Rapid synthesis of NADPH responsive CdSe quantum dots from selenium nanoparticles†
Abstract
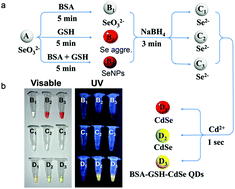
Herein we developed a new approach for CdSe quantum dots (QDs) synthesis. These bovine serum albumin (BSA) and glutathione (GSH) conjugated CdSe QDs (BSA–GSH–CdSe QDs) are of low cytotoxicity and NADPH responsive. The whole synthetic process can be completed within 8 minutes at room temperature.

 Please wait while we load your content...
Please wait while we load your content...