Synthesis and up/down conversion luminescence properties of Na0.5R0.5MoO4:Ln3+ (R3+ = La, Gd), (Ln3+ = Eu, Tb, Dy, Yb/Er) thin phosphor films grown by pulsed laser deposition technique†
Abstract
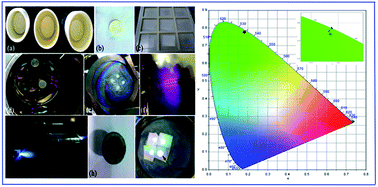
Single crystalline nano thin phosphor films of (Na0.5R0.5)MoO4:Ln3+ (R3+ = La, Gd), (Ln3+ = Eu, Tb, Dy, Yb/Er) have been deposited on quartz substrates by pulsed laser deposition (PLD) technique. In this work, we clearly explained the target preparation, pre-deposition cleaning of substrates using piranha cleaning, and optimized growth conditions of the thin films. The deposition was carried out using an Nd-YAG laser (λ = 1064 nm) in an ultra-high vacuum (UHV) with an oxygen back pressure of 300 mTorr at different substrate temperatures. To examine the crystal structure, 3D surface topography and film thickness, the as-deposited samples were characterized by X-ray diffraction (XRD), atomic force microscopy (AFM) and scanning electron microscopy (SEM), respectively. The thin phosphor films possess a scheelite tetragonal crystal structure with the space group I41/a. The down-conversion luminescence properties of Eu3+, Tb3+, Dy3+ doped (Na0.5R0.5)MoO4 phosphor films were studied in detail. Under 980 nm NIR laser excitation, the Yb3+/Er3+ doped (Na0.5R0.5)MoO4 thin films show intense up-converted green emission identified at 524 nm due to the transition from the populated 2H11/2 level to 4I15/2 ground level of the erbium ion. The fluorescence decay time for the major transitions in (Na0.5R0.5)MoO4 when doped with Eu3+ (5D0 → 7F2), Tb3+ (5D4 → 7F5), Dy3+ (4F9/2 → 6H13/2) and Yb3+/Er3+ (2H11/2 → 4I15/2) are determined and to assess the color richness, the CIE color coordinates were also calculated. The obtained results suggested that the potential (Na0.5R0.5)MoO4:Ln3+ thin phosphor films can serve as an excellent material for electro/cathodo-luminescence and display applications.

 Please wait while we load your content...
Please wait while we load your content...