Hardening process and properties of an epoxy resin with bio-based hardener derived from furfural
Abstract
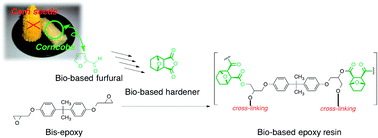
The development of bio-based plastics is an important research area because of its contribution to environmental preservation. Herein, we evaluated the properties of a bio-based epoxy resin synthesised using 2,2-bis(4-glycidyloxyphenyl)propane as the epoxy monomer and oxabicyclodicarboxylic anhydride (OBCA) as a bio-based hardener derived from furfural. The major hardening process of the resin at 130 °C, manifested in an exponential increase in the storage modulus, was complete within an hour, which was longer than that for a commercially available petroleum-based epoxy resin hardened using cis-cyclohexane dicarboxylic anhydride (CDCA). This indicated that OBCA was less reactive than CDCA, which has a similar structure. The bio-based epoxy resin showed excellent transparency in the visible light region and was thermally stable, with 5% weight loss at temperatures exceeding 270 °C. Glass transition temperatures were above 100 °C, and mechanical properties were moderately better than those of the commercially available epoxy resin. Moreover, the bio-based carbon content ranged from 21% to 53%, depending on the amount of OBCA added. Thus, the bio-based OBCA is a good hardener for a bio-based epoxy resin that can be used as a value-added material in industrial applications.

 Please wait while we load your content...
Please wait while we load your content...