Synthesis and structures of 5-nitro-salicylaldehyde thiosemicarb-azonates of copper(ii): molecular spectroscopy, ESI-mass studies, antimicrobial activity and cytotoxicity†
Abstract
The basic objective of this investigation is to explore potential metallo-organic antimicrobial agents based on metal–thiosemicarbazonates. This study acquires significance in the light of the antibacterial resistance exhibited by Gram-positive and Gram-negative bacteria which have become a serious global medical problem. The increasing drug resistant bacteria are responsible for various nosocomial infections and among these, methicillin resistant Staphylococcus aureus (MRSA) is the most frequent nosocomial pathogen. Likewise, Candida albicans (fungi) are found to have developed resistance against a number of antifungal agents. In this context, compounds of copper(II) with salicylaldehyde based thiosemicarbazones {5-R′-2-HO–C6H4–C2(H)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N3–N2H–C1(
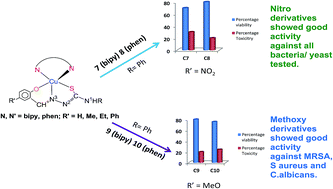
N3–N2H–C1(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S)–N1HR; R = H, Me, Et, Ph: H2L1, H2L2, H2L3, H2L4 respective thio-ligands] using bipyridines/phenanthrolines (L′) as co-ligands are being tested against various microorganisms (bacteria/fungi). For R′ = methoxy, several complexes (five coordinate complexes) were tested recently against Gram positive bacteria such as Staphylococcus aureus (MTCC740), methicillin resistant Staphylococcus aureus (MRSA), Gram negative bacteria, Shigella flexneri (MTCC1457), Klebsiella pneumoniae (MTCC109), P. aeruginosa (MTCC741) and yeast, Candida albicans (MTCC227). These complexes displayed significant growth inhibitory action even with low MIC (minimum inhibitory concenteration). A series of new copper(II) complexes (R′ = nitro, keeping R and co-ligands same) namely, [Cu(κ3-O,N,S–L)(κ2-N,N-L′)] {(L)2− = (L1)2−, L′ = bipy, 1, phen, 2; (L)2− = (L2)2−, L′ = bipy, 3, phen, 4; (L)2− = (L3)2−, L′ = bipy, 5, phen, 6; (L)2− = (L4)2−, L′ = bipy, 7, phen, 8} have been isolated and characterized by elemental analysis, infrared and electronic absorption spectroscopy, ESR spectroscopy, fluorescence, and single crystal X-ray crystallography. These copper(II) complexes have been investigated for their antimicrobial activity (antibacterial and antifungal activity), viable cell count studies through time kill assays and cellular toxicity testing using MTT assays against the above mentioned bacteria/fungi. Several complexes have shown bactericidal effects with low cytotoxicity towards living cells (sheep blood used).
S)–N1HR; R = H, Me, Et, Ph: H2L1, H2L2, H2L3, H2L4 respective thio-ligands] using bipyridines/phenanthrolines (L′) as co-ligands are being tested against various microorganisms (bacteria/fungi). For R′ = methoxy, several complexes (five coordinate complexes) were tested recently against Gram positive bacteria such as Staphylococcus aureus (MTCC740), methicillin resistant Staphylococcus aureus (MRSA), Gram negative bacteria, Shigella flexneri (MTCC1457), Klebsiella pneumoniae (MTCC109), P. aeruginosa (MTCC741) and yeast, Candida albicans (MTCC227). These complexes displayed significant growth inhibitory action even with low MIC (minimum inhibitory concenteration). A series of new copper(II) complexes (R′ = nitro, keeping R and co-ligands same) namely, [Cu(κ3-O,N,S–L)(κ2-N,N-L′)] {(L)2− = (L1)2−, L′ = bipy, 1, phen, 2; (L)2− = (L2)2−, L′ = bipy, 3, phen, 4; (L)2− = (L3)2−, L′ = bipy, 5, phen, 6; (L)2− = (L4)2−, L′ = bipy, 7, phen, 8} have been isolated and characterized by elemental analysis, infrared and electronic absorption spectroscopy, ESR spectroscopy, fluorescence, and single crystal X-ray crystallography. These copper(II) complexes have been investigated for their antimicrobial activity (antibacterial and antifungal activity), viable cell count studies through time kill assays and cellular toxicity testing using MTT assays against the above mentioned bacteria/fungi. Several complexes have shown bactericidal effects with low cytotoxicity towards living cells (sheep blood used).

 Please wait while we load your content...
Please wait while we load your content...