Spontaneous formation of giant vesicles with tunable sizes based on jellyfish-like graft copolymers
Ke-Jing Gao*a,
Xiao-Zhou Liub,
Guangtao Lic,
Bo-Qing Xuc and
Jianjun Yia
aPetrochina Petrochemical Research Institute, 100195-Beijing, China. E-mail: gaokejing@petrochina.com.cn; Fax: +86 10 62792905; Tel: +86 10 62978296
bPetroChina Refining & Chemicals Company, 100007-Beijing, China
cKey Lab of Organic Optoelectronics & Molecular Engineering, Department of Chemistry, Tsinghua University, 100084-Beijing, China
First published on 4th November 2014
Abstract
For self-assembly studies, a series of “jellyfish-like” graft copolymers with shorter backbones and longer branch chains were adopted in this work. Chitooligosaccharide (COS), the oligomer of chitosan, was chosen as the hydrophilic short rigid backbone and poly(ε-caprolactone) (PCL) as the hydrophobic long branch chain. It was found that these special graft copolymers in 1,4-dioxane–water mixture could self-assemble into giant vesicles with diameter in the range of 0.5–54 μm. The structure parameters of COS-g-PCLs have a significant effect on their self-assembly behavior. The average number of PCL chains per COS unit and the average degree of polymerization of the PCL branch chain are the key factors for the vesicles obtained. The greater the number of PCL chains and the longer PCL chains there are on the COS unit, the larger the vesicles become. In comparison to other copolymer vesicles, the obtained giant vesicles show unusual properties. One is that the polymer vesicles undergo sequential morphology changes upon heating. With the temperature increasing, the vesicles gradually disappear in the mixed solvent. But when the temperature is reduced, the giant vesicles appear again, with a sharper size distribution compared to the original vesicles. The other property is that the polymer vesicles have a great number of amino and hydroxyl groups on their surface. These groups could be utilized for the further modification of the obtained giant vesicles, such as cross-linking of vesicles, chelating of metal ions to vesicles and so on.
1. Introduction
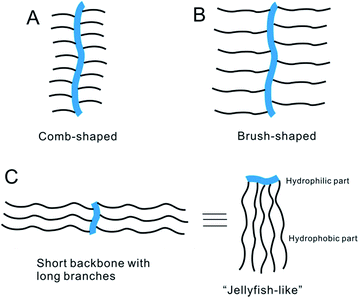
Supramolecular self-assembled vesicles have attracted great attention for their potential applications in drug delivery, gene therapy and model systems of biomembranes.1–3 In biology, lipids and proteins can spontaneously assemble into vesicles (liposomes) or vesicle-like structures, because of the driving forces such as electrostatics and hydrophobicity.4,5 Vesicles can also be formed from synthetic amphiphilic polymers.6–10 Compared to liposomes, polymer vesicles possess unique properties such as good stability and broader range of accessible solvents.11 Polymer vesicles have thus become attractive and promising research objects since the first observation of block copolymer vesicles by Eisenberg and co-workers,12 who called the aggregations of the block copolymer with small hydrophilic fractions (<20%) “crew-cut” micelles. Discher et al.13 reported a type of polymer giant vesicles formed from block copolymer and these were termed polymersomes. Polymer vesicles have also been prepared from other linear block copolymers such as polypeptides, rod–coil and rod–rod copolymers.13–19 Compared to linear block copolymers, relatively few works were conducted on amphiphilic graft copolymers. In most cases, amphiphilic graft copolymers tended to form large compound spherical micelles (LCMs).20–23 unimolecular micelles.24,25 In fact, the phenomena are due to the chemical structure of graft copolymers. Most of graft copolymers which were adopted to self-assemble usually focused on the traditional comb-shaped or brush-shaped graft copolymers based on longer main chains as backbones and shorter side chains as graft segments. It is believed that these structures easily undergo inter-molecular entanglement among the longer main chains or intra-molecular association so that they tend to form large compound spherical micelles (LCMs). If the structure of amphiphilic graft copolymer could be properly manipulated to decrease the above mentioned influence, it should be possible for a graft copolymer to organize into abundant morphologies in selective solvents.26,27So far, some attention has been devoted to developing the new kind of amphiphilic graft copolymers with a structure suitable for generating self-assembled morphologies other than LCMs.28–34 By adjustment of grafting density or side chain length, it was found that some graft copolymers can self-assemble successfully into vesicles at certain condition.
Herein, we synthesized a new type of amphiphilic graft copolymer based on a shorter rigid chain as backbone and a longer chain as branch (Scheme 1c). Chitooligosaccharide (COS), the oligomer of chitosan, was chosen as the hydrophilic short rigid main chain and poly(ε-caprolactone) (PCL) as the hydrophobic long side chain. The chemical structure of COS-g-PCL obtained is quite different from the conventional comb-shaped or brush-shaped graft copolymers (Scheme 1a and b). We termed it “jellyfish-like” structure.34–38 It was found that this type of graft copolymers in water–1,4-dioxane mixtures can self-assemble into vesicles with diameter in the range of 0.5–54 μm. As far as we know, our research group is the first one to demonstrate the giant vesicles self-assembled from the graft copolymers in solution.37 In the present paper, the influences of the structure parameters of COS-g-PCL molecule on the vesicle morphologies have been examined. The properties of polymer vesicles obtained and the mechanism of self-assembly have also been investigated.
2. Experimental section
2.1. Materials
Chitooligosaccharide (COS, Mn = 1800, the degree of deacetylation is 96%), purchased from Shenzhen Bright Way Novel Biomaterials Tech. Co. Ltd. (P. R. China), was dried at 60 °C under vacuum. ε-Caprolactone (Acros Organics, 99%)was purified by vacuum distillation over CaH2 and the fraction collected at 96–98 °C was used in polymerization. Triethylaluminum was obtained from Fluka Company and used as a 1.0 M solution in toluene. Chloroform and pyridine were purified by usual distillation method. Hexamethyldisilicane (HMDS) and other regents were used as received without further purification.2.2. Synthesis of COS-g-PCL copolymers
The graft copolymer of COS-g-PCL was synthesized according to the previous literature.36,37 Briefly, Chitooligosaccharide (COS, 5.0 g, 30.9 mmol pyranose) was placed in a dried glass reactor that was previously flushed with high purity N2 for 30 min. and added 50 ml of dimethyl sulfoxide (DMSO). Under vigorous stirring, the dispersion was heated at 70 °C for 6 h to form a clear solution and then allowed to cool down to room temperature. Predetermined amount of hexamethyldisilazane (the molar ratio of hexamethyldisilazane to pyranose unit was in the range of 2.0–4.0) were added, and after being heated at 80 °C for 12 h with stirring, the mixture was poured into 150 ml of acetone. The precipitate was filtered, washed with water, and dried in vacuum to give pale tan powder. The degree of substitution (DS) of COS, i.e. the average number of –Si(CH3)3 per COS unit, was calculated from the C/N value of elemental analysis.1.0 g trimethylsilylChitooligosaccharide (TMSCOS) with a certain degree of substitution was dissolved in Chloroform of 50 ml and then a certain amount of triethylaluminum in toluene (the molar ration of AlEt3 to OH contained in TMSCOS was 1.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) was added dropwise via a syringe through a rubber septum. The reaction was kept for two hours and then a desired amount of the ε-caprolactone monomer was transferred into the reactor. Under N2, the graft copolymerization was allowed to proceed for 24 h at ambient temperature while stirred. When the setting reaction time of 24 h elapsed, 5 ml mixture of isopropyl alcohol–H2O–HCl was added and the reactor was stirred for further 4 h to move the TMS group. The resulting polymer was purified by repeated precipitation (3 times) using 10 ml of chloroform and 300 ml alcohol as a solvent and precipitant pair. It was dried for 72 h at 50 °C in vacuum to a constant weight.
1) was added dropwise via a syringe through a rubber septum. The reaction was kept for two hours and then a desired amount of the ε-caprolactone monomer was transferred into the reactor. Under N2, the graft copolymerization was allowed to proceed for 24 h at ambient temperature while stirred. When the setting reaction time of 24 h elapsed, 5 ml mixture of isopropyl alcohol–H2O–HCl was added and the reactor was stirred for further 4 h to move the TMS group. The resulting polymer was purified by repeated precipitation (3 times) using 10 ml of chloroform and 300 ml alcohol as a solvent and precipitant pair. It was dried for 72 h at 50 °C in vacuum to a constant weight.
2.3. Measurement of COS-g-PCL copolymers
1H-NMR spectra were taken at room temperature in tetrahydrofuran-d8 or D2O/THF-d8 in by a Bruker AV400 spectrometer operating at 400. Elemental analyses were performed using ThermoQuest EA1112 elemental analyzer with a combustion temperature at 950 °C. The molecular weight (Mn) and molecular weight distribution (Mw/Mn) were measured with a Waters 515 GPC instrument equipped with Styragel columns (101, 102, and 103 nm pore sizes) with tetrahydrofuran (THF) as the mobile phase. Polystyrene and Waters Millennium 32 were used as calibration standards and data-processing software, respectively.2.4. The self-assembly of COS-g-PCL copolymers
The obtained COS-g-PCL copolymers were initially dissolved in 1,4-dioxane. Deionized water was added dropwise at a rate of 0.2% per minute to the COS-g-PCL solutions under vigorous stirring until the water contents were reached to ca. 25 wt%. The solution were stirred for at least 2 h except otherwise noted, and then observed with optical microscopy or TEM.2.5. Characterization of vesicle morphology and size
Environmental Scanning Electron Microscopy (ESEM) Measurements were recorded with model XL 30 ESEM FEG from Micro FEI Philips at room temperature. The lyophilized aggregate powders were deposited on a silicon wafer. A thin layer of gold was coated on the sample surface before measurement. Transferring Electron Microscopy (TEM) was performed on a JEOL JEM-1200 electron microscope at an acceleration voltage of 120 KV. A drop of the dialyzed was deposited on a copper grid coated with a carbon film and stained with a drop of phosphorous tungstenic acid in water (2 wt%). The copper grid was then dried at room temperature prior to measurement. Confocal Laser Scanning Microscopy (LSCM) measurements were conducted as follow: the pyrene solution in acetone (50 mM, prepared prior to use) was added to the suspension of GVs in dioxane–water mixture to make a pyrene concentration of 5 mM. Fluorescence images were record using the microscopy (Olympus BX 61, Olympus Optical Co., Tokyo, Japan) with a confocal optical scanner (Yokogawa Inc., Tokyo, Japan) and an argon ion laser with 405 nm excitation wavelength. Phase contrast micrographs were record using the phase contrast microscopy (Olympus BX 51, Olympus Optical Co., Tokyo, Japan) with a digital CCD camera, and operated by the software provided by the camera manufacturer. The average size of vesicle was calculated from 100 vesicles selected randomly.2.6. Thermal response experiments
The GVs suspension was transferred into an observation chamber and observed with a phase-contrast microscopy (Olympus BX-51), equipped with a JVC color video camera and connected to an image-recording system. To determine the effect of temperature on the stability of GVs. Slow uniform heating was achieved with a resistive heating stage constructed in our lab, consisting of a backside metal coated microscope coverslip and a current limiting standard power supply. Cooling was achieved by simple heat dissipation after turning off the heating stage. Temperature in the droplet close to the vesicle was measured using a calibrated micron-sized Cu/Ni thermoelement. The temperature measurement was time synchronized with the time readings on the videotape.3. Results and discussion
3.1. Vesicle formation dependence on the structure parameters of COS-g-PCLs
According to the synthesis method reported in the previous literature,36 six COS-g-PCL samples with different structure parameters were synthesized (denoted as g1–6). The details of the characterization of COS-g-PCLs are listed in Table 1.| Sample | DSa of COS | Mnb | DPc of CL | Diameterd/μm |
|---|---|---|---|---|
| a The average number of PCL chains per COS unit, determined by elemental analysis.b Number average molecular weight of COS-g-PCL, determined by GPC as described in the text.c DP: the average degree of polymerization of PCL single branch attached to COS chain, calculated by 1H NMR.d Determined visually from images for the average diameter of vesicles, based on the 100 vesicles. | ||||
| g1 | 1.0 | 1.4 × 104 | 61 | 0.5 |
| g2 | 1.0 | 2.6 × 104 | 100 | 35 |
| g3 | 1.0 | 7.7 × 104 | 178 | 54 |
| g4 | 0.5 | 1.4 × 104 | 71 | Cluster |
| g5 | 0.5 | 2.9 × 104 | 118 | 31 |
| g6 | 0.5 | 4.5 × 104 | 187 | 43 |
| g7 | 0.2 | 5.2 × 104 | 261 | Precipitation |
Using water as selective solvent and 1,4-dioxane as common solvent, the self-assembly behavior of COS-g-PCLs was investigated under stirring according to the methodology of Eisenberg et al. to create “crew-cut” aggregates.39–44 It was found that most of the jellyfish-like graft copolymers can self-assemble into vesicles with diameter great than 0.5 μm. Fig. 1a typically displays the micrographs of the fluorescence-labeled g2 aggregates observed by laser scanning focal microscope (LSCM). These images clearly illustrate the hollow interior of vesicular morphology, as evidenced by a significant decrease in fluorescence intensity toward the center of the spheres.45 The argument is also supported by the TEM measurements. Fig. 1b shows a representative TEM image of the g2 aggregates negatively stained by phosorous tungstenic acid in water (2 wt%). The contrast difference between the aggregate skin and the inner pool proved that the aggregates are vesicular in nature.
The morphologies of the aggregates are heavily dependent on the structure parameters of COS-g-PCL molecules. When DS of COS (the average number of PCL chains per COS unit) was 1.0, COS-g-PCLs can self-assemble into vesicles and the vesicle diameter increases with DP of CL (Table 1, Fig. 2a–c). When DP of CL was lower (sample g1 in Table 1), the diameters of vesicles obtained were less than 1 μm. The low resolution of microscopy limits direct imaging of the aggregates (Fig. 2a). So, TEM was used to observe the vesicles (Fig. 2a′). When DP of CL was higher (sample g3 in Table 1), the diameter of vesicles grew up to more than 50 μm. For COS-g-PCLs with DS of 0.5, the results were different slightly. When DP of CL was lower (sample g4 in Table 1), the vesicle-like aggregates were formed, accompanied frequently by clusters of vesicle-like aggregates (Fig. 2d). With the further increase in DP, the clusters disappeared and the vesicle diameter increased obviously (Fig. 2e and f). The appearing of cluster, resulting from vesicle aggregation, might attribute to the intervesicle hydrogen bonds due to a relative more –OH and –NH2 groups embedded in their vesicles. For the COS-g-PCLs with higher DP of CL (sample g5, g6), the hydrophobic interaction among PCL branches may become the main driving force for self-assembly and the intervesicle hydrogen bonds are hard to build up. As a consequence, the discrete vesicles are the only observed assemblies (Fig. 2e and f). In addition to DP of CL, the DS of COS was crucial for the formation of vesicle. When the DS was decreased to 0.2, no vesicles were obtained although the copolymers have the higher DP of CL (sample g7 in Table 1, Fig. 2g).
In short, the structure parameters of these copolymers exert a significant effect on formation of the aggregates. Only the COS-g-PCLs with the higher DP of CL and the higher DS of COS can self-assemble into giant vesicles with the diameter greater than 0.5 μm.
3.2. Properties of the vesicles in solution
 | ||
| Fig. 3 Caption sequence showing the heating (image a–d) and the cooling (image e–h) of g2 vesicles, which was observed with phase-contrast microscopy. | ||
As mentioned above, the vesicles from copolymer g4 aggregate into clusters due to the intervesicle hydrogen bonds among the –OH and –NH2 groups embedded in these vesicles. These –OH and –NH2 groups have a strong affinity to Cu2+. The intervesicle hydrogen bonds will be broke up in the presence of Cu2+, and clusters will disappear. As expected, with the presence of Cu2+ in the vesicle suspension, no clusters were observed, only leading to discrete vesicles (Fig. 5a).
Cucurbit[n]uril (CB[n] n = 5–8), a family of macrocyclic compounds comprising n glycoluril units, has a hydrophobic cavity that is accessible through two identical carbony-fringed portals.47 Supramolecular chemistry of CB[n], including their host–guest chemistry, has been studied extensively.48,49 In particular, CB[6] and its derivatives have been found to form exceptionally stable host–guest complexes with compound containing –NH2 groups. Many supramolecular assemblies such as one- (1D), two- (2D), three-dimensional (3D) structures and molecular necklaces have been reported.50 In our case, CB(6) in aqueous solution was introduced into the copolymer g2 vesicle suspension and stirred for 12 h at room temperature. It was found that the vesicle clusters were generated owing to the strong affinity of CB(6) toward –NH2 groups with their two identical carbony-fringed portals (Fig. 5b).
The –OH and –NH2 groups on the surface of vesicles could be used for further modification through covalent cross-linking, which is a acceptable means of stabilization. To achieve this, a certain amount of glutaraldehyde was added into the vesicle suspension and stirred for 4 h at temperature 40 °C. After cross-linking, the vesicle stability against heating was verified to promote greatly (Fig. 6). Even at high temperature of 70 °C, the vesicles remained their shapes (Fig. 6c). When temperature was increased to above 80 °C, the vesicles from copolymer g2 did not still dissolve in 1,4-dioxane–water mixture in spite of the fact that all of them collapsed due to the escape of dioxane–water mixture inside the vesicles (Fig. 6d). SEM images also support the enhanced stability of vesicles. Due to the enhanced stability of vesicle, the vesicles could stand the tough condition during the TEM preparation and the most of vesicles were not destroyed although collapsed, which are shown in Fig. 7.
 | ||
| Fig. 6 Phase contrast micrographs (a–d) at different temperature after copolymer g2 vesicles were cross-linked with gutaraldehyde. (a) 32.8 °C; (b) 65.1 °C; (c) 70.0 °C; (d) 80.0 °C. | ||
3.3. The mechanism of self-assembly
Taking account of the structure nature of COS-g-PCL, the new kind of graft copolymer has hydrophilic COS backbones and pendant hydrophobic PCL polyester chains. During the course of COS-g-PCL self-assembly driven by the hydrophobic interaction in 1,4-dioxane–water mixtures, the PCL branches would form the middle layer of the vesicle wall, and the COS backbones would form the outer layers.On the other hand, there are a lot of hydroxyl and amino groups along the backbones of COS, the formation of hydrogen bonds is another potential interaction for the self-assembly. To verify this argument, trimethylsilyl Chitooligosaccharide-graft-poly(caprolactone) (TMSCOS-g-PCL) were utilized to self-assemble. When the amino and hydroxyl groups were protected by trimethylsilylation, the formation of vesicles was not observed. Only ill-defined precipitation was detected (not shown).
Variable temperature FTIR was used to detect the hydrogen bonds in the resulting dried vesicles. The results are provided in Fig. 8. It was found that with increasing temperature from 25 °C to 80 °C, the peak of the hydroxyl groups gradually becomes weaker and shifts to the high wavelength side, which clearly indicates that a certain amount of hydroxyl groups have formed hydrogen bonds in the dried vesicles originated from the self-assembly of COS-g-PCL molecules.
As for the conformation of COS backbone in the aggregate, it is believed that they exist in parallel array to achieve as many hydrogen bonds as possible and the lowest energy.51 Conventionally, amphiphilic graft copolymers with the same molecular weight might form self-assemblies with small size (on nanometer scale). For our system, the graft copolymers can form large vesicles (on micrometer scale) spontaneously. This may be due to the tendency for the COS backbones to form parallel array driven by intra- and intermolecular hydrogen bonds originated from the groups of –OH and –NH2. Therefore, in the present case, the self-assembly mechanism should include two driving forces: one is the hydrophobic interaction which is attributed to the amphiphilic character of COS-g-PCL consisting of hydrophilic COS backbones and hydrophobic PCL branches, and the other is the formation of hydrogen bonds among the amino and hydroxyl groups remained on the COS backbones.
4. Conclusions
The size of the giant vesicles can be tuned in the range of 0.5–54 μm through the structure adjustment of COS-g-PCL molecules. Surprisingly, the formed vesicles exhibit high stability and can be stored at ambient temperature, even at elevated temperature (<50 °C) for 3 months. When the vesicle suspension is heated to above 50 °C, the vesicles dissolve or collapse in the mixture of 1,4-dioxane–water. But with temperature cooled down, the copolymer can self-assembled into giant vesicles again. Taking into account the structure of COS-g-PCL molecule, there are a great number of –OH and –NH2 groups along COS hydrophilic backbone. These groups, which exist on the surface of vesicles, could be utilized for the further modification of the obtained giant vesicles, such as cross-linking of vesicles, chelating of metal ion to vesicles and so on. In general, the work will shed new light on designing new type of polymer giant vesicles, self-assemble from Chitooligosaccharide-based graft copolymer in 1,4-dioxane–water, with properties different from other vesicles.Acknowledgements
This work was supported by National Natural Science Foundation of China (20473044, 20533050, 50673048 and 973 Program 2006CB806200). The authors thank Q. F. Zhao and B. Q. Xing for technical assistance, and S. Y. Tao for useful discussions.Notes and references
- S. Jain and F. S. Bates, Science, 2003, 300, 460 CrossRef CAS PubMed.
- S. Forster and T. Plantenberg, Angew. Chem., Int. Ed., 2002, 41, 688 CrossRef CAS.
- M. W. Neiser, S. Muth, U. Kolb, J. R. Harris, J. Okuda and M. Schmidt, Angew. Chem., Int. Ed., 2004, 43, 3192 CrossRef CAS PubMed.
- T. Ruysschaert, A. F. P. Sonnen, T. Haefele, W. Meier, M. Winterhalter and D. Fournier, J. Am. Chem. Soc., 2005, 127, 6242 CrossRef CAS PubMed.
- Q. Wang, T. W. Lin, L. Tang, J. E. Johnson and M. G. Finn, Angew. Chem., Int. Ed., 2002, 41, 459 CrossRef CAS.
- D. E. Discher and A. Eisenberg, Science, 2002, 297, 967 CrossRef CAS PubMed.
- A. Ranqoin, W. Versées, W. Meier, J. Steyaert and P. Van Gelder, Nano Lett., 2005, 5, 2220 CrossRef PubMed.
- A. Choucair, C. Lavigueur and A. Eisenberg, Langmuir, 2004, 20, 3894 CrossRef CAS.
- E. P. Holowka, D. J. Pochan and T. J. Deming, J. Am. Chem. Soc., 2005, 127, 12423 CrossRef CAS PubMed.
- J. Rodriguez-Hernández and S. Lecommandoux, J. Am. Chem. Soc., 2005, 127, 2026 CrossRef PubMed.
- P. P. Ghoroghchian, G. Li, D. H. Levine, K. P. Davis, F. S. Bates, D. A. Hammer and M. J. Therien, Macromolecules, 2006, 39, 1673 CrossRef CAS PubMed.
- L. Zhang and A. Eisenberg, Science, 1995, 268, 1728 CAS.
- B. M. Discher, Y. Y. Won, D. S. Ege, J. C. M. Lee, F. S. Bates, D. E. Discher and D. A. Hammer, Science, 1999, 284, 1143 CrossRef CAS; M. Antonietti and S. Förster, Adv. Mater., 2003, 15, 1323 CrossRef.
- F. S. Chécot, G. Lecommandoux and H. A. Klok, Angew. Chem., Int. Ed., 2002, 41, 1339 CrossRef.
- S. I. Stupp, V. LeBonheur, K. Walker, L. S. Li, K. Huggins, M. Keser and A. Amstutz, Science, 1997, 276, 384 CrossRef CAS.
- A. Kros, W. Jesse, G. A. Metselaar and J. J. L. M. Cornelissen, Angew. Chem., Int. Ed., 2005, 44, 4349 CrossRef CAS PubMed.
- P. Ghoroghchian, G. Li, D. Levine, K. Davis, F. Bates, D. Hammer and M. Therien, Macromolecules, 2006, 39, 1673 CrossRef CAS PubMed.
- J. Sun, X. Chen, C. Deng, H. Yu, Z. Xie and X. Jing, Langmuir, 2007, 23, 8308 CrossRef CAS PubMed.
- F. Meng, C. Hiemstra, G. Engbers and F. Jan, Macromolecules, 2003, 36, 3004 CrossRef CAS.
- J. H. Jeonga, H. S. Kangb, S. R. Yanga and J. D. Kim, Polymer, 2003, 44, 583 CrossRef.
- K. Kuroda, K. Fujimoto, J. Sunamoto and K. Akiyoshi, Langmuir, 2002, 18, 3780 CrossRef CAS.
- O. E. Philippova, E. V. Volkov, N. L. Sitnikova, A. R. Khokhlov, J. Desbrieres and M. Rinaudo, Biomacromolecules, 2001, 2, 483 CrossRef CAS PubMed.
- B. Nottelet, A. E. Ghzaoui, J. Coudane and M. Vert, Biomacromolecules, 2007, 8, 2594 CrossRef CAS PubMed.
- S. Yusa, A. Sakakibara, T. Yamamoto and Y. Morishima, Macromolecules, 2002, 35, 5243 CrossRef CAS.
- A. Kikuchi and T. Nose, Macromolecules, 1996, 29, 6770 CrossRef CAS; A. Kikuchi and T. Nose, Polymer, 1996, 37, 5889 CrossRef.
- H. Duan, M. Kuang, J. Wang, D. Chen and M. Jiang, J. Phys. Chem. B, 2004, 108, 550 CrossRef CAS.
- K. H. Kim, J. Huh and W. H. Jo, Macromolecules, 2004, 37, 676 CrossRef CAS.
- N. Xu, F. S. Du and Z. C. Li, J. Polym. Sci., Part A: Polym. Chem., 2007, 45, 1889 CrossRef CAS.
- C. Cai, J. Lin, T. Chen and X. Tian, Langmuir, 2010, 26, 2791 CrossRef CAS PubMed.
- Y.-C. Huang, M. Arham and J.-S. Jan, Soft Matter, 2011, 7, 3975 RSC.
- K.-H. Liu, S.-Y. Chen, D.-M. Liu and T.-Y. Liu, Macromolecules, 2008, 41, 6511 CrossRef CAS.
- B.-Y. Chen, Y.-F. Huang, Y.-C. Huang, T.-C. Wen and J.-S. Jan, ACS Macro Lett., 2014, 3, 220 CrossRef CAS.
- K.-H. Liu, B.-R. Chen, S.-Y. Chen and D.-M. Liu, J. Phys. Chem. B, 2009, 113, 11800 CrossRef CAS PubMed.
- Y.-C. Huang and J.-S. Jan, Polymer, 2014, 55, 540 CrossRef CAS PubMed.
- C. Wang, G. Li and R. Guo, Chem. Commun., 2005, 3591 RSC.
- K.-J. Gao, J. Yi, J. Mao, G. Li and B.-Q. Xu, J. Appl. Polym. Sci., 2013, 128, 1687 CAS.
- K.-J. Gao, G. Li, H. Shi, X. Lu, Y. Gao and B.-Q. Xu, J. Polym. Sci., Part A: Polym. Chem., 2008, 46, 4889 CrossRef CAS.
- K.-J. Gao, G. Li, X. Lu, Y. G. Wu, B.-Q. Xu and J.-H. Fuhrhop, Chem. Commun., 2008, 1449 RSC.
- L. Zhang and A. Eisenberg, J. Am. Chem. Soc., 1996, 118, 3168 CrossRef CAS.
- L. Zhang, K. Yu and A. Eisenberg, Science, 1996, 272, 1777 CAS.
- K. Yu and A. Eisenberg, Macromolecules, 1996, 29, 6359 CrossRef CAS.
- Y. Yu, L. Zhang and A. Eisenberg, Macromolecules, 1998, 31, 1144 CrossRef CAS.
- L. Zhang and A. Eisenberg, Polym. Adv. Technol., 1998, 9, 667 CrossRef.
- P. L. Soo and A. Eisenberg, J. Polym. Sci., Part B: Polym. Phys., 2003, 42, 923 Search PubMed.
- R. J. Thibault, T. H. Galow, E. J. Turnberg, M. Gray, P. J. Hotchkiss and V. M. Rotello, J. Am. Chem. Soc., 2002, 124, 15249 CrossRef CAS PubMed.
- Y. Zhou, D. Yan, W. Dong and Y. Tian, J. Phys. Chem. B, 2007, 111, 1262 CrossRef CAS PubMed.
- J. W. Lee, S. Samal, N. Selvapalam, H. J. Kim and K. Kim, Acc. Chem. Res., 2003, 36, 621 CrossRef CAS PubMed.
- Y. H. Ko, K. Kim, J. K. Kang, H. Chun, J. W. Lee, S. Sakamoto, K. Yamaguchi, J. C. Fettinger and K. Kim, J. Am. Chem. Soc., 2004, 126, 1932 CrossRef CAS PubMed.
- P. Mukhopadhyay, A. Wu and L. J. Isaacs, J. Org. Chem., 2004, 69, 6157 CrossRef CAS PubMed.
- J. Lagona, P. Mukhopadhyay, S. Chakrabarti and L. Isaacs, Angew. Chem., Int. Ed., 2005, 44, 4844 CrossRef CAS PubMed.
- K. Naka, R. Yamashita, T. Nakamura, A. Ohki and S. Maeda, Macromol. Chem. Phys., 1997, 198, 89 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2014 |