In situ synthesis of environmentally benign montmorillonite supported composites of Au/Ag nanoparticles and their catalytic activity in the reduction of p-nitrophenol
Biswajoy Bagchia,
Pradip Thakurb,
Arpan Koolb,
Sukhen Das*b and
Papiya Nandyb
aFuel Cell and Battery Division, Central Glass and Ceramic Research Institute, CSIR-CGCRI, Kolkata-700032, India
bPhysics Department, Jadavpur University, Kolkata-700032, India. E-mail: sdasphysics@gmail.com
First published on 7th November 2014
Abstract
In the present work, composites of montmorillonite clay supported silver and gold nanoparticles were synthesized by in situ chemical reduction method and characterized by X-ray diffraction (XRD), Fourier Transform Infrared Spectroscopy (FTIR), UV-vis spectroscopy and Transmission Electron Microscopy (TEM). The clay–nanoparticle composites were synthesized at two different temperatures (25 °C and 75 °C) where nanoparticle size was found to depend on synthesis temperature. The distribution of the catalytic nanoparticles was uniform in the clay matrix with sizes in the range of 20–45 nm (at 25 °C) and 5–15 nm (at 75 °C), respectively. Catalytic activity of the clay–nanoparticle composites were monitored by UV-visible spectroscopy using p-nitrophenol and NaBH4 as model reactants. The best catalytic efficiency was observed in the case of silver–clay nanocomposites with a rate constant of 5.6 × 10−3 s−1.
1. Introduction
The interest in noble metal nanoparticles (NPs) has grown tremendously over time due to their remarkable potential in diverse applications such as electronic, chemical, biological, and pharmaceutical fields.1,2 These NPs exhibit some unique physico-chemical properties which distinguishes them from their bulk counterparts. However, optimum utilization of such properties requires fine tailoring because they depend on size, shape, morphology, and surface charge, which in turn depend on precursor molecules, synthesis route, nature of reducing agent and coating agent used.3,4Out of several synthesis route reported, wet chemical synthesis has been widely used for producing metal NPs because of high chemical purity, homogeneity and yield of products. The process is also very simple and allows for high degree of versatility leading to tunable metal NPs. However, several hazardous organic and inorganic reagents (NaBH4, CTAB etc.) are used in the wet synthesis technique which has raised concerns regarding the impact of chemically grown metal NPs on living organisms and the environment. Consequently, new synthetic routes to produce metal NPs by nontoxic and environmentally acceptable way is gaining importance in research.5
One of the notable applications of noble metal nano clusters is the catalysis of chemical reactions. Due to their small size and high surface to volume ratio, these NPs exhibit unusually high activity in catalysis with respect to the inactive bulk material.6 This is of great importance because several toxic inorganic/organic chemicals are released in the environment and accelerated removal of such pollutants by nano-catalysis holds promise for a better ecology.
Especially, reduction of p-nitrophenol (4-NP) which is a non biodegradable toxic environmental pollutant has gained considerable concern.7 The reaction is also favored because the product 4-aminophenol (4-AP) is of commercial importance serving as intermediate in the synthesis of drugs, anti corrosion agent, photographic developer, hair dyes etc.8
Catalytic reduction of p-nitrophenol by CTAB stabilized Au nanoparticles were well studied by Fenger et al. and reported dependency of catalytic activity on particle size.6 Similar size dependency was observed by Gangula et al., where they used biogenic gold and silver nanoparticles to reduce 4-NP.5 Catalytic activity of chitosan stabilized silver nanoparticle was studied by Murugadoss and Chattopadhyay, where they reported linear dependency of catalytic activity on nanoparticle concentration.9 Similar studies were carried out on cationic polynorbornene stabilized silver nanoparticles by Baruah et al.10 A somewhat different observation was reported by Pozun et al., where they studied the catalytic effect of different dendrimer encapsulated bimetallic nanoparticles including Au/Cu and Au/Pt on nitro-aromatic compound degradation and reported that the activity is depended on binding energy of 4-NP to nanoparticle surface.11 Thus the catalytic activity of gold and silver nanoparticles depends on different factors like nanoparticle size, concentration, nature of nanoparticles and catalyst support.
Nanoparticles have a strong tendency to aggregate which reduces their catalytic efficiency and lifetime. Given the high reactivity of metal NPs, it is therefore almost customary to use some coating agents to inhibit aggregation of particles. Again as mentioned above, these coating agents generally includes polymers, inorganic molecules that are toxic to environment and may in some cases, may affect the catalytic property of the NPs designed for specific uses.12 Bearing this in mind, synthesis routes have been developed in which the NPs have been attached or grown on a solid inert support which not only act as a protective matrix but also provides high surface area to the overall composite by dispersing the particles.13 Corma and Serna studied selective hydrogenation of nitro groups by gold nanoparticles supported on TiO2 and Fe2O3.14 Perret et al., reported enhancement of catalytic activity in Mo2C and Mo2C/Al2O3 supported Au nanoparticles.15
Additionally, some oxide supports participate in charge transfer reactions with the catalyst further enhancing their activity.16,17 Thus the choice of catalyst support is very important for achieving catalytic optimal activity.
In this respect, metal nanoparticle–clay composite systems are a novel breed of composites in the sense that all the characteristics of the nanoparticles are fully retained with added advantage of being nontoxic to the environment due to the eco-friendly clay matrix. The clay matrix not only supports the metal nanoparticle but also protects the particles from oxidation and aggregation increasing stability and lifetime. Clays (especially montmorillonite) have a tendency to absorb a variety of compounds on their surface. Additionally, they can also intercalate large number of organic or inorganic cations. This is because, initially, negatively-charged interlayer sites of clays are balanced by small cations like Na+, Ca2+ etc. Over time, the clay may replace any foreign species or molecules in between the stacked silicate layers of the mineral causing expansion and swelling or contraction. This property called cation exchange capacity is the basis for the preparation of clay supported nano-composites.18–22 Further clays can also act as Bronsted and Lewis acids which in combination with the nanoparticles can serve as bi-functional catalysts.19
In the present work, a facile synthesis route has been developed for synthesis of montmorillonite based nano-composites of gold and silver and characterized by UV-vis spectroscopy, XRD, TEM and FTIR. Stable nano-composites were obtained which showed uniformly distributed nanoparticles. Catalytic activity of the hybrids has also been evaluated by reduction of p-nitrophenol by NaBH4.
2. Result and discussions
2.1 UV-vis spectroscopy
Fig. 2 shows the UV-visible absorption spectra of gold and silver clay nano-composites respectively. Both the nano-composites showed prominent surface plasmon bands indicating that the metal nanoparticles are effectively dispersed in the clay matrix without aggregation. Depending on the synthesis temperature (25 °C and 75 °C), nano-composites showed corresponding red shifts in the plasmon band. This relates to the formation of smaller sized particles (for Au75 and Ag75 (Fig. 2b and d)) at 75 °C and relatively larger sized particles at lower temperature of 25 °C (Fig. 2a and c). | ||
| Fig. 2 UV-visible absorption spectra of nanoparticle–MMT composites: (a) Au75, (b) Au25, (c) Ag75 and (d) Ag25. | ||
In the MMT-nanoparticle system, since both the clay particles and nanoparticles have less mobility at a low temperature therefore adsorbed particles may coalesce forming agglomerates after in situ reduction process. But at elevated temperature such inter particle interactions are minimized (due to increased kinetic energy of the precursor molecules) giving reduced particle size and uniform distribution in the clay matrix.23
2.2 X-ray diffraction
Phase characterizations of the nano-composites were carried out by X-ray diffraction technique. Fig. 3 shows the XRD pattern of nanoparticle loaded clay composites. Pristine MMT (black) showed characteristic reflections in the range 2θ = 4 to 70°.For nanoparticle loaded MMT, reflections due to clay crystallites are reduced and flattened while prominent reflections of metal nanoparticles appear for characteristic crystal planes (111, 200 and 220). This shows that the clay structure is somewhat disrupted after nanoparticle formation but majority of nanoparticles are primarily on the surface of clay platelets as evident from XRD reflections.19,24 It is to be noted that during synthesis, MMT is first treated with hydrazine which is reported to cause disruption and separation of layered structure resulting in MMT platelets. In the present case, in situ formed of nanoparticles are deposited on the clay platelets. It was also observed that the clay structure was retained more in case where the smaller size particles are formed i.e. for Au75 and Ag75 (Fig. 3 blue and magenta). Therefore, the size of the nanoparticles formed may also have effect on the stability of clay structure.
2.3 Transmission electron microscopy (TEM)
TEM micrographs of nano-composites are shown in Fig. 4. The nanoparticles were well dispersed in the clay matrix which indicated minimum inter-particle aggregation. The synthesized nanoparticles in the composite were in the range of 10–15 nm (Au75) (Fig. 4b) and 5–7 nm (Ag75) (Fig. 4d) at 75 °C. The same nano-composites showed larger nanoparticle inclusions for Au25 (25–45 nm) and Ag25 (10–15 nm) at 25 °C (Fig. 4a and c). Fig. 5 shows the corresponding particle size distribution of the nanocomposites.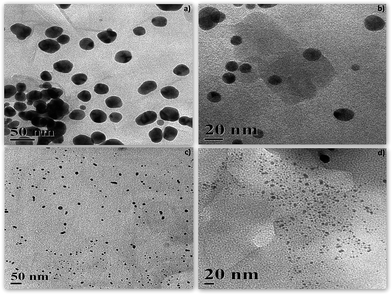 | ||
| Fig. 4 Transmission electron micrographs (TEM) of nanoparticle–MMT composites: (a) Au25, (b) Au75, (c) Ag75 and (d) Ag25. | ||
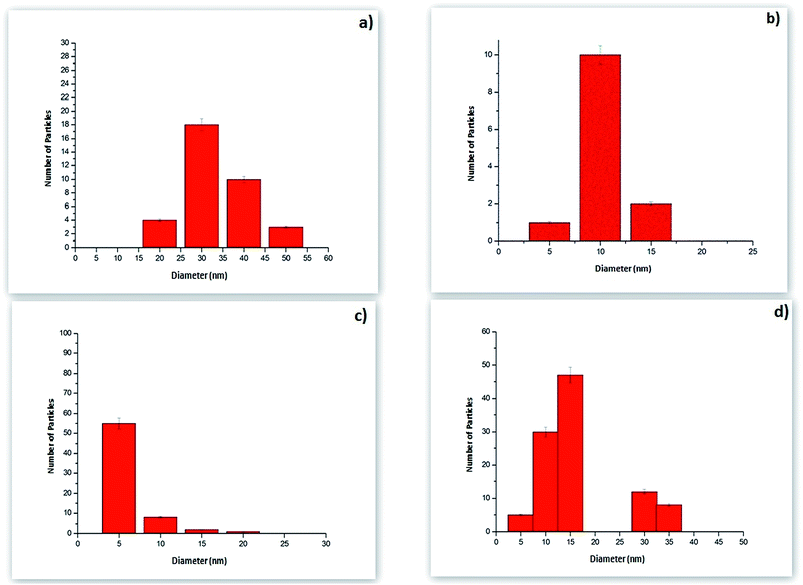 | ||
| Fig. 5 Particle size distribution of nanocomposites (a) Au25, (b) Au75, (c) Ag75 and (d) Ag25 determined from statistical analysis of the TEM images. | ||
This also supports the red shift in plasmon resonance bands of nanoparticles synthesized at low temperature in UV-visible spectroscopy. The gold nanoparticles were much larger at both the synthesis temperature (25 °C and 75 °C) and showed inter particle aggregation tendency while silver nanoparticles were smaller with uniform dispersion in the clay matrix. Both the nanoparticles showed more or less spherical morphologies with some oval shaped particles for gold. Since no coating agent was used during synthesis it is therefore evident that the clay matrix plays a vital role in stabilizing the nanoparticles for a sufficient period of time without affecting characteristic properties of individual nanoparticles.
2.4 FTIR
The FTIR spectra of MMT and nanoparticle–MMT composites are given in Fig. 6. For all the samples, characteristic bands of MMT were present around 3633 cm−1 (O–H stretching), 3452 cm−1 (inter-layer O–H stretching, H-bonding), 1641 cm−1 (H–O–H bending), 1126 and 1042 cm−1 (Si–O stretching), 917 and 799 cm−1 (Al–OH stretching), 525 cm−1 and 464 cm−1 for Si–O bending mode vibrations (Fig. 6).20 This indicates that the overall structure of montmorillonite is retained in the nano-composites.However, in case of these nanoparticle–MMT composites, the bands are somewhat shifted to higher wavelengths and is attributed to probable van der Waals interaction between the oxygen atoms in MMT and metal nanoparticles (Fig. 6). The appearance of a new band around 1402 cm−1 in the composites is due to the conformational changes in the metal nanoparticle linked clay.18,20,25,26 This band is observed to be more prominent in case of silver nanoparticle–clay composites (Ag25 and Ag75) than gold indicating a more intimate interaction of silver nanoparticles with the clay matrix. This may explain the relatively smaller size and uniform distribution of silver nanoparticles in the clay matrix as observed through TEM (Fig. 4). As mentioned in the previous section, absence of any coating agent indicates direct interaction of clay particles with naked metal nanoparticles. In most of earlier reporting of clay nanoparticles composites, interaction is based on hydrogen bonding between functional groups on coated nanoparticles. Thus this type of metal–clay direct interaction is primarily based on van der Waals interaction though further investigation is needed on this subject.
2.5 Catalysis
The catalytic model reaction of p-nitrophenol (4-NP) to p-aminophenol (4-AP) by sodium borohydride with nanoparticles–MMT hybrids were investigated using a standard UV-vis setup.An initially yellow solution of p-nitrophenolate turned gradually colourless in a period of 0–10 minutes upon addition of Au/AgNp–MMT. An absorption band centered at approximately 298 nm evolves in the UV-vis spectra and indicates the formation of p-aminophenolate, the intensity of which correlates with the concentration of the p-aminophenolate. Catalytic reaction progressed almost instantaneously with no induction time after the addition of AuNp–MMT and AgNp–MMT. Generally, in system with unsupported metal nanoparticles a typical induction period of about 60 to 90 seconds is observed depending on the size of the nanoparticles. This induction time is attributed to diffusion processes as reported by Wunder et al.27 However, in the present case, since the metal nanoparticles are stationary on the clay matrix such diffusion process is minimized. As a catalytic blank system for reference purposes we mixed p-nitrophenol with sodium borohydride without the addition of gold or silver clay hybrids. The solution remained unchanged for many weeks.
Fig. 7–10, shows the catalytic behavior and reaction kinetics of Au75, Au25, Ag75 and Ag25 respectively at three different concentrations of catalyst (in terms of volume). With the addition of catalyst, the absorption peak (at 415 nm) corresponding to 4-NP gradually decreased within a short interval of 10 minutes. The peak at around 300 nm emerges showing formation of 4-AP due to reduction of nitro functional group in 4-NP. All the reactions followed first order reaction kinetics. The rate constants (K) of the reactions are represented in Table 3.
| Sample | MMT–hydrazine (mg) | Water (mL) | Au/Ag ion conc. (mM) | Synthesis temperature (°C) | Colour |
|---|---|---|---|---|---|
| Au25 | 20 | 15 | 7 | 25 | Blue |
| Au75 | 20 | 15 | 7 | 75 | Red |
| Ag25 | 20 | 15 | 0.1 | 25 | Reddish yellow |
| Ag75 | 20 | 15 | 0.1 | 75 | Yellow |
| Au25/Au75 (μL) | Ag25/Ag75 (μL) | PNP (μL) | NaBH4 (μL) | Water (μL) | |
|---|---|---|---|---|---|
| Set 1 | 20 | 30 | 200 | 2750 | |
| Set 2 | 50 | 30 | 200 | 2720 | |
| Set 3 | 100 | 30 | 200 | 2670 | |
| Set 4 | 5 | 30 | 200 | 2765 | |
| Set 5 | 10 | 30 | 200 | 2760 | |
| Set 6 | 15 | 30 | 200 | 2755 |
| Sample | Volume added (μL) | K (×10−3) s−1 |
|---|---|---|
| Au25 | 20 | 0.302 |
| 50 | 0.72 | |
| 100 | 0.027 | |
| Au75 | 20 | 0.884 |
| 50 | 4.597 | |
| 100 | 4.298 | |
| Ag25 | 5 | 5.187 |
| 10 | 5.626 | |
| 15 | 0.701 | |
| Ag75 | 5 | 5.476 |
| 10 | 5.067 | |
| 15 | 2.272 |
As evident, all the nano-composites showed good catalytic activity with optimum at a particular concentration. In case of gold nanoparticle hybrids, Au75 (Fig. 7d) showed a maximum K value of 4.597 × 10−3 s−1 for 50 μL whereas, for Au25 a value of 7.2 × 10−4 s−1 was observed (Fig. 8d). The observed decrease in K value for Au25 is due the large particle size due to inter-particle aggregation as observed through TEM.
However, highest catalytic activity was observed for Ag25 (K = 5.6 × 10−3 s−1) at 10 μL (Fig. 9d). It has been reported by several authors, that the mechanistic pathway of catalytic reduction of p-nitrophenol involves co-adsorption of the reactant and reducing agent (BH4−) on the surface of nanoparticle catalyst and subsequent electron relay from BH4− to the reactant mediated by catalyst.5,24,28 Fenger et al., reported a size of 13 nm to be ideal for catalytic gold nanoparticles to adsorb p-nitrophenols.6 Considering this, Au75 and Ag25 both have particle size in the range of 13 nm and hence shows good catalytic activity. However, increasing the concentration (volume) of the catalyst decreased the K value for both the nano hybrids (Fig. 7–10).
This is probably because of increased amount of clay particles masking the nanoparticles from the reactant.
Earlier report of comparative studies between catalytic AuNp/AgNp showed that AuNp is more active than AgNp as latter is prone to oxidation.5 But in the present case, the opposite was observed where AgNp–MMT hybrids showed maximum catalytic activity with reaction almost complete in less than 3 minutes. This is due to very small average particle size of AgNps compared to Au and is a direct consequence of initial interaction with the clay matrix. During synthesis, Ag+ ions from AgCl can easily enter the clay lamellae by cation exchange where they are reduced by hydrazine leading to very small particle size and increased protection by clay matrix. In case of gold, the reacting species is AuCl4− which is loosely adsorbed and is therefore reduced before entering the clay matrix which causes agglomeration. This is reflected in large particle size, instant AuNp formation (compared to AgNp) and more importantly low catalytic activity of AuNp–MMT hybrids.
It is well known that clay matrix (especially 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 layered clays) is effective in not only providing protection to adsorbed nanoparticles but also creates attachment sites for various molecules.20 Thus the increased catalytic activity of Ag25 observed may be attributed to a combination of multiple factors like protection of Ag nanoparticles from local oxidizing environment, prevention of inter-particle aggregation due to strong attachment to clay surface (it is to be noted from FTIR spectra that AgNps showed more intimate interaction with the clay matrix than AuNps) and most importantly, the clay matrix may itself may act as attachment site for adsorption of p-nitrophenolate ions and NaBH4 creating environment for formation of localized catalyst–reactant–reducing agent complexes facilitating catalysis (Fig. 11).
1 layered clays) is effective in not only providing protection to adsorbed nanoparticles but also creates attachment sites for various molecules.20 Thus the increased catalytic activity of Ag25 observed may be attributed to a combination of multiple factors like protection of Ag nanoparticles from local oxidizing environment, prevention of inter-particle aggregation due to strong attachment to clay surface (it is to be noted from FTIR spectra that AgNps showed more intimate interaction with the clay matrix than AuNps) and most importantly, the clay matrix may itself may act as attachment site for adsorption of p-nitrophenolate ions and NaBH4 creating environment for formation of localized catalyst–reactant–reducing agent complexes facilitating catalysis (Fig. 11).
 | ||
| Fig. 11 Schematic diagram showing probable mechanistic pathway for the catalytic activity of the nanoparticle–MMT composites. | ||
In fact, it has been proposed by Zhou et al., that the p-nitrophenol intercalates between the siloxane layer of montmorillonite and bonds through the ditrigonal space of the siloxane hexagonal units to the inner OH units29 and clay minerals, especially sepiolite and montmorillonite, are considered to be excellent supports for catalytic molecules. They can also exhibit Brönsted and Lewis acidities, which can be combined with the redox reactivity of metallic nanoparticles adsorbed on their surfaces to produce bi-functional catalysts.30–32 These interactions bring p-nitrophenolate in close proximity to the adsorbed nanoparticles creating the desired microenvironment for rapid catalysis.
3. Experimental
3.1 Materials
Montmorillonite clay (MMT) (<1 μm) (procured from NanoCor, USA), hydrogen tetrachloroauric acid (HAuCl4) (Merck), silver nitrate (AgNO3) (Sigma-Aldrich), hydrazine hydrate (N2H4) (Merck), p-nitrophenol (PNP) (Sigma-Aldrich), sodium borohydride (NaBH4), (Merck, India). All the chemicals are >99% pure.3.2 Methods
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (v/v) hydrazine hydrate (99–100%). The dispersion was then vigorously stirred overnight at 50 °C. The resulting mixture was centrifuged at 15
1 (v/v) hydrazine hydrate (99–100%). The dispersion was then vigorously stirred overnight at 50 °C. The resulting mixture was centrifuged at 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 10 minutes and then vacuum dried to form MMT–hydrazine complex.
000 rpm for 10 minutes and then vacuum dried to form MMT–hydrazine complex.In case of silver, 100 μL of AgNO3 (0.1 mM) was added similarly. This time the solution turned yellow after 5 minutes. The solutions were then centrifuged at 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 15 minutes. The resulting pellets were re-suspended in 1 mL of distilled water. Synthesis was also carried out at 25 °C keeping all the parameters same where blue and reddish yellow colored suspensions were observed for gold and silver respectively. A schematic diagram of the synthesis route is given in Fig. 1. It is to be noted that the reducing agent (hydrazine hydrate) used during nanoparticle reduction produces only N2 and H2O as byproduct which are inert.33 This in conjunction with montmorillonite makes the nanoparticle–clay hybrid system ecologically compatible. Table 1 designates the synthesized nano-composites with compositions.
000 rpm for 15 minutes. The resulting pellets were re-suspended in 1 mL of distilled water. Synthesis was also carried out at 25 °C keeping all the parameters same where blue and reddish yellow colored suspensions were observed for gold and silver respectively. A schematic diagram of the synthesis route is given in Fig. 1. It is to be noted that the reducing agent (hydrazine hydrate) used during nanoparticle reduction produces only N2 and H2O as byproduct which are inert.33 This in conjunction with montmorillonite makes the nanoparticle–clay hybrid system ecologically compatible. Table 1 designates the synthesized nano-composites with compositions.
Powder X-ray diffraction (XRD) patterns of Ag75 and Au75 were recorded using a Bruker AXS (Model D8, WI, USA) setup with CuKα radiation (1.5409 Å) and scan speed of 5° min−1 and scanning range from 3 to 70 (2θ). Pristine MMT clay was used as reference.
Fourier transform infrared spectroscopy (FTIR) (FTIR-8400S, Shimadzu) was also performed to determine the phases in the composite. Samples were prepared by KBr disk method,20 in which 0.2 g of KBr (spectroscopy grade) was thoroughly mixed with sample powder (1 wt% of KBr) and then made into disks by uni-axial pressing. Measurements were taken by an FTIR-8400S model (Shimadzu, Tokyo) with scanning range set from 400 to 2000 cm−1 under Happ–Genzel configuration.
Morphological characteristics of the hydrogels were observed by transmission electron microscope (TEM) (JSM 2100, JEOL Ltd., Japan). A minute quantity of samples were first suspended in aqueous solution and sonicated for 15 min before observation.
4. Conclusions
A montmorillonite clay based Au/Ag nanoparticle hybrid system is synthesized by in situ reduction process. The hybrids showed excellent stability with uniform nanoparticles distribution in the clay matrix with dimensions in the range of 5–50 nm depending on the synthesis temperature. Good catalytic activity was exhibited by both Au/Ag–clay hybrids but AgNp–clay hybrids were more active than AuNp. It was also observed that hybrids containing finer sized (∼5–10 nm) nanoparticles showed best results. Increased activity of Ag–clay nano hybrids may be attributed to the protective role of the clay matrix with the nanoparticles and increased reactant–catalyst interaction on the clay surface.Acknowledgements
We are grateful to Council of Scientific and Industrial Research (CSIR) and Department of Science and Technology (DST), Government of India for financial assistance. The authors are also indebted to Dr Kausik Dana, CSIR-CGCRI for his helpful discussion on the research work.References
- M. C. Daniel and D. Astruc, Chem. Rev., 2004, 104, 293–346 CrossRef CAS PubMed.
- A. Muller and A. K. Cheetham, in The Chemistry of Nanomaterials. Synthesis, Properties and Applications, ed. C. N. R. Rao, Wiley-VCH, Weinheim, 2004 Search PubMed.
- S. Coe, W. K. Woo, M. Bawendi and V. Bulovic, Nature, 2002, 420, 800–803 CrossRef CAS PubMed.
- D. Varade and K. Haraguchi, Soft Matter, 2012, 8, 3743–3746 RSC.
- A. Gangula, R. Podila, M. Ramakrishna, L. Karanam, C. Janardhana and A. M. Rao, Langmuir, 2011, 27, 15268–15274 CrossRef PubMed.
- R. Fenger, E. Fertitta, H. Kirmse, A. F. Thünemann and K. Rademann, Phys. Chem. Chem. Phys., 2012, 14, 9343–9349 RSC.
- T. L. Lai, K. F. Yong, J. W. Yu, J. H. Chen, Y. Y. Shu and C. B. Wang, J. Hazard. Mater., 2011, 185, 366–372 CrossRef CAS PubMed.
- P. Deka, R. C. Deka and P. Bharati, New J. Chem., 2014, 38, 1789–1793 RSC.
- A. Murugadoss and A. Chattopadhyay, Nanotechnology, 2008, 19, 15603 CrossRef CAS PubMed.
- B. Baruah, G. J. Gabriel, M. J. Akbashev and M. E. Booher, Langmuir, 2013, 29(13), 4225–4234 CrossRef CAS PubMed.
- Z. D. Pozun, S. E. Rodenbusch, E. Keller, K. Tran, W. Tang, K. J. Stevenson and G. Henkelman, J. Phys. Chem. C, 2013, 117, 7598–7604 CAS.
- P. K. Khanna, P. More, J. Jawalkar, Y. Patil and N. K. Rao, J. Nanopart. Res., 2009, 11, 793–799 CrossRef CAS.
- C. Xu, X. Lai, G. Zajac and D. W. Goodman, Phys. Rev. B: Condens. Matter Mater. Phys., 1997, 56, 13464–13482 CrossRef CAS.
- A. Corma and P. Serna, Science, 2006, 313, 332 CrossRef CAS PubMed.
- N. Perret, X. Wang, L. Delannoy, C. Potvin, C. Louis and M. A. Keane, J. Catal., 2012, 286, 172–183 CrossRef CAS PubMed.
- F. Cárdenas-Lizana, S. Gómez-Quero, N. Perret and M. A. Keane, Gold Bull., 2009, 42, 124 CrossRef.
- E. Florez, F. Viñes, J. A. Rodriguez and F. Illas, J. Chem. Phys., 2009, 130, 244706 CrossRef PubMed.
- V. Belova, H. Möhwald and D. G. Shchukin, Langmuir, 2008, 24, 9747–9753 CrossRef CAS PubMed.
- L. Zhu, S. Letaief, Y. Liu, F. Gervais and C. Detellier, Appl. Clay Sci., 2009, 43, 439–446 CrossRef CAS PubMed.
- B. Bagchi, S. Kar, S. K. Dey, S. Bhandary, D. Roy, T. K. Mukhopadhyay, S. Das and P. Nandy, Colloids Surf., B, 2013, 108, 358–365 CrossRef CAS PubMed.
- J. Y. Kim, K. J. Ihn and J. S. Na, J. Ind. Eng. Chem., 2011, 17, 248–253 CrossRef CAS PubMed.
- G. Nagendrappa, Appl. Clay Sci., 2011, 53, 106–138 CrossRef CAS PubMed.
- L. Ai and J. Jiang, Biotechnol., 2013, 132, 374–377 CAS.
- M. Venkatesham, D. Ayodhya, A. Madhusudhan, N. V. Babu and G. Veerabhadram, Appl. Nanosci., 2014, 4, 113–119 Search PubMed.
- R. Das, S. S. Nath and R. Bhattacharjee, J. Fluoresc., 2011, 21, 1165–1170 CrossRef CAS PubMed.
- M. B. Ahmad, K. Shameli, M. Darroudi, W. M. Zin, W. Yunus and N. A. Ibrahim, Am. J. Appl. Sci., 2009, 6(12), 2030–2035 CrossRef.
- S. Wunder, F. Polzer, Y. Lu, Y. Mei and M. Ballauff, J. Phys. Chem. C, 2010, 114, 8814–8820 CAS.
- K. Esumi, R. Isono and T. Yoshimura, Langmuir, 2004, 20, 237–243 CrossRef CAS.
- Q. Zhou, R. L. Frost, H. He and Y. Xi, J. Colloid Interface Sci., 2007, 314, 405–414 CrossRef CAS PubMed.
- J. B. d'Espinose de la Caillerie and J. J. Fripiat, Catal. Today, 1992, 14, 125–140 CrossRef.
- O. S. Ahmed, Langmuir, 2003, 19, 5540–5541 CrossRef CAS.
- C. H. Zhou, D. S. Tong, M. Bao, Z. X. Du, Z. H. Ge and X. N. Li, Top. Catal., 2006, 39, 213–219 CrossRef CAS PubMed.
- M. Groushko, A. Kamyshny, K. Ben-Ami and S. Magdassi, J. Nanopart. Res., 2009, 11, 713–716 CrossRef.
| This journal is © The Royal Society of Chemistry 2014 |







