Bark depolymerization during submerged fermentation using monofloral honey, a natural mediator substitute, and integration between laccases vs. bark biopolymers, characterized by Py-GC-MS
M. Ferhan*a,
N. Yanab and
M. Sainabc
aCentre for Biocomposites and Biomaterials Processing (CBBP), Faculty of Forestry, University of Toronto, 33 Willcocks Street, Toronto, ON M5S 3B3, Canada. E-mail: muhammad.ferhan@utoronto.ca; Fax: +1 416 978 3834; Tel: +1 416 946 3122
bDepartment of Chemical Engineering and Applied Chemistry, University of Toronto, 200 College Street, Toronto, ON M5S 3E5, Canada
cKing Abdulaziz University (KAU), Jeddah, Saudi Arabia
First published on 12th December 2014
Abstract
Due to increasing waste production and disposal problems arising from synthetic polymer production, there is a critical need to substitute materials with biodegradable and renewable resources. Attempts to use laccases as a catalyst to enhance the catalytic properties of enzymes have shown them to be a promising solution for bark depolymerization. In this study, eight different fungal strains were tested for laccase enzyme production during submerged fermentation (SF), and the Pleurotus species were shown to be the best producers among the competing fungal strains. P. pulmonarius mainly produces laccase enzyme in production medium (PM) at initial conditions of pH 5.5 and 30 °C. Bark depolymerization was conducted in SF and we identified polyphenols/polyaromatic compounds after four weeks when the PM was induced with 50 mg per 100 mL of each bark during the lag-phase. During SF where honey was used as a natural mediator substitute (NMS) in the PM, laccase activities were about 1.5 times higher than those found in comparable cultures without honey in the PM. These samples were analyzed by GC-MS/MS. The laccase enzyme was purified using UNO® sphere Q-1 anion exchange chromatography and the molecular weight was determined to be ∼50 kDa on 10% SDS-PAGE. The laccase kinetic parameters Vmax, Km, and turnover number (Kcat) were found to be 76.9 μM min−1, 909 μM and 739 min−1, respectively, from a Lineweaver–Burk plot. Furthermore, laccases are suitable for biotechnological applications that transform bark biomass into high value bark biopolymers/biochemicals. The differences observed among the identified aromatic compound MS/MS profiles were due to the utilization of two different bark species. Py-GC-MS analysis of bark showed differing effects of fungal activity on bark composition. Polyphenolics were separated in reverse-phase mode using HPLC with a pinnacle DB Biphenyl, C18 column, and UV detector. Two recognition wavelengths of 290 and 340 nm were selected to improve the separation of each single compound in monofloral honey and bark-fermented samples. This study is novel because it replaces natural mediators (NM) with monofloral honey in PM and bark materials impregnated with honey, and studies the effects of fungi-derived laccases on bark biopolymers.
1. Introduction
The biobased economy relies on sustainable resources, and is likely to have an enormous impact comparable to the fossil-based economy. The biobased economy is not about simply utilizing renewable resources and applying them to cutting-edge innovations; its principles can be used to modify procedures with far-reaching effects on society.1 Nations have set managed focuses to replace certain fuel and chemical commodities with biomass to help the biobased economy. Woody biomass provides lignocellulosic feedstock for the energy sector and chemical industry. Because of its inexhaustible accessibility, useful chemical composition, and reasonably low costs, woody biomass can improve products economically and sustainably. Global annual lignocellulosic biomass production was reported by Zhang2 to be about 200 bnt. Haveren et al.3 found that 0.3 bnt is differentiated to synthetic chemicals every year.In Europe and North America, P. pulmonarius is the most cultivated fungal species,4 and the most commonly found throughout the world, particularly in temperate and subtropical forests. This species is frequently found on hardwoods in eastern North American forests; however, it is also found on conifers in the western United States.5 The cultivation method, which is comparable to that of other Pleurotus species, is by spreading spores to grain and then dispersing the seeds to cellulosic biomass as substrate, like straw, coffee grounds, wood chips, sawdust and cardboard.
Pleurotus is considered an effective lignin degrader that can grow fairly fast on different types of lignocellulosic biomass. Laccase (EC 1.10.3.2) belongs to the multicopper oxidase family. Fungal growth conditions, media composition and cultivation method play an important role in laccase production.6 Laccase production in many fungi had certain effects on alteration of diverse polyphenolic compounds containing lignin and humic substances.7
The composition of honey, a natural bee product, depends mainly on the botanical source, topographical origin, and dispensing and atmospheric conditions. Due to similar antioxidant contents of many fruits and vegetables, it functions as a natural food antioxidant.8–10 In honey, the main antioxidant compounds are polyphenolics, flavonoids, enzymes (catalase, glucose oxidase), organic acids, ascorbic acid, carotenoid-like substances, amino acids and proteins. The antioxidant value varies significantly depending on the floral source.11 Several phenolic compounds including syringic acid (SA) and methyl syringate (MS) are found in honey. Recently, a compound was found having substantial antibacterial activity against Staphylococcus aureus.12
Mixed balsam fir (Abies balsamea) is monoecious and considered a valuable conifer from the boreal forest. It is mainly used in pulp and in light frame construction, and as a food source and as shelter for wildlife (http://www.borealforest.org/).
Mixed aspen (Populus tremuloides) is one of the fundamental source species and is considered to be essential for managing biodiversity in the western and boreal zones in North America.13 So as to better comprehend the fungal interaction and bark depolymerization, it is vital to investigate and think about the impacts of polyaromatics/polyphenolics reflected as natural mediators. Polyaromatics are hydrocarbons containing mainly C and H with multiple aromatic rings in which the electrons are delocalized. They are primarily found in fossil fuels (oil, coal and tar deposits) and produced due to partial burning of organic matter.
Laccase alone does not polymerize, nor depolymerize or delignify pulp until it consolidates with a mediator like hydroxybenzotriazole (HBT). Poppius et al.14 reported that the maximum degree of delignification up to 40% in pulp was examined using HBT as mediator. The laccases possessing high redox-potential in basidiomycetes from genus Trametes assists with lignin removal when it is joined with HBT from complete15 and preserved16 lignocellulosic biomass, producing cellulose available to hydrolysis. Generally examined mediators are synthetic compounds based on nitrogen heterocycles. Because of high toxicity and price, it is hard to realize laccase-mediator systems (LMS) on an industrial scale.
The phenolic compounds as redox mediator's oxidation is a typical mechanism using laccases to enhance, envision and switch enzymatic lignin-based biotransformation routes. The enzymatic oxidation of syringyl-type phenolics identified as natural mediators, i.e. methyl syringate (MS), acetosyringone (AS) and syringaldehyde (SA), directs to phenolics oxidation contingent on negatively charged residues similar to a substrate binding site of the enzyme.
Natural mediators in the presence of laccase facilitate the oxidation of non-phenolics but it depends on the phenolic compound structure, as well as the reactivity beside stability of the phenoxy radicals produced (MS˙ > AS˙ > SA˙) reported by Tania et al.17 Due to high multiplicity and the variable structure and composition in woody biomass, researchers are trying to profile radical-coupling routes that are involved in the formation of different phenolic species and identified as mediators. Its structure and properties biochemically, phenotypically and through the exploration of its molecular properties depend on the capacity of plant cell walls to resist deconstruction because of fungal laccases.18
Molecular properties of bark/biomass combustion (BC) can be assessed by pyrolysis-gas chromatography-mass spectrometry (Py-GC-MS).19–22 Heat-affected reactions and the resulting changes produce structures that may be like the pyrolysis artifacts of BC.23,24 Py-GC-MS is considered as a quick and inexpensive method for the bark characterization. The hydrolyzable bonds are cleaved and, subsequently, CO2H and OH groups are changed in situ amid by Py-GC-MS directly to the related methyl esters and methyl ethers, respectively, which are more acquiescent to GC than their underivatized complements.25,26 It also provides supplementary data on structure over position of derivatized multifunctional rings.26
There is need to focus on bark depolymerization mechanisms and molecular changes as a function of carbonizing temperature aside from recognizing the effect of honey in production media where it can mainly be utilized as a replacement for natural mediators.
2. Experimental methods
2.1. Materials
All chemicals were purchased from Sigma-Aldrich. Laccase Novozym®51003 (E.C.1.10.3.2) from Myceliophthora thermophila (1000 LAMU per g = 3.57 IU mL−1 min) was obtained from Novozymes (Franklinton, NC, USA). Mixed balsam fir and aspen barks were supplied by FP Innovations, (Vancouver, BC, Canada) and milled to particle size less than 0.212 mm (US70 mesh size). All chemicals used in this study were reagent-grade and consumed without further purification.2.2. Methods
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm and the supernatant was used for laccase assays.
000 rpm and the supernatant was used for laccase assays.The fluid flow in the system was incrementally changed to Buffer B (25 mM Tris–HCl pH 8.1 + 0.5 M NaCl) to elute the proteins captured in the column. At this phase, the laccase activity resembled a peak of absorbance monitored at 280 nm and eluted as a single peak. Fractions were observed at 280 nm using ChromLab™ software. The FPLC system (Bio-Rad, USA) comprised a Biologic Duoflow pump system, a BioFrac fraction collector, and a UV detector. The purified and concentrated enzyme was preserved at −20 °C and did not show any significant loss of enzymatic activity over several months. The molecular weight of laccase enzyme was determined by running 10% SDS-PAGE as previously described by Höfer.30 The protein gel was stained with Coomassie brilliant blue R-250 and commercially available prestained protein ladder (Fermentas, USA) was used as a standard.
2.3. Characterization techniques
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm. The supernatant was filtered through a 0.45 μm filter and analyzed by HPLC.
000 rpm. The supernatant was filtered through a 0.45 μm filter and analyzed by HPLC.The fermented samples were induced with 50 mg of each bark sample during the lag-phase and polyphenolics were determined spectrophotometrically and characterized based on their reported retention time (tr) values. Chromatographic separation was performed with gradient elution and the following steps were required:35 70% eluent A + 30% eluent B by an isocratic elution for 0–15 min, 60% eluent A + 40% eluent B by a linear increase for 16–20 min, 55% eluent A + 45% eluent B by a linear increase for 21–30 min, 40% eluent A + 60% eluent B by a linear increase for 31–50 min, 20% eluent A + 80% eluent B by a linear increase for 51–52 min, 10% eluent A + 90% eluent B by a linear increase for 52–60 min, 10% eluent A + 90% eluent B by an isocratic elution for 61–63 min, 70% eluent A + 30% eluent B by a linear increase for 64–73 min and lastly 70% eluent A + 30% eluent B by an isocratic elution for 74–75 min.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 15 min) to detach cell biomass. Supernatants were acidified to pH 2.5–3 with concentrated HCl and then completely separated with three volumes of CH3COOC2H5. An organic layer was collected and moisture was removed over anhydrous Na2SO4. The samples were then filtered and dried overnight in an oven kept at 50 °C. Using the Lundquist method,37 the ethyl acetate extracts residues were examined as trimethyl silyl (TMS) derivatives. Dioxane and pyridine (10
000 rpm for 15 min) to detach cell biomass. Supernatants were acidified to pH 2.5–3 with concentrated HCl and then completely separated with three volumes of CH3COOC2H5. An organic layer was collected and moisture was removed over anhydrous Na2SO4. The samples were then filtered and dried overnight in an oven kept at 50 °C. Using the Lundquist method,37 the ethyl acetate extracts residues were examined as trimethyl silyl (TMS) derivatives. Dioxane and pyridine (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) was added to the samples, which were then by silylated with 5 mL of the silylation derivatizing reagent trimethylchlorosilane (TMCS). To dissolve residual particles, the mixture was heated to 60 °C for 15 min with regular shaking.
1 v/v) was added to the samples, which were then by silylated with 5 mL of the silylation derivatizing reagent trimethylchlorosilane (TMCS). To dissolve residual particles, the mixture was heated to 60 °C for 15 min with regular shaking.An aliquot of 1 mL of silylated compound was injected into a Saturn 2100T GC-MS/MS (Varian, Inc., USA) equipped with Varian 3900 GC oven and Saturn® 200 MS workstation software. A PE-5MS capillary column (20 m × 0.18 mm i.d; 0.18 mm film thickness) was used and helium was the carrier gas with a flow rate of 1 mL min−1. Column temperature was maintained at 50 °C for 5 min, and then at 50–300 °C (10 °C min−1, hold time for 5 min). 3 min was chosen for solvent delay. The exchange line and particle source temperatures were sustained at 200 and 250 °C.
In full-examine mode, electron ionization (EI) mass spectra within the scope of 30–550 (m/z) were recorded at an electron energy of 70 eV.38 Characterization of lignin-related low molecular weight (LMW) compounds, which are isolated from fungal treatment, was interpreted by comparing their retention time (tr) with an existing database of the original compounds.
The GC instrument was fixed with non-polar 5% phenyl, 95% dimethylpolysiloxane (HP-5MS) column (30 m × 0.25 mm internal diameter; film thickness 0.25 μm) and helium was used as a carrier gas (flow rate 1 mL min−1). The GC oven remained heated from 50 to 325 °C (held for 5 min) at 20 °C min−1. The transfer line of the GC-MS was maintained at 325 °C. The ion source (electron impact mode, 70 eV) of the 5975 MSD (Agilent Technologies, Palo Alto, USA) was controlled at 230 °C and scanning of quadrupole detector at 150 °C, a range between m/z 50 and 550. Relative proportions of the pyrolysis products were estimated from their peak areas, built on one or two characteristic or major fragment ions. The aggregate of peak areas (total quantified peak area, TQPA) was fixed 100%.
3. Results and discussion
3.1. Extracellular laccases and screening of potential fungal strain
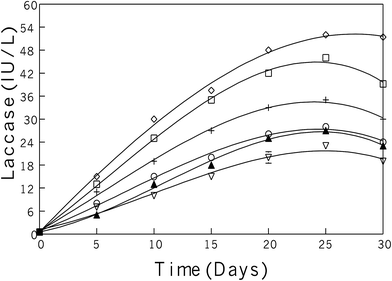
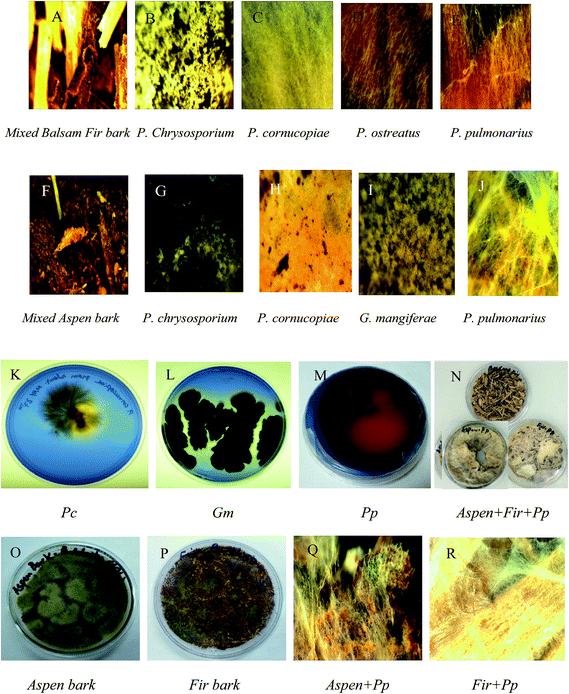
Extracellular laccase was produced during submerged growth conditions and screening of the best fungal strain was done based on the laccase production and growth conditions. These experiments were conducted both in solid as well as in liquid media. Among other competitor fungal strains, the P. pulmonarius was screened for further examination due to it possessing a strong laccase activity, which was determined on the malt extract agar media plates (malt extract 25 g; agar 20 g; distilled water 1 L, and 0.05% of the dye Remazol Brilliant Blue R), and wrapped with aluminum foil to keep out light and put in the incubator at 30 °C for two weeks. Ligninolytic activity was assessed on agar plates by observing decolorizing of polyaromatic anthraquinone dye and scoring attainable halos surrounding the fungus-growing colonies as shown in Fig. 2(K–M).After the agar plate prescreening, all fungal strains were transferred into the production media and fungal growth was observed during submerged fermentation. Each bark contained mixed aspen and balsam fir (50 mg per 100 mL of each bark) and was induced during the lag-phase, means start to produce laccase activity, which appears after five days, when transfer inoculum into the production medium (PM). Maximum laccase activity was recorded after 25 days in submerged fermentation as 52 U in P. pulmonarius, 46 U in P. cornucopiae, 35 U in P. ostreatus, 28 U in P. chrysosporium, 27 U in T. versicolor, and 23 U in G. mangiferae, as shown in Fig. 1.
Bark polyphenolics might possibly seem to be lignin-like, polymeric substances with carbohydrates, celluloses, hemi-celluloses, and polyoses (non-cellulose).41,42 By utilizing fungal degrading enzymes, the polyphenolic–carbohydrate complex compounds changed into smaller and more attainable moieties. As lignin retains high antioxidant activity,43,44 therefore, a hypothetical decrease in size of lignin-like, polyphenolics degraded through fungus is able to increased soluble bark phenolic contents. Instead of metallic ions, fungal activates molecular oxygen which involved in the phenolic moieties might reduce to antioxidant activity of bark polyphenols.45–47
It was observed that fungal growth increased through the mid (10–25 days) to late (20–30 days) phases, with improved soluble phenolic contents. P. pulmonarius had the maximum virtual laccase activity among other investigated fungal strains. In general, the phenolic compounds oxidized easily due to phenoxy radical formation as compared with non-phenolic compounds.
The increased phenolic concentration and oxygen availability assists polymerization. The degrading rate of lignin, via a feedback control mechanism of laccases with polyphenolics, directs an enzyme into a latent catalytic state.48 That is, the enzyme activity stars to decline after a certain time period owing to feedback inhibition due to accumulation of secondary metabolites and toxic compounds to a certain level. Morphological characterization of different fungal growth patterns on each bark was observed using an AmScope-WF25X/9 (magnification 0.5×) equipped with a 150 W cold-light source haloid lamp (Fig. 2).
3.2. Laccase purification and kinetics
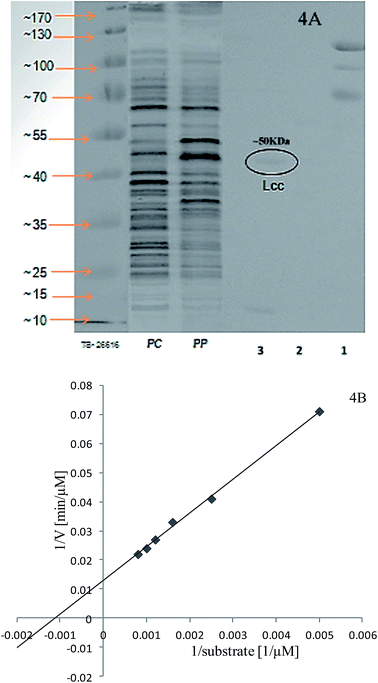
The extracellular laccases from P. pulmonarius cultivated under SF were purified almost to homogeneity level. The purification method has already been described in the methodology section. The sequential purification steps are shown in Fig. 3, and its purification to visible on 10% SDS-PAGE stained with Coomassie Blue R-250 (Fig. 4A). With the help of a Lineweaver–Burk plot, the Km and Vmax values of laccase from Pleurotus pulmonaris were obtained as 909 μM and 76.9 μM min−1, respectively (Fig. 4B). The turnover number (Kcat) and specificity constant (Kcat/Km) were 739 min−1 and 0.81 μM−1 min−1 respectively. As compared to literature, the similar specific constant of laccase enzyme was investigated from P. ostreatus. As a result of high catalytic efficiency and binding affinity with substrate (ABTS), we were able to compare with other laccase producing microorganisms.49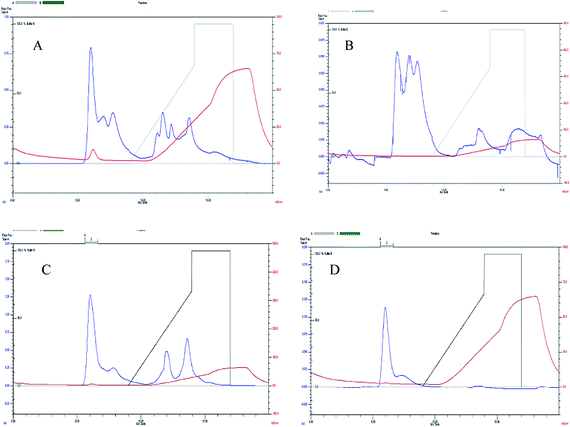 | ||
| Fig. 3 Laccase purification using FPLC by UNO® sphere Q-1 anion exchange column where (A–D) representing sequential purification steps. | ||
3.3. Bark decomposition by TG/DTG
Thermal degradation of bark samples was estimated using TGA. All experiments were done under nitrogen atmosphere. Both control and fungal treated bark samples were examined (Fig. 5). Cellulose decomposition is subjugated by the main DTG peak, while the shoulder peak mainly to hemicellulose decomposition due to its less stable structure, assigned at lower temperature (around 160 °C)50 while lignin starts to decompose from lower to wide temperature range between 200–400 °C.51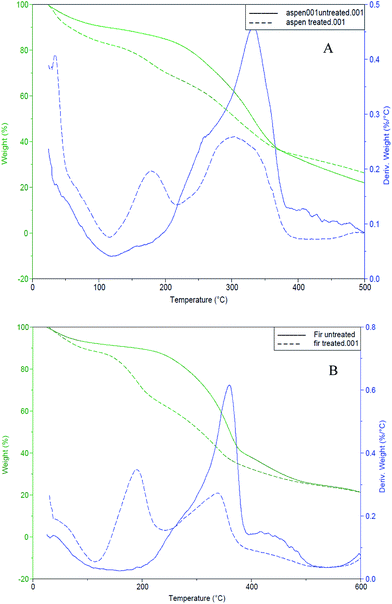 | ||
| Fig. 5 TG/DTG curves for control/untreated (solid-lines) and fungal-treated (dotted-lines) bark samples (A): mixed aspen, (B): mixed balsam fir. | ||
Mixed aspen and fungal treated aspen bark samples started decomposing at about 190 °C and 140 °C, while mixed balsam fir and fungal treated fir bark samples were activated to decay at about 200 °C and 170 °C, respectively. The solid lines in both TG/DTG thermograms shown in Fig. 5 indicate control or untreated barks whereas the dotted lines indicate the fungal-treated bark samples. The weight loss of fungal degraded bark samples was faster between 140–400 °C.52
3.4. Mass spectrometry (GC-MS/MS, MALDI-TOF/MS)
Mainly lower molecular weight compounds, including polyaromatics and polyphenolics, from each fungal-treated bark species were identified and analyzed by GC-MS/MS and MALDI-TOF/MS methods. In this study, GC-MS/MS was used to analyze LMW compounds liberated from fungal decayed bark. The total ion chromatograph (TIC) relating to ethyl acetate extracted compounds from acidified supernatants53 attained from both control and fungal treated bark samples with P. pulmonarius are shown in Fig. 6, and the major identified peak (tr) values are marked (in green) while all others (in black) are summarized in Table 1.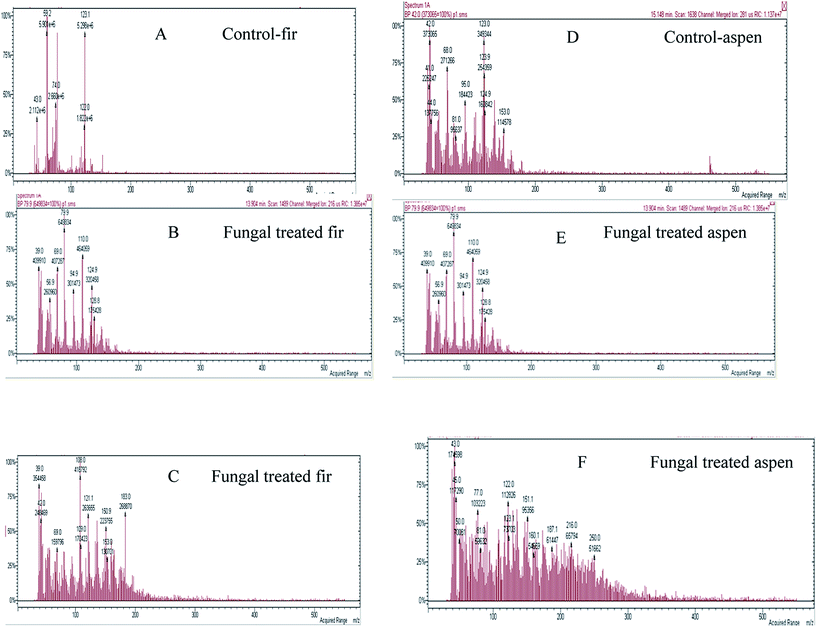 | ||
| Fig. 6 TIC and MS of identified compounds w.r.t. their tr-values characterized from both control and each fungal treated bark species are listed in Table 1: where (A) balsam fir, (B and C) fungal treated fir, while, (D) aspen and (E and F) fungal treated aspen. The fungal treated samples of each bark exhibited a variable number of new peaks due to fungal degradation and changes in chemical composition. | ||
| m/z | Polyaromatics/polyphenols | m/z | Polyaromatics/polyphenols |
|---|---|---|---|
| 39, 39.6 | CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH anion, propyne CH anion, propyne |
124.9 | Guaiacol, 4-methoxy-1-oxide isocyanato- |
| 41.2 | Methyl isocyanide | 127.83 | 2-Propenoic acid, oxiranylmethyl ester, 4-pentenoic acid |
| 42.1 | Propene | 128.8 | Isoquinoline, 2-propanoic acid |
| 43.01, 44 | Iso-cyanic acid | 139.97 | 1-Propanol, 3-phenoxy- |
| 56.9 | CH2COCH3 | 143.84 | 2-Butenedioic acid, dimethyl ester |
| 59 | CH3COO−, glyoxal | 150.9, 151 | Benzaldehyde, benzoic acid |
| 68 | 1,3-Butadiene, 2-methyl- | 128.8 | Isoquinoline, 2-propanoic acid |
| 69 | Vinyl isocyanate | 153 | Vanillin, biphenylene protonated ethyl ester, 3,4-dimethoxy- |
CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCH CHCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHO anion CHO anion |
128.8 | Isoquinoline, 2-propanoic acid | |
| 74 | Methyl propyl ether | 160.1 | Succinic acid, isopropyl-propanedioic acid, ethyl-dimethyl ester, 1,3-propanediol, tert-butyl propanedioic acid |
| 77 | Phenyl radical, isopropyl methyl-d3-ether | 182.1 | Syringaldehyde (SA) |
| 79.9 | 2-Vinyl-1,3-butadiene | 183 | 1,2-Benzenediol |
| 81 | 2-Furanyl-CH2 anion, C6H9 | 187.1 | 2-Ethylhexyl ester |
| 87.9 | 2-Butene-1,4-diol | 196.2 | Acetosyringone (AS) |
| 96 | Furaldehyde | 199 | Propyl ester |
| 97 | Isooxazole, 3,5-dimethyl- | 202.83 | Butanoic acid, valeric acid, trimethyl silyl ester |
| 98 | 1,3-Butadiene-1-carboxylic acid | 212.23 | Methyl syringate (MS), phenylmethyl ester |
| 104 | Propanedioic acid | 216 | Benzoic acid, benzal-barbituric acid, diethyl methyl isopropyl malonate, malonic acid, di-isobutyl ester, glutaric acid, ethyl isobutyl ester |
| 108, 112.88, 113.42 | Cresol, quinone, propanoic acid | 250 | Adipic acid, cinnamic acid, coniferyl aldehyde, trimethyl silyl ester, glyconic acid |
| 110 | Catechol, resorcinol, HQ, 1,2-benzenediol, furan, 2,3,5-trimethyl- | 260.86 | L-Valine |
| 121.1, 121.94 | 3,5-Dimethyl- | 326.82 | Behenic alcohol, isonipecotic acid, N-isobutoxy carbonyl, heptyl ester |
| 122, 123 | Carbamic acid | Weblink | http://webbook.nist.gov/chemistry/mw-ser.html |
The ethyl acetate extracted compounds were ascribed to chemical oxidation of bark because of aeration during microbial fermentation (Fig. 6). The fungal treated chromatographic profiles look different than the control, implying a strong biochemical ability of fungus on bark to alter bark composition. The previous studies have shown that the Paenibacillus sp. has minimal colour reduction as compared to Aneurinibacillus aneurinilyticus and Bacillus sp.54 Apart from an aldehyde and ketone-types, many acid-type complexes were also investigated due to microbial degradation of lignin.55
MALDI-TOF analyses were done in order to determine the molecular weight distribution (MWD) of each bark species treated by P. pulmonarius shown in Fig. 7, and the main peak retention time (tr) values are listed in Table 1 in blue. It was observed that bark mostly comprised LMW compounds with molecular weight less than 275 g mol−1. The identical molecular weight distribution (MWD) pattern was very similar within each bark species that revealing correlated depolymerization reactions which indicate to separate identical compounds.56
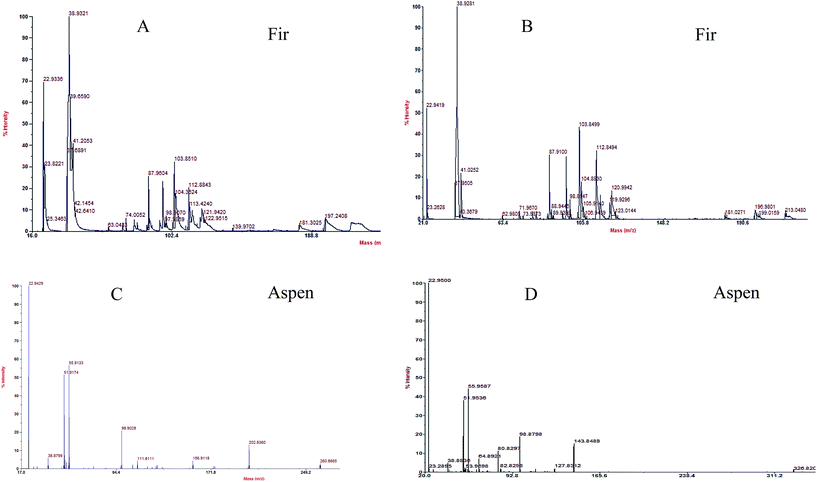 | ||
| Fig. 7 MWD of each bark species treated by P. pulmonarius analyze from MALDI-TOF/MS, and the values are enlisted in Table 1. Chemical changes observed in fugal treated (A and B) balsam fir, and (C and D) aspen bark samples, when subjugated with α-cyano-4-hydroxy cinnamic acid used as matrix compound. | ||
3.5. Honey in PM as natural mediator substitute (NMS)
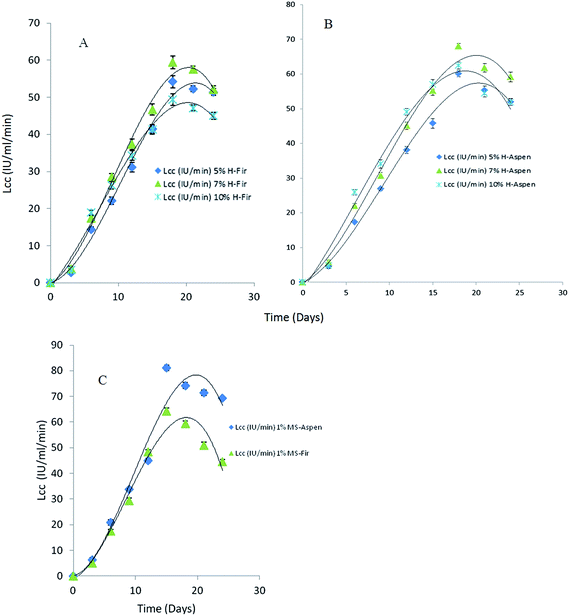
The effects of different honey concentrations on each bark species, extracellular laccase activity, and total polyphenolic contents were determined during a time course study in P. pulmonarius. Extracellular laccase activity by P. pulmonarius in response to different bark species is shown in Fig. 8. A significant increase in the laccase activity was observed in aspen bark compared to fir and methyl syringate used as a natural mediator. The obtained results from fermented aspen bark species are comparable with MS but better than fir samples.In the presence of different applied honey concentrations and after 18 days of incubation, the laccase activity slightly decreased. In our study, we used three different (%, w/v) concentrations of honey but we observed the highest laccase activity 68 IU mL−1 min at 7% (w/v) honey concentration after 18 days of growth (Fig. 8). During fermentation, a higher substrate concentration in the production medium leads to catabolite repression which ultimately affects enzyme productivity yield. In contrast, it has been suggested that laccase production in the presence of phenolic compounds causes toxicity that brings about their oxidation to form quinones which are considered as toxic for the fungal growth.57,58
Furthermore, induction with bark during lag-phase may decrease the extracellular proteolytic activity as well as ligninolytic enzymes including LiP, and MnP; therefore, it may upturn the laccase activity. Thus, we can propose laccase activity can be improved owing to readily available phenolics and aromatic compounds in fungal degraded aspen bark which might help to improve enzyme stability.59
In the presence of ligninases, the oxidation mechanism of synthetic mediators like hydroxybenzotriazole (HBT) is similar to that of phenolic type mediators such as methyl syringate (MS). During oxidation, the highly reactive phenoxy radicals are produced, which assist in removing one proton and one electron from the target substrate.60 The ability of these phenolate ions, alleviate to oxidizing intermediates, which probably control the role of phenol as mediator, which, reorganized and evenly distributed via steric interferences.61 Bark related free radicals and reactive oxygen species (ROS) are associated with laccase and laccase mediator system where honey used as a natural mediator substitute.
3.6. Polyphenolic chromatograms
HPLC detection mainly depends on the measurement of UV-Vis absorption at a specific wavelength. Qualitative identification of each separated analytes precisely analyzed through UV-Vis detector and identify its own characteristic retention time (tr). The performance of separation of analytes improves radically when different polyphenolic compounds are mixed in one sample. At selected suitable wavelength, it enhances separation of all groups with an utmost sensitivity. When the samples have similar compounds, it is hard to detect by any regular method. Most flavonoids comprise phenolic hydroxyl groups having oxidation potential and can be assessed by a UV-Vis method.62–65Romani et al.65 compared electrochemical detection methods with HPLC for polyphenolics in natural extract. The HPLC procedure was found to be more precise than a differential pulse voltammetry method, which was suitable for fast screening.
Polyphenolics separation was conducted in each fermented bark sample containing honey that mainly functioned as a natural mediator. The chromatograms are shown in Fig. 9. Remarkably, it was noticed that the standard methyl syringate (MS) peak appeared at 290 nm but vanished at 340 nm. Similarly, the diluted honey sample had an MS peak at 290 nm, but, when we ran the same sample at 340 nm, the peak was missing and new, different peaks formed. Our HPLC results confirmed that the wavelength at λ = 290 nm was found to be more suitable for the separation of MS natural mediator compounds. All major peaks in the chromatograms were compared and characterized based on their tr values of honey polyphenolic compounds as previously reported by Pyrzynska et al.36
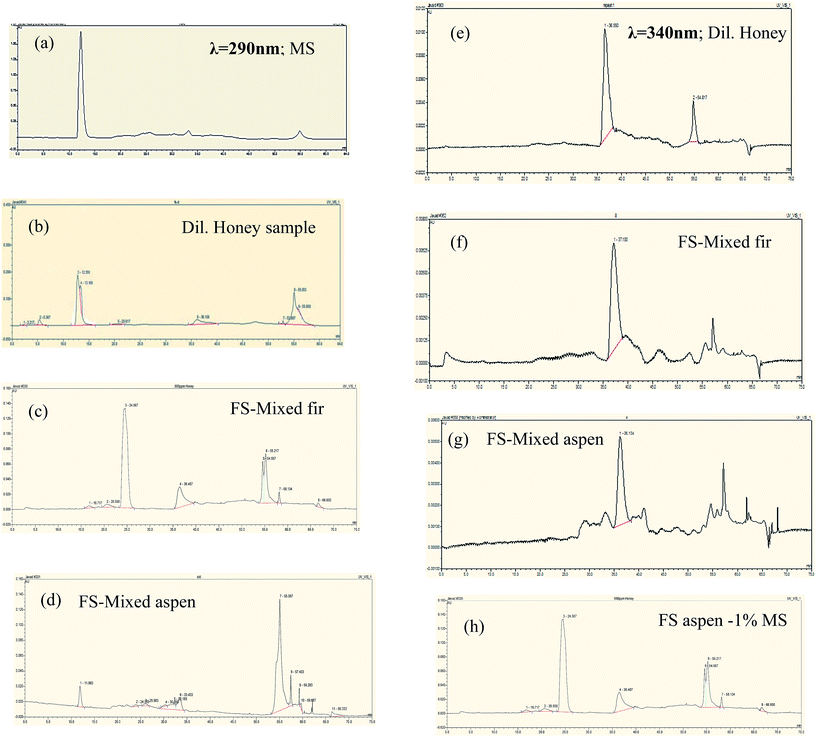 | ||
| Fig. 9 HPLC chromatograms phenolic profiles of bark fermented samples at 7% honey in production media (a–d at λ290), and (e–h at λ340). Peak identification: methyl syringate (12.017), pinobanksin (13.100), 8-methoxykeampferol (24.567), pinocembrin (36.467), chrysin (39.62), pinocembrin 7-Me (55.217), tetochrysin (57.142). All major peaks were characterized based on their tr values of honey polyphenolics was previously reported by Pyrzynska et al.36 | ||
Table 2 presents the total phenolic (TP) content in fermented samples when induced with 50 mg of each bark into 100 mL of PM during the lag-phase. The minor variation observed during data collection is possibly owing to some experimental errors. Total phenolic content (0.114 ± 0.09 mg cat equiv. per g) was found in a buckwheat monofloral honey. Wood degrading fungi plays an important role by attacking protein–polyphenolic complexes which possibly change the substrate properties.66 Polyphenolics, mainly condensed tannin with protein complexes, assist in developing fungal growth.67 During fermentation in PM with honey, it was also noticed that total polyphenols in each bark species were significantly degraded as compared to glucose and natural mediator (MS) samples because of fungal biomass accumulation.
| Sample | Mean total polyphenolics (mg cat equiv. per 100 mL) | |
|---|---|---|
| a Mean ± S.D. (n = 5). | ||
| Aspen | PM-Gluc | 18.4 ± 0.54 |
| PM-H | 23.7 ± 0.61 | |
| PM-MS | 27.8 ± 0.48 | |
| Balsam fir | PM-Gluc | 20.1 ± 0.81 |
| PM-H | 28.8 ± 0.36 | |
| PM-MS | 32.1 ± 0.74 | |
3.7. Py-GC-MS
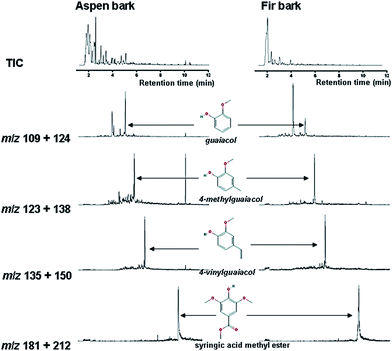
Py-GC-MS total ion chromatograms (TIC) are presented in Fig. 10, while the major peaks in TIC-fingerprints are the pyrolysis products as reported in Table 3 with corresponding retention times (tr), characteristic ion fragments (m/z) utilized for the quantification and their relative proportions. Flags are used to organize the compounds and mainly divided into seven groups, i.e. ALIPH = aliphatic compound, CARB = carbohydrate product, LIG = lignin, MAH = monocyclic aromatic hydrocarbon, NCOMP = nitrogen-containing compound, PHEN = phenol, SESQUI = sesquiterpenoid. Total quantified peak area (TQPA) values of each group are shown in Table 4, and the values of each group were calculated based on Table 3.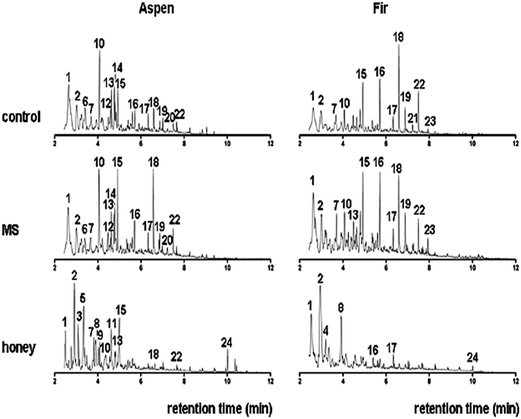 | ||
| Fig. 10 Total ion chromatograms (TIC) of untreated (control) bark samples of aspen and fir, MS-treated and bark fermented samples in honey production medium. Peak labels refer to peak numbers in Table 3. | ||
| Peak no. | Pyrolysis product | Retention time (tr) min | m/z | Flag | Mixed aspen bark | Mixed balsam fir bark | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Control | MS treated samples | Honey treated samples | Control | MS treated samples | Honey treated samples | |||||
| 1 | Toluene | 2.535 | 91, 92 | MAH | 23.2 | 19.7 | 31.4 | 13.0 | 20.2 | 25.1 |
| 2 | 3/2-Furaldehyde | 2.918 | 95, 96 | CARB | 15.0 | 11.0 | 24.9 | 18.5 | 18.1 | 46.9 |
| 3 | C1-Pyridine | 3.107 | 93, 66 | NCOMP | 0.0 | 0.0 | 2.9 | 0.0 | 0.0 | 0.0 |
| 4 | C2-Benzene | 3.107 | 91, 106 | MAH | 7.3 | 5.6 | 2.2 | 3.7 | 6.6 | 5.3 |
| 5 | 2-Furanmethanol | 3.147 | 98, 97 | CARB | 0.0 | 0.0 | 8.0 | 0.0 | 0.0 | 2.0 |
| 6 | Styrene | 3.404 | 104, 78 | MAH | 2.1 | 1.8 | 1.8 | 0.9 | 1.4 | 2.0 |
| 7 | 2,3-Dihydro-5-methylfuran-2-one | 3.896 | 98, 55 | CARB | 6.9 | 7.0 | 3.6 | 11.4 | 10.2 | 1.4 |
| 8 | 5-Methyl-2-furaldehyde | 3.939 | 110, 109 | CARB | 1.8 | 1.0 | 4.5 | 2.5 | 3.1 | 9.0 |
| 9 | 2-Methyl-2-cyclopenten-1-one | 4.091 | 96, 67 | CARB | 0.9 | 0.7 | 2.2 | 8.9 | 1.6 | 0.4 |
| 10 | Phenol | 4.234 | 94, 66 | PHEN | 15.0 | 15.9 | 6.8 | 4.2 | 4.9 | 1.8 |
| 11 | 3-Hydroxy-2-methyl-2-cyclopenten-1-one | 4.486 | 112, 55 | CARB | 2.4 | 3.5 | 3.5 | 3.9 | 4.5 | 0.8 |
| 12 | 2-Hydroxybenzaldehyde | 4.583 | 121, 122 | CARB | 2.3 | 1.1 | 0.3 | 0.2 | 0.6 | 0.2 |
| 13 | 4-Methylphenol | 4.749 | 107, 108 | PHEN | 4.2 | 4.6 | 2.9 | 2.0 | 2.6 | 0.6 |
| 14 | Unidentified aliphatic compound | 4.766 | 57, 70 | ALIPH | 6.5 | 5.7 | 0.5 | 1.4 | 2.6 | 0.2 |
| 15 | Guaiacol | 5.046 | 109, 124 | LIG | 5.0 | 8.7 | 2.4 | 8.4 | 7.9 | 1.3 |
| 16 | 4-Methylguaiacol | 5.784 | 123, 138 | LIG | 1.7 | 2.6 | 0.2 | 6.1 | 5.5 | 0.9 |
| 17 | 4-Ethylguaiacol | 6.380 | 137, 152 | LIG | 0.9 | 1.3 | 0.1 | 1.7 | 1.7 | 1.7 |
| 18 | 4-Vinylguaiacol | 6.643 | 150, 135 | LIG | 2.6 | 6.5 | 0.3 | 8.1 | 4.6 | 0.4 |
| 19 | C3-Guaiacol | 6.912 | 164, 149 | LIG | 0.5 | 0.9 | 0.1 | 1.4 | 1.1 | 0.1 |
| 20 | Alkene | 7.015 | 55, 69 | ALIPH | 0.7 | 0.4 | 0.3 | 0.1 | 0.2 | 0.3 |
| 21 | C3-Guaiacol | 7.244 | 164, 149 | LIG | 0.3 | 0.5 | 0.0 | 0.6 | 0.4 | 0.1 |
| 22 | C3-Guaiacol | 7.530 | 164, 149 | LIG | 0.8 | 1.5 | 0.1 | 2.8 | 1.7 | 0.2 |
| 23 | cf. α-muurolene (sesquiterpenoid) | 7.833 | 105, 204 | SESQUI | 0.0 | 0.0 | 0.0 | 0.1 | 0.3 | 0.0 |
| 24 | Unidentified aliphatic compound | 10.010 | 81, 95 | ALIPH | 0.0 | 0.0 | 1.2 | 0.1 | 0.1 | 0.3 |
| Flag | Aspen (%TQPA) | Fir (%TQPA) | ||||
|---|---|---|---|---|---|---|
| Control | MS | Honey | Control | MS | Honey | |
| ALIPH | 8.1 | 6.2 | 2.0 | 1.6 | 2.9 | 0.8 |
| CARB | 30.2 | 23.2 | 46.7 | 45.2 | 37.5 | 60.5 |
| LIG | 13.2 | 22.0 | 3.2 | 29.0 | 22.9 | 3.7 |
| MAH | 24.5 | 27.0 | 35.4 | 17.7 | 28.2 | 32.3 |
| NCOMP | 0.0 | 0.0 | 2.9 | 0.0 | 0.0 | 0.0 |
| PHEN | 24.0 | 21.6 | 9.9 | 6.4 | 8.1 | 2.6 |
| SESQUI | 0.0 | 0.0 | 0.0 | 0.1 | 0.3 | 0.0 |
The MS-treated bark sample from aspen produced a set of pyrolysis products that are rather similar to that of the control sample, even though there are minor differences such as a smaller relative proportion of phenols and higher proportion of guaiacols, especially guaiacol (15) and 4-vinylguaiacol (18). This might suggest that the material that is primarily affected by fermentation in MS is the phenol precursors while the lignin component is relatively unaffected.
The pyrolysis fingerprint of the aspen bark sample fermented in honey medium was very different from the control and MS-treated analogues. It is dominated by a set of furans, furaldehydes and cyclopentenones (2, 5, 7–9, and 11), which are typical products of polysaccharides.69 Guaiacol represents a lignin fraction that is relatively unaffected, but the intensities of the guaiacols are much lower than in the control and MS-treated samples.
Moreover, several N-containing pyrolysis products such as methyl pyridine (compound 3), in combination with the chitin marker acetamide (intensity too low for representation in Fig. 10, but identified unambiguously), are indicative of a major increase in the amount of microbial biomass in this sample. This makes it very likely that the carbohydrate products do not represent a recalcitrant polysaccharide component but rather that they originate from microbial sugars as well. Despite the small proportion of the lignin products, they can be traced from partial ion chromatograms (PIC) as shown in Fig. 11.
The fir bark material submerged in honey medium produced a very different set of pyrolysis products, dominated by MAHs (1 and 4) and furaldehydes (2 and 8). Some remains of lignin can still be recognized from the total ion chromatograms (TIC), e.g. 4-methylguaiacol (compound 16) and 4-ethylguaiacol (17), and additional compounds can be traced from the partial ion chromatograms (Fig. 11), but it is clear that this fermentation treatment almost completely degraded the original polyphenolic structures and the sample became dominated by microbial biomass. The lignin products are very abundant in control/untreated and MS-treated samples, and they can be identified directly from the TIC (with the peak labels).
There would be no added scientific value from presenting the partial ion chromatograms (PIC) of these compounds. By contrast to the honey-treated samples, they are not very abundant and, therefore, the PIC are useful. Possibly, the polyphenols in honey production medium might be below the detection limit of Py-GC-MS or these polyphenols could have been degraded by pyrolysis, producing aromatics, such as toluene and benzene. Traces of catechol can be detected by the Folin–Denis method but it should be below approximately ∼1 mg per 100 mg of catechol and then it would be below the detection limit of the pyrolysis method.
Both control samples produced typical pyrolysis fingerprints of bark materials consisting primarily of lignin and polysaccharides. The MS-treatment seemed to preferentially degrade a non-lignin component in the aspen bark, causing the enrichment of guaiacol markers from lignin, while the opposite trend was observed for the fir bark fermented in MS medium. This difference may be explained by the differences in original sample composition, with the fir bark being composed almost purely of lignin while the aspen bark sample also contains polyphenolic precursors producing phenols rather that guaiacols and a more abundant aliphatic component. These additional components in fir bark materials are probably more heavily affected by fermentation in MS than the lignin component. Moreover, the effects of fermentation on the pyrolysis fingerprints of both bark samples were rather small.
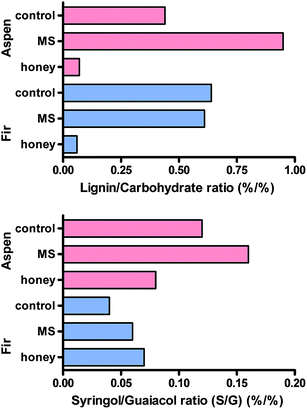
By contrast, fermentation in honey medium eliminated most of the recognisable polyphenols (lignin) and both samples were almost completely converted into microbial tissue, composed mainly of carbohydrates as shown in Fig. 12 – lignin/carbohydrate index. Even though most of the syringol products were found below the detection limit, the syringol was quantified at m/z 154 and 139 at 6.8 min to estimate the relative proportions of guaiacols and syringols using the syringol/guaiacol (S/G) ratio as also shown in Fig. 12. The lignin is strongly dominated by G-type lignin, and it can be concluded even though samples have a small proportion of S-type lignin (i.e. below 0.2 in all samples). MS-treated samples seem to slightly increase this ratio while the honey medium has opposite effects.
4. Conclusions
In the present study, we demonstrated that monofloral honey can be used as a natural mediator substitute in PM during submerged fermentation for the production of fungal laccases. We also found a methyl syringate in the diluted honey sample through HPLC analysis that separated at a wavelength of 290 nm. The time course study showed diverse laccase production among different fungal cultures. Laccase kinetics play an important role in biotechnological development when major constraints reduce catalytic efficiency to an inactive state. Moreover, GC-MS/MS and MALDI-TOF/MS analyses were found to be the most suitable methods to analyze LMW polyaromatics and polyphenolics due to fungal degradation of bark. Methyl syringate is considered to be the most effective natural mediator for the oxidation of non-phenolic lignin units, but our results during submerged fermentation with 7% honey in PM were comparable to the NM. Py-GC-MS of bark fermented samples in a honey medium indicated evidence of a profound effect on biotic degradation to an extent that a large proportion of the pyrolysate can be traced back to microbial biomass. This microbial tissue may be related to the formation of the thermolabile component detected by thermogravimetry. The abundance of phenols and unidentified products combined with the polysaccharide fingerprint of degraded material indicate significant fungal degradation and depolymerisation of bark biopolymers. So, finally, biotechnological procedures have effectively been used for the conversion of bark biomass into high-value bark biopolymers/biochemicals.Acknowledgements
The authors would like to acknowledge the financial support from ORF-RE (Bark Biorefinery Project), industry partners and Universidade de Santiago de Compostela (USC), Spain for access to the Pyrolysis-GC-MS facility and are extremely grateful to Cruz Ferro-Vazquez from (Incipit-CSIC) and Joeri Kaal for his time, technical assistance and help in data acquisition.References
- H. Langeveld, J. Sanders and M. Meeusen, The Biobased Economy: Biofuels, Materials and Chemicals in the Past-oil Era, ed. J. W. A. Langeveld, Earthscan Publications, UK, 2010 Search PubMed.
- Y. H. P. Zhang, J. Ind. Microbiol. Biotechnol., 2008, 35, 367 CrossRef CAS PubMed.
- J. V. Haveren, E. L. Scott and J. P. M. Sanders, Biofuels, Bioprod. Biorefin., 2008, 2, 41 CrossRef.
- Pleurotus-Fungal Guide, Pleurotus pulmonarius, Landcare Research, New Zealand, retrieved 26 January 2012, ed. P. Kummer, 1871, Der Führer in die Pilzkunde, C. Luppe Search PubMed.
- P. Stamets, Growth Parameters for Gourmet and Medicinal Mushroom Species, Growing gourmet and medicinal mushrooms, Ten Speed Press, Berkeley, California, USA, 3rd edn, 2000, ch. 21, pp. 316–320, ISBN 978-1-58008-175-7 Search PubMed.
- I. Y. Lee, K. H. Jung, C. H. Lee and Y. H. Park, Biotechnol. Lett., 1999, 21, 965 CrossRef CAS.
- S. S. Desai and C. Nityanand, Asian J. Biotechnol., 2011, 3, 98 CrossRef CAS.
- J. M. Alvarez-Suarez, S. Tulipani, S. Romandini, E. Bertoli and M. Battino, Med. J. Nutrition Metab., 2010, 3, 15 CrossRef.
- L. Chen, A. Mehta, M. Berenbaum, A. R. Zangerl and N. J. Engeseth, J. Agric. Food Chem., 2000, 48, 4997 CrossRef CAS PubMed.
- L. Vela, C. De Lorenzo and R. A. Perez, J. Sci. Food Agric., 2007, 87, 1069 CrossRef CAS.
- N. Gheldof, X. Wang and N. J. Engeseth, J. Agric. Food Chem., 2002, 50, 5870 CrossRef CAS PubMed.
- J. M. Alvarez-Suarez, S. Tulipani, D. Dıaz, Y. Estevez, S. Romandini, F. Giampieri, E. Damiani, P. Astolfi, S. Bompadre and M. Battino, Food Chem. Toxicol., 2010, 48, 2490 CrossRef CAS PubMed.
- E. H. Hogg, J. P. Brandt and B. Kochtubajda, Can. J. For. Res., 2005, 35, 610 CrossRef.
- K. Poppius-Levlin, W. Wang, T. Tamminen, B. Hortling, L. Viikari and M. L. Niku-Paavola, J. Pulp Pap. Sci., 1999, 25, 90 CAS.
- A. Gutiérrez, J. Rencoret, E. M. Cadena, A. Rico, D. Barth, J. C. del Río and A. T. Martínez, Bioresour. Technol., 2012, 119, 114 CrossRef PubMed.
- Q. Chen, M. N. Marshall, S. M. Geib, M. Tien and T. L. Richard, Bioresour. Technol., 2012, 117, 186 CrossRef CAS PubMed.
- R. Tania, B. Pedro, K. Kamila, V. C. Ana, M. Paula-Robalo and O. M. Lígia, Bioresour. Technol., 2012, 124, 371 CrossRef PubMed.
- M. Foston and A. J. Ragauskas, Ind. Biotechnol., 2012, 8, 191 CrossRef CAS.
- J. M. De la Rosa, H. Knicker, E. López-Capel, D. A. C. Manning, J. A. González-Pérez and F. J. González-Vila, Soil Sci. Soc. Am. J., 2008, 72, 258 CrossRef CAS.
- J. Kaal and C. Rumpel, Org. Geochem., 2009, 40, 1179 CrossRef CAS.
- J. Kaal, A. Martínez Cortizas and K. G. J. Nierop, J. Anal. Appl. Pyrolysis, 2009, 85, 408 CrossRef CAS.
- D. Fabbri, C. Torri and K. A. Spokas, J. Anal. Appl. Pyrolysis, 2012, 93, 77 CrossRef CAS.
- C. Saiz-Jiménez, Naturwissenschaften, 1994, 81, 451 Search PubMed.
- T. P. Wampler, J. Chromatogr. A, 1999, 842, 207 CrossRef CAS PubMed.
- P. G. Hatcher, K. J. Dria, S. Kim and S. W. Frazier, Soil Sci., 2001, 166, 770 CrossRef CAS.
- F. Shadkami and R. Helleur, J. Anal. Appl. Pyrolysis, 2010, 89, 2 CrossRef CAS.
- M. Ferhan, A. L. Leao, S. I. de Melo, N. Yan and M. Sain, Ferment. Technol., 2012, 1–106, DOI:10.4172/2167-7972.1000106.
- K. M. G. Machado and D. R. Matheus, Braz. J. Microbiol., 2006, 37, 468 CrossRef CAS.
- M. M. Bradford, Anal. Biochem., 1976, 72, 248 CrossRef CAS PubMed.
- C. Höfer and D. Schlosser, FEBS Lett., 1999, 451, 186 CrossRef.
- L. Kaichang, X. Feng and L. E. Karl-Erik, Appl. Environ. Microbiol., 1999, 65, 2654 Search PubMed.
- A. Mishra and S. Kumar, Biochem. Eng. J., 2009, 46, 252 CrossRef CAS.
- M. Ferhan, N. Tanguy, N. Yan and M. Sain, ACS Sustainable Chem. Eng., 2014, 2, 165 CrossRef CAS.
- Y.-P. Tan, Master's thesis, Louisiana State University, 2011.
- J. Lachman, A. Hejtmankova, J. Sykora, J. Karban, M. Orsak and B. Rygerova, Czech J. Food Sci., 2010, 28, 412 CAS.
- K. Pyrzynska and M. Biesaga, Trends Anal. Chem., 2009, 28, 893 CrossRef CAS.
- K. Lundquist and T. K. Kirk, Acta Chem. Scand., 1971, 25, 889 CrossRef CAS PubMed.
- A. Raj, M. M. Krishna Reddy and R. Chandra, Int. Biodeterior. Biodegrad., 2007, 59, 292 CrossRef CAS.
- J. Kaal, A. M. Cortizas and K. G. J. Nierop, J. Anal. Appl. Pyrolysis, 2009, 85, 408 CrossRef CAS.
- E. Eckmeier, M. Rösch, O. Ehrmann, M. W. I. Schmidt, W. Schier and R. Gerlach, Holocene, 2007, 17, 539 CrossRef.
- M. A. Bernards and F. A. Razem, Phytochemicals, 2001, 57, 1115 CrossRef CAS.
- G. Henriksson, G. Johansson and G. Pettersson, J. Biotechnol., 2000, 78, 93 CrossRef CAS PubMed.
- J. M. Cruz, J. M. Dominguez, H. Dominguez and J. C. Parajo, J. Agric. Food Chem., 2001, 49, 2459 CrossRef CAS PubMed.
- T. Oki, M. Masuda, M. Kobayashi, Y. Nishiba, S. Furuta, I. Suda and T. Sato, J. Agric. Food Chem., 2002, 50, 7524 CrossRef CAS PubMed.
- R. Cohen, L. Persky and Y. Hadar, Appl. Microbiol. Biotechnol., 2002, 58, 582 CrossRef CAS PubMed.
- P. Jeandet, A. C. Douillet-Breuil, R. Bessis, S. Debord, M. Sbaghi and M. Adrian, J. Agric. Food Chem., 2002, 50, 2731 CrossRef CAS PubMed.
- P. McCue and K. Shetty, Food Biotechnol., 2003, 17, 27 CrossRef CAS.
- P. J. Harvey, G.-F. Gilardi, M. L. Goble and J. M. Palmer, J. Biotechnol., 1993, 30, 57 CrossRef CAS.
- G. Palmieri, P. Giardina, C. Bianco, A. Scaloni, A. Capasso and G. Sannia, J. Biol. Chem., 1997, 272, 31301 CrossRef CAS PubMed.
- G. Várhegyi, E. Jakab, F. Till and T. Székely, Energy Fuels, 1989, 3, 755 CrossRef.
- E. Jakab, O. Faix and F. Till, J. Anal. Appl. Pyrolysis, 1997, 40/41, 171 CrossRef.
- E. Meszaros, E. Jakab, G. Varhegyi, P. Szepesvary and B. Marosvolgyi, J. Anal. Appl. Pyrolysis, 2004, 72, 317 CrossRef CAS.
- M. Ksibi, S. B. Amor, S. Cherif, E. Elaloui, A. Houas and M. Elaloui, J. Photochem. Photobiol., A, 2003, 154, 211 CrossRef CAS.
- R. Chandra, A. Raj, H. J. Purohit and A. Kapley, Proceedings of the 25th International Symposium on Toxicology/Environmental and Occupational Health, Industrial Toxicology Research Centre, Lucknow, India, 2005, p. 18 Search PubMed.
- M. Hernandez, M. J. C. Hernandez-Coronado, M. D. Montiel, J. Rodriguez and M. E. Arias, J. Chromatogr. A, 2001, 919, 389 CrossRef CAS.
- X. Erdocia, R. Prado, M. A. Corcuera and J. Labidi, Biomass Bioenergy, 2014, 66, 379 CrossRef CAS.
- A. Haars and A. Huttermann, Arch. Microbiol., 1980, 125, 233 CrossRef CAS.
- D. S. Arora and D. K. Sandhut, Enzyme Microb. Technol., 1985, 7, 405 CrossRef CAS.
- Y. Yang, F. Wei, R. Zhuo, F. Fan, H. Liu, C. Zhang, L. Ma, M. Jiang and X. Zhang, PLoS One., 2013, 8, e79307, DOI:10.1371/journal.pone.0079307.
- G. Cantarella, C. Galli and P. Gentili, J. Mol. Catal. B: Enzym., 2003, 22, 135 CrossRef CAS.
- F. d'Acunzo, C. Galli, P. Gentili and F. Sergi, New J. Chem., 2006, 30, 583 RSC.
- K. Inoue, S. Murayama, F. Seshimo, K. Takeba, Y. Yoshimura and H. Nakazawa, J. Sci. Food Agric., 2005, 85, 872 CrossRef CAS.
- D. Zielinska, L. Nagles and M. K. Piskuła, Anal. Chim. Acta, 2008, 617, 22 CrossRef CAS PubMed.
- G. Chen, H. Zhang and J. Ye, Anal. Chim. Acta, 2000, 423, 69 CrossRef CAS.
- A. Romani, M. Minunni, N. Mulinacci, P. Pinelli and F. F. Vinecieri, J. Agric. Food Chem., 2000, 48, 1197 CrossRef CAS PubMed.
- D. G. Bending and D. J. Read, Mycol. Res., 1997, 101, 1348 CrossRef.
- R. Mutabaruka, K. Hairiah and G. Cadisch, Soil Biol. Biochem., 2007, 39, 1479 CrossRef CAS.
- A. Velez Marquez and H. Pereira, J. Anal. Appl. Pyrolysis, 2013, 100, 88 CrossRef.
- A. D. Pouwels, G. B. Eijkel and J. J. Boon, J. Anal. Appl. Pyrolysis, 1989, 14, 237 CrossRef CAS.
- J. Ralph and R. D. Hatfield, J. Agric. Food Chem., 1991, 39, 1426 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2015 |