First reusable ligand-free palladium catalyzed C–P bond formation of aryl halides with trialkylphosphites in neat water†
Abstract
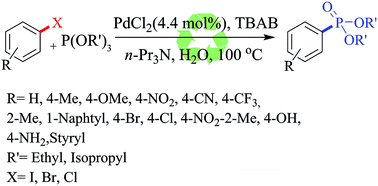
A reusable ligand-free palladium catalyzed phosphonation of aryl iodides, bromides and chlorides with trialkylphosphites is described for the first time in neat water. The aryl phosphonates are obtained in good to excellent yields. The reaction can be also performed with Ni(II) with longer reaction time. The role of tetrabutylammonium bromide in this reaction as reducing agent for generation of Pd(0) at room temperature is also demonstrated. Pd(0)/TBAB was easily reused for three runs without decreasing the efficiency.

 Please wait while we load your content...
Please wait while we load your content...