evidential use.1 The hydrolysis of α-napthyl phosphate within Brentamine reagent by prostate-secreted acid phosphatase is widely used as an initial colorimetric semen screening assay but suffers from the inability to visually locate discrete fluid deposits upon a large evidential surface.1 The autofluorescence of intra-seminal molecules upon excitation by Polilight or Wood's lamp lighting techniques may improve localisation but often generates false positive and negative results due to its complex interpretation.2 Our research group recently utilised a fluorogenic peptide substrate labelled with 4-amino-7-trifluoromethylcoumarin to overcome these challenges, with fluorescence production upon substrate cleavage allowing the simultaneous localisation and identification of seminal deposits in situ.3
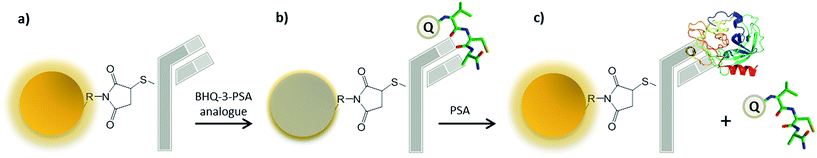
Despite the substrate demonstrating both high fluid specificity and sensitivity, subsequent laboratory confirmation is still likely to be required after reagent application. This is currently achieved through either the microscopic observation of sperm cells4 or the detection of Prostate Specific Antigen (PSA), a semen-specific protein secreted by the prostate, by immuno-chromatographic testing strips.5 However, several limitations to both of these methods have also been recognised. Microscopic examination cannot be applied to samples from suspected azoospermic or vasectomised males in which sperm cells may be absent. In such cases, PSA has been demonstrated to be a valid alternative marker6,7 and it may be detected by immunological lateral flow assay, such as the SERATEC® PSA Semiquant or ABAcard® p30 systems. These kits utilize the migration of mobile complexes formed by PSA and dye-linked antibodies to generate colorimetric signals within a testing zone. However, oversaturation of the device by PSA may prevent complex binding, leading to weak or false negative results. These ‘high-dose hook effects’ are likely to occur at PSA concentrations above 0.1–0.5 mg mL−1.8,9 Consequently, the identification of undiluted seminal fluid samples, which have a recorded average PSA concentration of 0.82 mg mL−1,10 may be difficult.5 Furthermore, the 200 μL of semen required for analysis cannot be recovered following absorption to the testing strip, potentially sacrificing a precious amount of available genetic material. Biosensors have gained considerable attention in forensic analysis,11 finding application in the detection of illicit drugs,12 fingerprints13 and explosives.14 Furthermore, the identification of human blood by fluorescent sensors specific to erythrocyte membrane protein Glycophorin A has already been demonstrated.15 However, whilst these sensors were able to signify Glycophorin interaction through emission quenching, an ‘off–on’ fluorescence system would likely result in a superior analyte presence confirmation. This study therefore documents the development of a turn-on sensor for the detection of seminal fluid. Firstly, quantum dot nanoparticles (QDots) are conjugated to PSA-specific antibodies to form an initially fluorescent biological recognition complex. Constructs are then temporarily quenched by the moderate binding of an acceptor labelled peptide analogue to PSA, which is brought within the 10 nm distance required for the absorbance of the QDot emission via Förster resonance energy transfer (FRET). In the presence of seminal fluid, peptides are displaced by higher affinity antigen native protein, relieving FRET transfer and restoring fluorescence (Fig. 1). Other platforms based on FRET are available for forensic use. For example, fluorogenic substrates, which produce a fluorescent signal upon cleavage by the target molecule, can be used to detect specific body fluid enzymes hence allowing their identification. Such an approach can, however, only be applied to biological molecules that display enzymatic activity. Conversely, the Ab-based approach presented here benefits from a higher level of flexibility as antibody can be virtually raised against any target of interest. Moreover, their well known specificity represents a considerable advantage especially within a forensic context.
The selection of an appropriate analogue ligand that is able to undergo indefinite antibody binding until preferential displacement in the presence of a target molecule may be considered the most challenging aspect of sensor construction. We envisage that due to a lack of secondary folding structure, synthetic peptide sequences based upon the isolated amino acids responsible for protein–antibody binding are likely to display a lower binding affinity than their protein counterparts and may be used as ideal moderately bound ligands for the attachment of fluorescence acceptors. Furthermore the small length of peptides sequences ensures that donor and acceptor molecules are brought within a suitable distance for efficient FRET quenching to occur. Both antibody selection and ligand construction was therefore based on research conducted by Piironen et al.16 in which the antigenic epitopes of PSA for 25 monoclonal antibodies were determined. Antibody 8301 (Abcam, UK), labelled in Piironen as E86, was selected for sensor use after demonstrating considerable specificity and binding affinity for free PSA. This antibody was further shown to interact exclusively with amino acids 158–164 of PSA (H–DVCAQV–NH2), which were subsequently chosen to constitute our peptide ligand. H–DVCAQV–NH2 peptide was synthesised using solid-phase peptide synthesis and following removal from the solid support it was purified using semi-preparative RP-HPLC and finally characterised by ESI-MS (ESI Fig. S2†).
Differences in interaction affinity between antibody 8301 with native PSA protein and synthetic peptide were monitored through Biacore surface plasmon resonance. Small response unit increases observed upon 40 nM peptide injection indicated the successful binding of synthetic peptide H–DVCAQV–NH2 to immobilized anti-PSA antibodies, whilst PSA injection showed much larger interaction, requiring 3 injections of 10 mM glycine–HCL (pH 1.5) to remove bound PSA. This reflects the significantly large affinity constant of 1 × 1011 L mol−1 given by the antibody manufacturer. A second peptide (H–DVSAQV–NH2), in which the cysteine residue was replaced by a serine, was additionally synthesised. Such AA substitution was chosen as the two residues are known to be structurally similar. This was to anticipate the possibility of peptide dimerization by exposed thiol disulphide bridge formation which could affect the assay performance.
However, with dimerization of the initial sequence remaining unobserved, small decreases in binding efficiency exhibited by sequence H–DVSAQV–NH2 excluded its use in final sensor constructs. Peptide H–DVCAQV–NH2 was subsequently conjugated to Black Hole Quencher-3 (BHQ-3, Biosearch Technologies, USA) according to a method reported by Stefflova et al.17 to form a displaceable quenching ligand (Fig. 2b), which was analysed via MALDI-TOF MS (see ESI Fig. S2†). QDots emitting at 625 nm were also chosen for sensor use after demonstrating greater spectral overlap with BHQ-3 absorbance compared to their 605 nm counterparts (Fig. 2a), which is likely to result in a more efficient FRET transfer process. Anti-PSA antibody 8301 was conjugated to amine functionalized 625 quantum dots using a sulfhydryl-reactive QDot Antibody conjugation kit (Invitrogen, Paisley, UK), allowing the site-specific labelling of hinge region thiol groups. This was chosen over more traditional EDC chemistry to avoid potential interference often associated with non-specific amine labelling of the antibody binding region. Successful conjugation was confirmed using agarose gel electrophoresis (see ESI Fig. S3†).
The ability of this sensor construct to indicate PSA presence as a displacement assay was then tested via spectrofluorimetry (see ESI S4†).
In a preliminary optimization study, different dilutions of Ab–QDot conjugates were tested to determine the minimum concentration needed to generate a recordable signal suitable for the assay. A 10 nM conjugate solution was to be the optimum operating condition for the analysis. Subsequently, variable concentrations of BHQ-labelled peptide were incubated with 10 nM of Ab–QDot conjugates for 1 hour, with ligand binding demonstrated through increased quenching of QDot emission. The subsequent addition of 160 nM PSA to quenched conjugates resulted in immediate restorations of Ab–QDot fluorescence, comparable to pre-incubation emission intensities (Fig. 3), indicating the successful displacement of peptide ligands and establishing the potential of this sensor as a viable assay for confirming semen presence. Analysis (Wilcoxon matched pairs test) was performed to compare the datasets from pre- and post-incubation across all the concentrations used and the results showed statistically significant differences (p-value 0.018). The PSA solution employed in the analysis corresponds to a dilution of about forty times the concentration of PSA found in human semen; this provides a preliminary indication of the sensitivity of the assay and of its potential applicability to forensic samples.
 | ||
| Fig. 3 Fluorescence of Ab–QDot conjugates incubated with quencher-labelled peptide before (■) and after (●) PSA addition. | ||
Whilst being the most abundant fluid within sexual assault casework, seminal fluid is encountered less often within violent crime, where depositions of blood and saliva are more frequent. The interchangeable nature of quantum dots and characterised fluid antibodies may allow for the detection of other fluid targets by this sensor to be realised. This would likely depend not only elucidating a number amino acid sequences attributed to antibody binding in forensically relevant proteins for use as other moderately bound ligands, but may require a number of sensing optimisations to maximise assay performance. With FRET quenching occurring over an ideal distance of 1–10 nm, larger peptide ligand use will lead to some reduction in energy transfer efficiency. Resolution to this issue may arise from conjugating only portions of the antibody, namely the Fab′ binding regions, in order to decrease donor–acceptor distance. Ideal application is likely to involve the exploitation of several different Ab–QDot complexes, each with a separate emission wavelength for the simultaneous detection of several analytes in a multiplex fluid sensing system.
Whilst we have already established that the application of fluorogenic substrates to biological fluids samples has no effect on downstream genetic profiling processes,3 it is important to confirm this is the case for every assay construction. Therefore Ab–QDot complexes were applied in solution to a biological fluid source before undergoing standard forensic DNA extraction, quantification, amplification and electrophoresis processes (ESI S5†). A full genetic profile, which was found to be indistinguishable from an identically processed reference sample, was obtained.
The work presented here focussed on specific aspects surrounding the design and development of the new method. To this end, the promising results obtained in this primary developmental phase would justify further work. Validation studies would encompass a comprehensive evaluation of cross-reactivity, limit of detection, variability, comparison with other available tests as well as the inclusion of casework samples; aspects that are all part of any forensic evaluation.
Displaying both an immediate and specific response to PSA presence, this biosensor construct may have the potential to accurately confirm semen presence within a matter of seconds and with no expense to genetic material. Serious thought should therefore be given to the development of biosensor assays as replacements to current confirmatory testing techniques.
Acknowledgements
The authors would like to thank the Metropolitan Police Service Directorate of Forensic Services for providing technical and financial assistance throughout the project.Notes and references
- K. Virkler and I. K. Lednev, Forensic Sci. Int., 2009, 188, 1–17 CrossRef CAS PubMed.
- H. J. Kobus, E. Silenieks and J. Scharnberg, J. Forensic Sci., 2002, 47, 819–823 Search PubMed.
- J. Gooch, B. Daniel and N. Frascione, Talanta, 2014, 125, 210–214 CrossRef CAS PubMed.
- B. C. M. Pang and B. K. K. Cheung, Forensic Sci. Int., 2007, 169, 27–31 CrossRef CAS PubMed.
- M. N. Hochmeister, B. Budowle, O. Rudin, C. Gehrig, U. Borer, M. Thali and R. Dirnhofer, J. Forensic Sci., 1999, 44, 1057–1060 CAS.
- I. Sato, M. Sagi, A. Ishiwari, H. Nishijima, E. Ito and T. Mukai, Forensic Sci. Int., 2002, 127, 71–74 CrossRef CAS.
- J. P. Simich, S. L. Morris, R. L. Klick and K. Rittenhouse-Diakun, J. Forensic Sci., 1999, 44, 1229–1231 CAS.
- M. M. Hobbs, M. J. Steiner, K. D. Rich, M. F. Gallo, L. Warner and M. Macaluso, Contraception, 2010, 82, 291–295 CrossRef PubMed.
- M. Yokota, T. Mitani, H. Tsujita, T. Kobayashi, T. Higuchi, A. Akane and M. Nasu, Leg. Med., 2001, 3, 171–176 CrossRef CAS.
- J. Lovgren, C. Valtonen-Andre, K. Marsal, H. Lilja and A. Lundwall, J. Androl., 1999, 20, 348–355 CAS.
- N. Frascione, J. Gooch and B. Daniel, The Analyst, 2013, 138, 7279–7288 RSC.
- J. P. Hilton, T. H. Nguyen, R. Pei, M. Stojanovic and Q. Lin, Sens. Actuators, A, 2011, 166, 241–246 CrossRef CAS PubMed.
- M. Wood, P. Maynard, X. Spindler, C. Lennard and C. Roux, Angew. Chem., Int. Ed., 2012, 51, 12272–12274 CrossRef CAS PubMed.
- S. Singh, J. Hazard. Mater., 2007, 144, 15–28 CrossRef CAS PubMed.
- N. Frascione, V. Pinto and B. Daniel, Anal. Bioanal. Chem., 2012, 404, 23–28 CrossRef CAS PubMed.
- T. Piironen, B. O. Villoutreix, C. Becker, K. Hollingsworth, M. Vihinen, D. Bridon, X. Qiu, J. Rapp, B. Dowell, T. Lovgren, K. Pettersson and H. Lilja, Protein science : a publication of the Protein Society, 1998, 7, 259–269 CrossRef CAS PubMed.
- K. Stefflova, J. Chen, H. Li and G. Zheng, Mol. Imaging, 2006, 5, 520–532 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra14066d |
| This journal is © The Royal Society of Chemistry 2015 |