Ag/ZnO nanoparticles thin films as visible light photocatalysts
Abstract
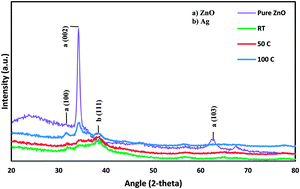
Silver (Ag) and zinc oxide (ZnO) nanoparticles were simultaneously deposited on a glass substrate using the radio frequency (RF) sputtering technique at different substrate temperatures. Detailed characterization of the co-sputtered Ag/ZnO thin films was performed by X-ray diffraction (XRD), field emission scanning electron microscopy (FESEM) and X-ray photoelectron spectroscopy (XPS). The as synthesized thin films were tested using UV-Vis diffuse reflectance spectroscopy to evaluate their optical properties. The obtained SEM results show a uniform dispersion of Ag nanoparticles within the ZnO matrix. These nanoparticles have average particle size of 20 nm. The optical band gap value was calculated from UV transmission spectra of Ag/ZnO thin films deposited at various substrate temperatures. This value was observed to be in the visible light range (i.e., 2.7–3.1 eV), which is much smaller than that of pure ZnO (3.37 eV). The photocatalytic activity of the produced thin films was evaluated through visible light photodegradation of 2-chlorophenol (2-CP) which has been used as a pollutant model in water. The synthesized thin films showed enhanced visible light photocatalytic efficiency towards 2-CP degradation at elevated substrate temperature and retained their catalytic efficiency with only 8% loss in efficiency after four reuse cycles. Kinetic parameters involved in the degradation process were investigated by applying a pseudo-second-order kinetic model.

 Please wait while we load your content...
Please wait while we load your content...