Nanoparticle mediated delivery of a GST inhibitor ethacrynic acid for sensitizing platinum based chemotherapy
Qiang Yang†
a,
Haihua Xiao†bc,
Jing Caia,
Zhigang Xie*b,
Zehua Wang*a and
Xiabin Jingb
aDepartment of Obstetrics and Gynecology, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, 1277 Jiefang Avenue, Wuhan 430022, China. E-mail: zehuawang@163.net; Tel: +86-2785351649
bState Key Laboratory of Polymer Physics and Chemistry, Changchun Institute of Applied Chemistry, Chinese Academy of Sciences, Changchun 130022, China. E-mail: xiez@ciac.ac.cn; Tel: +86-43185262775
cUniversity of Chinese Academy of Sciences, Beijing 100049, China
First published on 6th November 2014
Abstract
Drug resistance is a major obstacle for platinum based chemotherapy, and one of the most common reasons is GSH-mediated detoxification. To overcome this problem, we proposed ethacrynic acid (EA), a GST inhibitor, to reduce the conjugation of GSH with platinum agents so as to sensitize cancer cells to chemotherapy. We constructed biodegradable nanoparticles to co-deliver EA and DACHPt, a precursor of oxaliplatin. In vitro studies showed that the hybrid nanoparticles could release EA and DACHPt faster in intracellular-like environments than in blood circulation, enhancing the cellular Pt accumulation and Pt–DNA adduct formation. Most importantly, it showed a synergy effect between EA and DACHPt, resulting in an enhancement of up to 4.6 fold of the anticancer efficacy. We also verified this approach in vivo, taking into account both anticancer efficacy and systemic toxicity, and the nanoparticles were much better than the simple combination of free drugs. The results demonstrated that the nanoparticles we constructed could be a promising approach to overcome the drug resistant problem in cancer chemotherapy.
Introduction
Since the discovery of the therapeutic potential of cis-diamminedichloroplatinum(II) (cisplatin) by Barnett Rosenberg,1,2 platinum compounds have become the backbone of cancer chemotherapy, either alone or combined with other anticancer drugs. Nowadays, patients with a variety of cancers including sarcomas, cancers of soft tissue, bones, muscles, and blood vessels are treated with platinum-based regimens.3,4 Despite of the wide use of platinum analogues in cancer therapy, great disadvantages exist which hamper the further application of platinum-based chemotherapy. These disadvantages mainly are: (a) serious side effects may occur during chemotherapy; (b) inherent or acquired drug-resistance happens, which results in the failure of chemotherapy.3–6A key reason for both inherent and acquired drug resistance as well as the failure of chemotherapy can be ascribed to the over-expressed glutathione (GSH), an important abundant antioxidant and detoxicant, at a concentration of 5–10 mM in cancer cells.7,8 GSH plays an important role in regulating Pt activities and their ultimate cell killing ability. Over-expression of GSH in cancer cells leads to the drug resistance and failure of treatment.4–8
One important enzyme family responsible for the GSH mediated detoxification of Pt drugs is glutathione-S-transferases (GSTs). GSTs are a family of phase II detoxification enzymes, catalyze the conjugation of glutathione (GSH) to a wide variety of endogenous and exogenous electrophilic compounds including Pt based drugs, forming an important cellular defense mechanism.7,9 Study also found that GST enzymes, specifically GST-π isozymes, are over-expressed in cisplatin resistant cells10,11 and inhibition of these enzymes can retard the reaction of GSH with Pt drugs, reduce the drug pump-out, resulting in the reversal of the drug resistance and recalling the sensitivity of Pt drugs.12 For this specific reason, a variety of GST inhibitors with varying ability of inhibiting the conjugation of GSH with Pt drugs or lowering the GSH levels directly were systematically evaluated to sensitize the cancer cells to Pt drugs.9,13 Particularly, ethacrynic acid (EA) was such a drug which can effectively inhibit GST isozymes and deplete GSH directly via conjugating the thiol groups of GSH.14,15 Moreover, clinic trails on EA in combination with chlorambucil and thiotepa against a variety of cancers have made progresses.16,17
Drug delivery systems (DDS) are engineered approaches transporting pharmaceutical compounds to the body/organs as needed, such as targeted delivery and/or controlled release.18–22 Targeting delivery of combination of anticancer drugs or siRNA can also be realized.23,24 Moreover, this can exploit the ‘enhanced permeability and retention’ (EPR) effect for preferential extravasation within tumor tissues, resulting in enhanced efficacy and/or reduced systemic toxicity of anticancer agents.25,26 Based on the promise of EA on GSTs inhibition, specifically, the possible chance of reducing GSH-mediated detoxification, we propose here for the first time to use a biodegradable polymer platform to co-deliver EA and 1,2-diaminocyclohexane platinum(II) (DACHPt), a precursor of oxaliplatin (a third generation Pt drugs widely used on clinic), aiming at sensitizing the Pt drugs via EA inhibiting GSH-mediated detoxification. In doing so, by conjugating EA and chelating DACHPt to biodegradable polymers, both polymer conjugates of EA (P(EA) and DACHPt (P(Pt))) can be obtained with ease. Mixing the two conjugates and varying the ratios can lead to composite micelles (M(EA/Pt)) loading with both EA and Pt at desirable ratios via simple assembly (Scheme 1b and c). M(EA/Pt) can be internalized into cancer cells via endocytosis. The lower pH within cancer cells facilitates the rapid release of both DACHPt and EA. The released EA inhibits the activity of GST enzymes, thus suppresses the conjugation of GSH with DACHPt, resulting in less detoxification and relatively more active oxaliplatin species, which ultimately sensitizes cancer cells to Pt and thus increases the efficacy of Pt.
Results and discussion
Synthesis of polymer conjugates P(Pt) and P(EA)
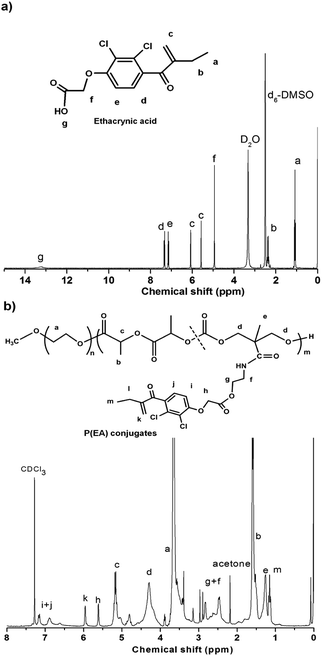
Our previous work reported chelating Pt(II) onto the pendant carboxyl groups of a biodegradable amphiphilic polymer carrier, methoxyl-poly(ethylene glycol)-block-poly(l-lactide-co-2-methyl-2-carboxyl-propylene carbonate) (MPEG-b-P(LA-co-MCC)), to get a polymer–Pt(II) conjugate P(Pt).27 When assembling the P(Pt) into micelles, the hydrophobic P(LA-co-MCC/Pt) segment forms the core of the micelles, protects the Pt species from environment. Meanwhile, the MPEG corona reduces the adsorption of proteins so as to allow a slowed clearance of the micelles from the blood. P(Pt) used here was prepared and characterized as previously and will not be discussed in detail. The content of platinum in P(Pt), determined by ICP-MS, was 10 wt%.In a similar way, we acquired P(EA) by conjugating EA with MPEG-b-P(LA-co-MCC-OH) via simple DCC/NHS chemistry. The P(EA) was first characterized by 1H NMR (Fig. 1). As compared with the 1H NMR of free EA in CDCl3, the major peaks at 5.6, 6.1, 7.11 and 7.29 ppm can be assigned to EA, indicating the successful conjugation of EA to the polymer chains. By comparing the integration at 7.11 and 7.29 ppm (d and e in EA) with protons in PEG at 3.65 ppm (–CH2CH2O– in polymer), we deduced the EA content in P(EA) of 14 wt%, corresponding to a conjugation efficiency of 68%.
Moreover, as EA has showed UV absorbance with the peak at 262 nm, we can further confirm the drug content via UV spectrum. We collected the UV absorbance of a series solutions in CHCl3 of EA (Fig. 2a) and P(EA) conjugates (Fig. 2b) and calculated the EA content in P(EA) to be 11.6 w/w% via the standard curve (Fig. 2c; A = 0.37777 + 0.0549C, R2 = 0.9943). This slight difference from the 1H NMR result was due to the slight change of the UV absorption behavior of EA upon conjugation onto the polymer chain. In fact, the peak of EA was shifted to 268 nm after conjugation (Fig. 2b).
Preparation and characterization of polymer micelles M(Pt), M(EA) and M(EA/Pt)
Micelles were prepared as previously described.27 The Pt-loaded micelles M(Pt) and EA-loaded micelles M(EA) were obtained from conjugates P(Pt) and P(EA), respectively. Due to the amphiphilicity of the polymer carrier, the conjugates P(Pt) and P(EA) in acetone can self-assembled into micelles by adding enough water under stirring, the hydrophobic P(LA-co-MCC/EA) or P(LA-co-MCC/Pt) block develops the inner core of the micelles while the MPEG segment forms the shell.Due to identical polymer backbones, it is possible and easy to co-assemble the P(Pt) and P(EA) to form hybrid micelles, M(EA/Pt), containing both EA and DACHPt in each micelle. As EA and DACHPt were conjugated to the polymer, the hydrophobic property of the P(LA-co-MCC) segment ensures them reside in the core of the micelles (Scheme 1), hence free from the blood clearance. In order to determine the proper dose ratio of EA and Pt, three typical molar ratios were considered, i.e., 0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and 2
1, and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, coded as M(EA/Pt = 0.5), M(EA/Pt = 1) and M(EA/Pt = 2), correspondingly.
1, coded as M(EA/Pt = 0.5), M(EA/Pt = 1) and M(EA/Pt = 2), correspondingly.
The micelles M(Pt), M(EA) and M(EA/Pt) at different EA/Pt ratios were characterized by TEM and DLS. As shown in Table 1, the M(Pt) had a mean diameter of 41 nm by DLS and 34 nm by TEM with a zeta potential of −12.8 mV and the M(EA) features a mean diameter of 51.4 nm by DLS and 41 nm by TEM with a zeta potential of −15.4 mV. M(EA/Pt = 0.5), M(EA/Pt = 1) and M(EA/Pt = 2) had mean diameters of 65 nm, 94 nm and 109 nm by TEM, respectively, increasing with the EA/Pt ratio. Keeping in mind that the particle size of M(Pt) is only 34 nm, the bigger size of M(EA/Pt) is attributed to the contribution of EA. The zeta potential of M(EA/Pt = 0.5), M(EA/Pt = 1), and M(EA/Pt = 2) were −9.1 mV, −5.6 mV and −4.2 mV, respectively. Fig. 2d and e are representative DLS and TEM images of M(EA/Pt = 1), which showed a spherical morphology.
Drug release of EA and Pt from M(EA/Pt)
It is vital that the hybrid M(EA/Pt)s can release both EA and DACHPt in a proper way under different conditions so as to acquire a better tolerance, an elongated circulation and an enhanced antitumor efficacy. To verify this, the in vitro drug release profiles of the M(EA/Pt = 1) were performed, by a dialysis method against pH 5.0 acetate buffered solution (ABS) and pH 7.4 phosphate buffered saline (PBS). The amount of released platinum and EA were determined by ICP-OES and UV-vis spectroscopy respectively.Fig. 2f shows the results. From these release profiles, some basic features can be deduced: (1) the release (of either EA or Pt) is pH sensitive. Both the EA and DACHPt are released faster in acidic environment. Taking the time point 7 h as an example, the cumulative released Pt at pH 5.0 is 56% while it is only 25% at pH 7.4. And those of EA are 43% and 15% respectively. It is believed that the as-seen pH dependence of drug release can be attributed to the hydrolysis of the drug linkage with the polymer backbone. Moreover, the polymer is biodegradable hence the breakage of the polymer backbone also contributes to this. (2) As the ratio of Pt to EA is 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, we can conclude that Pt is released faster than EA either at pH 5.0 or 7.4, which implies that the COO–Pt bonds in P(Pt) are much more susceptible to hydrolysis than the ester linkages in P(EA) as depicted in Scheme 1a. In other words, at a certain time after administration, there will be less released EA than Pt within cancer cells.
1, we can conclude that Pt is released faster than EA either at pH 5.0 or 7.4, which implies that the COO–Pt bonds in P(Pt) are much more susceptible to hydrolysis than the ester linkages in P(EA) as depicted in Scheme 1a. In other words, at a certain time after administration, there will be less released EA than Pt within cancer cells.
It is well recognized that the environment within cancer tissues is different from that of normal ones. One significant difference is that the cancer neoplasm is much more acidic than normal tissue.28,29 The relatively slower release of the Pt and EA in pH 7.4 can assure a longer half-life of M(Pt/EA)s in blood circulation and milder side effects on healthy organs. And once the micelles were internalized into cancer cells, the EA and DACHPt can be quickly released and play their roles.
In vitro cytotoxicity of the micelles
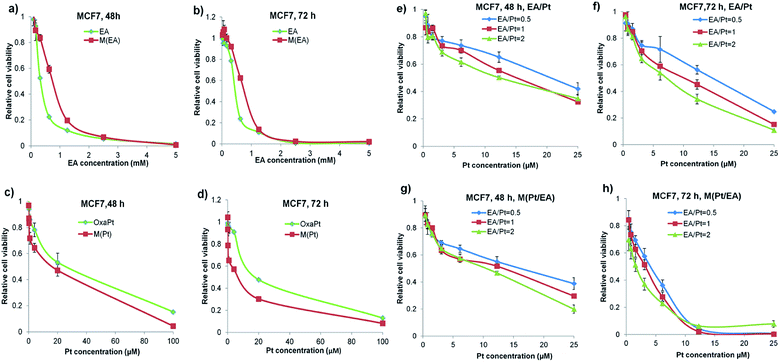
Cytotoxicity of each drug was evaluated by MTT assay on human breast cancer cell line MCF7. Our previous study has demonstrated that the MPEG-b-P(LA-co-MCC) polymer is nontoxic,27 hence the cytotoxicity observed here should be attributed to the released EA, DACHPt or both of them.As shown in Fig. 3a and b, both EA and M(EA) have negligible toxicity towards the cells. What's more, M(EA) showed less cytotoxic effect on MCF7 cells than free EA, both at 48 h and 72 h. This is possibly due to the conjugation of EA to the polymer chain. Then the cytotoxicity of oxaliplatin and M(Pt) was compared and as shown in Fig. 3c and d, M(Pt) was more toxic than oxaliplatin at 48 h and 72 h, in accordance with our expectation, as one of the nanodrugs' features is sustained release, which provides effective concentration for an extended period.
Next we evaluated three different combinations of free EA and DACHPt as well as the corresponding micellar formulations of the same ratios (EA/Pt = 0.5, 1 and 2). The results were illustrated in Fig. 3e–h. The abscissa is based on the Pt concentration, and the corresponding EA concentrations are 0.5, 1 or 2 times of Pt concentration according to the EA/Pt ratio. We can tell that: (1) compared to Fig. 3a–d, the combinations showed better antitumor efficacy than single drug usage, the details will be discussed later. (2) The curves shifted down as the ratio of EA/Pt increased, indicating that combining more EA in a reasonable range (that EA alone is nontoxic) can achieve a better sensitization to Pt drugs. (3) More importantly, the micellar formulations M(EA/Pt)s were much more effective than the corresponding free combinations both at 48 h and 72 h, which convincingly verified our strategy of co-delivery of EA and DACHPt by conjugating with a biodegradable polymer.
Sensitization of Pt drug efficacy via EA
To further clarify that the EA can sensitize cancer cells to DACHPt, the IC50 value of each group was collected and plotted in Fig. 4. As we can see in Fig. 4a and b, the IC50 values of oxaliplatin and M(Pt) at 48 h were 25.7 μM and 17.6 μM, respectively, and at 72 h, they were 19.1 μM and 7.8 μM. Longer incubation time benefited M(Pt), possibly because the degradation of the polymer and release of the drugs took time. As for the EA and M(EA), the IC50 for EA were 340 μM and 475 μM at 48 h and 72 h, respectively. Such high IC50 concentration indicated that the EA is quite low toxic. Moreover, after conjugation, the IC50 value of M(EA) changed to 775 μM, almost a 50% decrease in toxicity.As shown in Fig. 4c and d, the IC50 value of each combination group was significantly smaller than oxaliplatin and M(Pt) alone. For instance, oxaliplatin had an IC50 value of 25.7 μM at 48 h, whereas EA/Pt = 0.5, EA/Pt = 1 and EA/Pt = 2 had IC50 values of 20.5, 15.2 and 12.2 μM, respectively, an enhancement of 1.25, 1.69 and 2.1 fold. Considering that EA was almost non-toxic, the lowering of the IC50 values can be attributed to the specific sensitizing effect of EA. Similar results could be seen when the incubation time was prolonged to 72 h. As far as the M(EA/Pt) was concerned, the sensitizing of Pt drugs at 72 h was much more eminent. M(Pt) at 72 h had an IC50 value of 7.8 μM, whereas M(EA/Pt = 0.5), M(EA/Pt = 1) and M(EA/Pt = 2) had IC50 values of 4.2, 3.2 and 1.7 μM, corresponding to a sensitizing fold of 1.85, 2.44 and 4.59, respectively.
Combination index
To further understand the sensitization by EA, we also analyzed the combination index (CI). As described by Ting-Chao Chou,30 the effect of drug combination can be quantitatively defined as synergism (CI < 0.90), additive effect (CI = 0.90–1.10), and antagonism (CI > 1.10). And synergism can be subdivided into slight synergism (CI = 0.85–0.90), moderate synergism (CI = 0.7–0.85), synergism (CI = 0.3–0.7), strong synergism (CI = 0.3–0.1) and very strong synergism (CI < 0.1). As shown in Fig. 4e–h, all the CI values for EA/Pt combinations are ranging from 0.8 to 0.4, suggesting that EA/Pt combinations generated moderate synergism to synergism. As for M(EA/Pt)s, the CI values of 72 h were lower than the corresponding free EA/Pt combinations, showing synergism to strong synergism. The CI value for M(EA/Pt = 2) at 72 h was 0.22, showing the strongest synergism, although the real EA dose was far below the IC50 of EA or M(EA). Therefore, it can be concluded that our combination strategy is effective, and the nanoparticles mediated approach shows much better synergism than simply co-administering the two free drugs.Pt intracellular uptake and Pt–DNA adducts
It is known that the platinum agents function as anticancer drugs majorly by finally forming Pt–DNA adducts, and one of the most common mechanisms of platinum resistance is the reduced platinum accumulation.3–6 Thus, we evaluated the levels of both the intracellular Pt and Pt–DNA adducts to see whether our strategy overcame the obstacle.For intracellular uptake, MCF-7 cells were exposed to different drug formulations with equal amount of platinum of 10 μM for 4 h, and for Pt–DNA adduct determination, that was 24 h with 2 μM. The results are shown in Fig. 5. First, the Pt content in oxaliplatin + EA treated cells is 5.3 ng Pt mg−1 protein, nearly 8 times as much as that in oxaliplatin alone treated cells (0.7 ng Pt mg−1 protein), convincingly indicating that EA can assist cancer cells in preserving oxaliplatin. This may possibly due to the inhibition of GST enzyme activity. As described previously, GST catalyzes the combination of GSH with Pt, after which the GS–Pt–SG complex could be pumped out, thus leading to detoxification of Pt drugs.4 Second, not surprisingly, internalized by endocytosis, the nanoparticle formulation M(Pt) got a much better result than small molecule drug. Third, the M(EA/Pt) group achieved 10.5 ng Pt per mg protein, almost 15 times as much as that of free oxaliplatin alone, and twice as much as oxaliplatin + EA or M(Pt), indicating our strategy of delivering both EA and DACHPt in hybrid micelles can take advantage of both the EA and the nanoparticle mediated approach.
 | ||
| Fig. 5 Intracellular uptake of Pt and Pt–DNA adducts formation. The Pt contents in proteins or DNAs were tested by ICP-MS. | ||
Furthermore, in Fig. 5b, we can see the amount of Pt–DNA adducts formed in the combination groups (oxaliplatin + EA, 14.4 pg Pt per μg DNA and M(EA/Pt), 10.2 pg Pt per μg DNA) were about 4 to 5 times as much as that of oxaliplatin alone. This proved that EA can also assist oxaliplatin to form more Pt–DNA adducts in cancer cells.
Taken together, the cellular uptake and Pt–DNA adduct experiments gave a reasonable explanation to the enhancement of the cytotoxicity of M(EA/Pt) shown in Fig. 3 and 4, and further corroborated that this enhancement was due to efficient internalization of the M(EA/Pt) nanoparticles by the cancer cells via endocytosis on one hand, and due to the inhibitory effect of EA specie released from the micelles towards the GST enzyme and consequently towards the GSH-detoxification effect on the other hand.
In vivo antitumor study
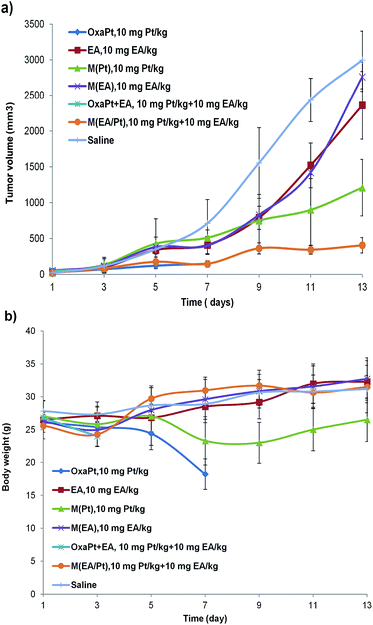
At last, to evaluate the antitumor efficacy of the hybrid micelles in vivo, we established subcutaneous H22 xenograft models on five-week-old KM mice. The mice were randomly grouped when tumor size reached ∼50 mm3 (7 groups, 10 mice per group), and subjected to intravenous administration of the drugs on day 1, 3, and 5, naming the first injection day as day 1. Then the body weight and tumor size were monitored every other day for two weeks. The results were depicted in Fig. 6.As shown in Fig. 6a, we can tell: (1) the EA group and M(EA) group (10 mg kg−1) were least effective, which was in accordance with our expectation and the in vitro results. (2) The oxaliplatin + EA group (10 mg Pt per kg + 10 mg EA per kg) and the oxaliplatin alone group (also 10 mg Pt per kg) showed great systemic toxicity, all the mice died before day 3 and day 7 respectively. Apparently 10 mg Pt per kg of free oxaliplatin is too high to dose a mouse. (3) At the same time, the equal Pt dosage of M(Pt) group showed much better tolerance with moderate tumor inhibition. (4) Our hybrid nanodrug M(EA/Pt = 1) exhibited the best antitumor efficacy, it almost halted the tumor growth since day 9, several days after the 3rd injection. Moreover, there were no deaths throughout the procedure. This powerfully validated that the M(EA/Pt) approach works in vivo better than oxaliplatin and EA or their combination, when both the efficacy and toxicity are taken into account.
Fig. 6b shows the average body weight of every group. It is clear that the oxaliplatin group and EA/Pt combination group are too toxic to proceed the experiment, the mice rapidly lost body weight after the administration, and died in one week. On the contrary, although weight loss was observed during the injection period, the M(Pt) group and the M(EA/Pt) group gradually regained body weight as much as the saline group, and survived the entire procedure. This corroborated the safety of micellar hybrid nanoparticles M(EA/Pt).
Materials and methods
Reagents
Oxaliplatin was purchased from Qilu Pharmaceutical, Co., Ltd. (Jinan, China). Ethacrynic acid (EA) was purchased from Sigma-Aldrich (USA). 1,2-Diamine-cyclohexane-platinum(II) dichloride (DAHPt(II)) was prepared as previously described in our published paper.23 The block copolymers poly(ethylene glycol)-block-poly(l-lactide-co-2-methyl-2-carboxyl-propylene carbonate) (MPEG-b-P(LA-co-MCC)) and poly(ethylene glycol)-block-poly(l-lactide-co-2-methyl-2-carboxyl-propylene carbonate-ethanol amine) (MPEG-b-P(LA-co-MCC-OH)) were synthesized as previously described.18,23 The molecular formula of the polymers used in this paper were MPEG5000-b-P(LA1000-co-MCC960) and MPEG5000-b-P(LA1000-co-MCC960-OH), respectively by proton nuclear magnetic resonance (1H NMR). The subscript denotes the molecular weight of each block. N,N-Dicyclohexylcarbodiimide (DCC) and 4-dimethylaminopyridine (DMAP) were purchased from Sigma-Aldrich (USA). Other chemicals and solvents were obtained commercially and used directly as needed.Cell lines and cell culture
MCF7 (human breast cancer) cells were purchased from Sigma (USA) and cultured in DMEM (Life Technologies, USA) supplemented with 10% fetal bovine serum (Life Technologies), 2 mM L-glutamine and 1% penicillin/streptomycin in 5% CO2 at 37 °C. H22 cells (murine liver cancer cells, a generous gift from Tongji Hospital, Wuhan, China) were cultured in RMPI-1640 (Life Technologies, USA) containing 10% fetal bovine serum, 2 mM L-glutamine and 1% penicillin/streptomycin in 5% CO2 at 37 °C.Synthesis of MPEG-b-P(LA-co-MCC/Pt) conjugates (P(Pt))
P(Pt) was prepared as previously described in our published paper.23Synthesis of MPEG-b-P(LA-co-MCC/EA) conjugates (P(EA))
EA was conjugated to the polymer MPEG-b-P(LA-co-MCC) with carboxyl groups with ease using DCC/DMAP. Briefly, EA (0.151 g, 0.5 mmol) was put into a pre-dried round flask, to which 30 mL of dried dichloromethane (DCM) was added to fully dissolve it. Then MPEG-b-P(LA-co-MCC) (0.33 g, 0.282 mmol carboxyl group) CH2Cl2 was added to the flask. The flask was then transferred to ice bath immediately. After that, DCC (0.103 g, 0.3 mmol) and DMAP (0.030 g, 0.25 mmol) were added into the mixed solution. The reaction mixture was left in ice bath for 12 hours and then was filtered to remove DCU formed and precipitated into ethyl ether. The solid product was collected by filtration, washed thoroughly with ether and vacuum-dried to get white powders. Then the product was dissolved in dimethyl sulfoxide (DMSO) and the solution was dialyzed against water to remove trace un-reacted EA, and finally lyophilized to obtain P(EA) conjugate (0.25 g, yield: 60.9%).Preparation of drug loaded micelles (M(Pt), M(EA) and M(EA/Pt))
The hybrid micelles M(EA/Pt) were prepared by co-precipitation method with a molar ratio of EA/Pt = 0.5, 1 and 2, respectively. Taking the ratio of EA/Pt = 1 as an example, P(EA) and P(Pt) with a molar ratio of EA/Pt equal to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 were mixed and dissolved in a flask containing an prescribed amount of acetone (total polymer concentration 10% w/v), and then water of double volume of the acetone used was added drop-wise into the flask under stirring to form a micellar solution. The solution was rotary evaporated to remove acetone and then freeze-dried to obtain the M(EA/Pt). The individual micelles of P(EA) and P(Pt) were prepared in a similar way. To simplify the nomination of all the micelles, “M” is used to stand for micelles. Therefore, P(EA) micelles can be written as “M(EA)” and M(EA/Pt) stands for a hybrid micelle of P(EA) and P(Pt), i.e., a combination of P(EA) and P(Pt).
1 were mixed and dissolved in a flask containing an prescribed amount of acetone (total polymer concentration 10% w/v), and then water of double volume of the acetone used was added drop-wise into the flask under stirring to form a micellar solution. The solution was rotary evaporated to remove acetone and then freeze-dried to obtain the M(EA/Pt). The individual micelles of P(EA) and P(Pt) were prepared in a similar way. To simplify the nomination of all the micelles, “M” is used to stand for micelles. Therefore, P(EA) micelles can be written as “M(EA)” and M(EA/Pt) stands for a hybrid micelle of P(EA) and P(Pt), i.e., a combination of P(EA) and P(Pt).
Drug release from hybrid micelles M(EA/Pt = 1)
50 mg M(EA/Pt = 1) in 5 mL of phosphate buffer solution (PBS, 0.01 M, pH 7.4) was put into a dialysis bag (molecular weight cutoff of 3.5 kDa), which was then immersed into 45 mL PBS (0.01 M, pH 7.4). The dialysis was performed at 37 °C in a shaking culture incubator. At desired time intervals, 1.5 mL of samples was withdrawn from outside the dialysis bag and 1.5 mL of fresh PBS was supplemented into the incubation medium. The same procedure was performed in acetate buffer solution (pH 5.0, 0.01 mM). Platinum and EA released from the hybrid micelles were measured by ICP-OES and UV-vis (wavelength: 262 nm), respectively. Each of the drugs released from the micelles was expressed as cumulative percentage of the drug outside the dialysis bag to the total drug in the original micelles.In vitro toxicity assay
In vitro efficacy was evaluated by MTT(3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl-tetrazoliumbromide) assay. MCF7 cells were seeded in 96-well plates at a density of 104 cells per well and exposed to various drug formulations for 48 h or 72 h, 20 μL of MTT solution (5 mg mL−1) was added and incubated for another 4 h, then replace the media with 150 μL DMSO to dissolve the formazan crystals and read absorbance at 495 nm. In terms of the drug concentrations, for oxaliplatin and M(Pt), the concentrations tested were from 0.0064 to 100 μM (5 × dilution); for EA and M(EA), the concentrations tested were set from 0.078 mM to 5 mM (2 × dilution); for drug combinations, the drug concentrations were set from 0.382 to 25 μM (2 × dilution) based on Pt.Pt intracellular uptake and Pt–DNA adducts formation in vitro
MCF7 cells were seeded in 6-well plates at a density of 106 cells per well and treated with 10 μM Pt of every drug formulation for 4 h, washed with cold PBS three times to remove surface-bound drugs, and harvested by trypsinization. Each cell pellet was divided into two portions. The first portion was for protein extraction, using Membrane and Cytosol Protein Extraction Kit (Beyotime, China), and the protein concentrations were tested by BCA assay. The second one was lysed with 1 mL 70% nitric acid, evaporated to near-dry on 95 °C heating panel and then dissolve in 0.5 mL deionized water, then the Pt content was determined by ICP-MS. The uptake of drugs was expressed as “ng Pt per mg protein”.As to the Pt–DNA adducts formation, the cells were treated with every drug formulation with 2 μM Pt for 24 h, washed five times with cold PBS, then harvested by trypsinization. Genomic DNA was extracted and purified using DNAZOL (Life Technologies, NY, USA) according to the manufacturer's instruction and concentrations were measured by Nanodrop 2000 (NanoDrop Technologies, Wilmington, DE). An aliquot of DNA (60 μL) was digested with 70% nitric acid (64 μL) in a 65 °C water bath overnight. And then diluted with 776 μL water containing indium and Triton X-100 to achieve a final concentration of 5% acid (final concentration of 1 ppb for indium and 0.05% Triton X-100). The Pt concentration was determined by ICP-MS.
In vivo antitumor study
All animal experiments were approved by the Animal Care and Use Center of Tongji medical college, Huazhong University of Science and Technology. Female KM mice (5–6 week, 18–20 g) were purchased from Experimental Animal Center, Huazhong University of Science and Technology (Wuhan, China). For preparation of subcutaneous model, 0.2 mL H22 cells (1 × 105 in PBS/100 μL) were injected subcutaneously into the right flank of the KM mice. When the tumor nodules grew to ca. 50 mm3, mice were randomly grouped into 7 with 10 mice per group. Then, the mice were injected with EA (10 mg kg−1), oxaliplatin (10 mg kg−1 of Pt), EA plus oxaliplatin (10 mg kg−1 of EA plus 10 mg kg−1 of Pt), M(Pt) (5 mg kg−1), M(EA) (10 mg kg−1), and M(EA/Pt) (10 mg kg−1 of EA plus 10 mg kg−1 of Pt) and saline, respectively. Mice were intravenously injected three times on day 1, 3, and 5, respectively. Tumor length (maximum axis of the tumor) and width (axis vertical to length) were measured with calipers. Body weight and tumor volume of each mouse were measured every two days over a period of 2 weeks. The tumor volume was calculated using the following equation: Tumor volume (V) = length × width2/2, as previously described.23,24 Tumor growth and relative body weight curves were plotted using the average tumor volume and mean relative body weight in each group.Conclusions
In summary, we constructed a platform based on biodegradable polymer self-assembled nanoparticles loading with both EA and DACHPt, a precursor of oxaliplatin. EA was acting as a GST inhibitor, lowering GSH-mediated Pt detoxification and ultimately, sensitizing cancer cells to Pt drugs. Representative M(EA/Pt = 1)s possess a spherical morphology with mean diameter of 119 nm and zeta potential of −5.6 mV. In vitro study showed M(EA/Pt)s can take advantage of both EA and nanoparticles mediated approach: M(EA/Pt)s can release drugs within cancer cells faster than that in the blood circulation, and EA can sensitize cancer cells to platinum and can increase the cellular Pt accumulation and Pt–DNA adduct formation. In vivo studies validated that M(EA/Pt) was much better than oxaliplatin or even the oxaliplatin + EA combination, as it could effectively inhibit tumor growth with a milder systemic toxicity. In conclusion, we showed a promising approach to sensitize cancers to platinum based chemotherapy.Acknowledgements
This work was supported by National Natural Science Foundation of China (81272860), Wuhan Science and Technology Bureau (2014060101010042) and China Postdoctoral Science Foundation (2012T50650).References
- B. Rosenberg, L. VanCamp and T. Krigas, Nature, 1965, 205, 698 CrossRef CAS.
- B. Rosenberg, L. VanCamp, J. E. Trosko and V. H. Mansour, Nature, 1969, 222, 385 CrossRef CAS.
- S. Dasari and P. Bernard Tchounwou, Eur. J. Pharmacol., 2014, 740, 364 CrossRef CAS PubMed.
- L. Kelland, Nat. Rev. Cancer, 2007, 7, 573 CrossRef CAS PubMed.
- Z. H. Siddik, Oncogene, 2003, 22, 7265 CrossRef CAS PubMed.
- C. A. Rabik and M. E. Dolan, Cancer Treat. Rev., 2007, 33, 9 CrossRef CAS PubMed.
- N. Traverso, R. Ricciarelli, M. Nitti, B. Marengo, A. L. Furfaro, M. A. Pronzato, U. M. Marinari and C. Domenicotti, Oxid. Med. Cell. Longevity, 2013, 2013, 972913 Search PubMed.
- F. Michelet, R. Gueguen, P. Leroy, M. Wellman, A. Nicolas and G. Siest, Clin. Chem., 1995, 41, 1509 CAS.
- M. Schultz, S. Dutta and K. D. Tew, Adv. Drug Delivery Rev., 1997, 26, 91 CrossRef CAS.
- S. Hamada, M. Kamada, H. Furumoto, T. Hirao and T. Aono, Gynecol. Oncol., 1994, 52, 313 CrossRef CAS.
- S. Goto, T. Iida, S. Cho, M. Oka, S. Kohno and T. Kondo, Free Radical Res., 1999, 31, 549 CrossRef CAS.
- W. H. Ang, I. Khalaila, C. S. Allardyce, L. Juillerat-Jeanneret and P. J. Dyson, J. Am. Chem. Soc., 2005, 127, 1382 CrossRef CAS PubMed.
- C. H. Wang, H. T. Wu, H. M. Cheng, T. J. Yen, I. H. Lu, H. C. Chang, S. C. Jao, T. K. Shing and W. S. Li, J. Med. Chem., 2011, 22, 8574 CrossRef PubMed.
- J. H. Ploemen, A. Van Schanke, B. Van Ommen and P. J. Van Bladeren, Cancer Res., 1994, 54, 915 CAS.
- J. H. Ploemen, B. Van Ommen, J. J. Bogaards and P. J. Van Bladeren, Xenobiotica, 1993, 23, 913 CrossRef CAS.
- M. Petrini, A. Conte, F. Caracciolo, A. Sabbatini, B. Grassi and G. Ronca, Br. J. Haematol., 1993, 85, 409 CrossRef CAS PubMed.
- P. J. O'Dwyer, F. LaCreta, S. Nash, P. W. Tinsley, R. Schilder, M. L. Clapper, K. D. Tew, L. Panting, S. Litwin, R. L. Comis and R. F. Ozols, Cancer Res., 1991, 51, 6059 Search PubMed.
- H. Xiao, R. Qi, S. Liu, X. Hu, T. Duan, Y. Zheng, Y. Huang and X. Jing, Biomaterials, 2011, 32, 7732 CrossRef CAS PubMed.
- Z. Yu, R. M. Schmaltz, T. C. Bozeman, R. Paul, M. J. Rishel, K. S. Tsosie and S. M. Hecht, J. Am. Chem. Soc., 2013, 135, 2883 CrossRef CAS PubMed.
- C. Bhattacharya, Z. Yu, M. J. Rishel and S. M. Hecht, Biochemistry, 2014, 53, 3264 CrossRef CAS PubMed.
- H. Gao, Q. Zhang, Z. Yu and Q. He, Curr. Pharm. Biotechnol., 2014, 15, 210 CAS.
- H. Rosen and T. Abribat, Nat. Rev. Drug Discovery, 2005, 4, 381 CrossRef CAS PubMed.
- H. Xiao, H. Song, Q. Yang, H. Cai, R. Qi, L. Yan, S. Liu, Y. Zheng, Y. Huang, T. Liu and X. Jing, Biomaterials, 2012, 33, 6507 CrossRef CAS PubMed.
- R. Qi, S. Liu, J. Chen, H. Xiao, L. Yan, Y. Huang and X. Jing, J. Controlled Release, 2012, 159, 251 CrossRef CAS PubMed.
- H. Maeda, J. Wu, T. Sawa, Y. Matsumura and K. Hori, J. Controlled Release, 2000, 65, 271 CrossRef CAS.
- H. Xiao, W. Li, R. Qi, L. Yan, R. Wang, S. Liu, Y. Zheng, Z. Xie, Y. Huang and X. Jing, J. Controlled Release, 2012, 163, 304 CrossRef CAS PubMed.
- H. Xiao, D. Zhou, S. Liu, Y. Zheng, Y. Huang and X. Jing, Acta Biomater., 2012, 8, 1859 CrossRef CAS PubMed.
- K. Engin, D. B. Leeper, J. R. Cater, A. J. Thistlethwaite, L. Tupchong and J. D. McFarlane, Int. J. Hyperthermia, 1995, 11, 211 CrossRef CAS PubMed.
- R. J. Gillies, N. Raghunand, G. S. Karczmar and Z. M. Bhujwalla, J. Magn. Reson. Imag., 2002, 16, 430 CrossRef PubMed.
- T. C. Chou, Pharmacol. Rev., 2006, 58, 621 CrossRef CAS PubMed.
Footnote |
| † These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2014 |