Effect of collagen sponge incorporating Macrotyloma uniflorum extract on full-thickness wound healing by down-regulation of matrix metalloproteinases and inflammatory markers†
Abstract
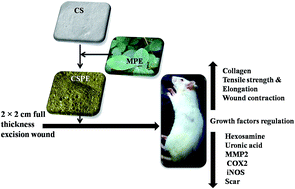
Collagen sponge (CS) was prepared using fish scales, which are a biological waste product in the marine food industry. CS was prepared so as to incorporate separately the drug mupirocin (thus designated CSM) and Macrotyloma uniflorum plant extract (CSPE). CS, CSM and CSPE were applied to the experimental wounds of rats and the healing pattern was observed using various biological and physicochemical techniques. CSPE enhanced wound healing and was involved in the up-regulation of growth factors such as vascular endothelial growth factor (VEGF), fibroblast growth factor (FGF), epidermal growth factor (EGF) and transforming growth factor β (TGF-β). Increased levels of hydroxyproline, hexosamine and uronic acid were observed in the CSPE-treated group compared with the other groups. Treatment with CSPE reduced inflammation, the expression of matrix metalloproteinases (MMPs) and scar formation, thereby contributing to faster wound healing.

 Please wait while we load your content...
Please wait while we load your content...