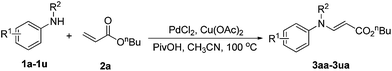
Palladium-catalyzed oxidative amination of activated olefins with N-alkyl anilines for synthesis of tertiary (E)-enamines†
Mi-Na Zhao,
Xiao-Li Lian,
Zhi-Hui Ren,
Yao-Yu Wang and
Zheng-Hui Guan*
Key Laboratory of Synthetic and Natural Functional Molecule Chemistry of Ministry of Education, Department of Chemistry & Materials Science, Northwest University, Xi'an 710127, P. R. China. E-mail: guanzhh@nwu.edu.cn
First published on 12th November 2014
Abstract
An efficient palladium-catalyzed oxidative amination of olefins with secondary anilines for the synthesis of tertiary (E)-enamines has been developed. Trimethylacetic acid (PivOH) played an important role in the reaction. This protocol tolerates a range of functional groups and is a reliable method for direct synthesis of tertiary (E)-enamines in high yields under mild conditions.
Enamines and their derivatives are prevalent in numerous natural products1 and have been widely applied in nucleophilic addition reactions for synthesis of chiral compounds2 or azaheterocycles.3–5 Conventionally, enamines were synthesized by condensation of aldehydes and ketones with amines, or Pd- or Cu-catalyzed cross-coupling of vinyl halides with amines.6 However, these methods often require rigorous conditions, such as exclusion of moisture and air. Therefore, novel and efficient methods for the direct synthesis of enamines have been and continue to be an active area of research.7
Oxidative amination reaction represents an attractive strategy for the construction of C–N bonds.8 Particularly, transition-metal-catalyzed oxidative amination of olefins is an important method to synthesize enamine derivatives. In recent years, palladium-catalyzed oxidative amination of olefins with nonbasic amination reagents such as amides, sulfonamides or imides, have been established for the synthesis of enamides.9 However, oxidative amination of olefins with anilines still remains challenging.10 The main issue of the reactions include: (a) the competed hydroamination of olefins occurred easily;11 (b) anilines could be oxidized to aromatic azo compounds;12 (c) olefins usually suffers from polymerization.13
Recently, utilizing an ortho-substituent strategy, Obora and co-workers have developed a palladium-catalyzed oxidative amination of olefins with ortho-substituted primary anilines for the synthesis of enamines.14 Palladium-catalyzed oxidative amination of olefins with primary anilines for the synthesis of enamines in the presence of LiBr have been reported by Jiang et al.15 However, these protocols are mainly focused on the synthesis of secondary (Z)-enamines. In connection with our continuous interest in the N-alkyl anilines and enamines' transformations,4,5 in this paper, we have developed an efficient palladium-catalyzed oxidative amination of olefins with secondary anilines for the synthesis of a series of synthetic useful tertiary (E)-enamines.5a
We commenced our study by investigating the palladium-catalyzed oxidative amination of N-methyl aniline 1a with n-butyl acrylate 2a (Table 1). The desired (E)-enamine 3aa was obtained in 15% yield in the presence of the Pd(OAc)2 catalyst and 1.2 equiv. of Cu(OAc)2 in CH3CN at 100 °C (Table 1, entry 1). To improve the reaction efficiency, various acids and bases were screened as additives. Experiments suggest that the acid additive plays an important role in the reaction (Table 1, entries 4–6). The yield of (E)-enamine 3aa was dramatically improved to 81% when PivOH was used as an additive (Table 1, entry 6). Optimization of various palladium precursors and solvents revealed that PdCl2 and CH3CN were suitable catalyst and solvent respectively (Table 1, entry 11). Finally, the reaction temperature was also varied, and 100 °C gave the best result (Table 1, entries 13 and 14).
| Entry | Catalyst | Additive | Solvent | T (°C) | Yield (%) |
|---|---|---|---|---|---|
| a Reaction conditions: 1a (0.2 mmol), 2a (0.3 mmol), [Pd] (5 mol%), Cu(OAc)2 (1.2 equiv.), additives (1.0 equiv.) in solvent (2 mL) for 10 h in air; isolated yields. | |||||
| 1 | Pd(OAc)2 | — | CH3CN | 100 | 15 |
| 2 | Pd(OAc)2 | KOAc | CH3CN | 100 | 27 |
| 3 | Pd(OAc)2 | K2CO3 | CH3CN | 100 | 45 |
| 4 | Pd(OAc)2 | p-TsOH | CH3CN | 100 | 30 |
| 5 | Pd(OAc)2 | HOAc | CH3CN | 100 | 60 |
| 6 | Pd(OAc)2 | PivOH | CH3CN | 100 | 81 |
| 7 | Pd(OAc)2 | PivOH | Toluene | 100 | 61 |
| 8 | Pd(OAc)2 | PivOH | DCE | 100 | 70 |
| 9 | Pd(OAc)2 | PivOH | THF | 100 | 65 |
| 10 | Pd(OAc)2 | PivOH | DMSO | 100 | 15 |
| 11 | PdCl2 | PivOH | CH3CN | 100 | 84 |
| 12 | PdCl2(PPh3)2 | PivOH | CH3CN | 100 | 38 |
| 13 | PdCl2 | PivOH | CH3CN | 80 | 48 |
| 14 | PdCl2 | PivOH | CH3CN | 120 | 37 |
With the optimized reaction conditions established, the scope of the reaction was investigated (Table 2). This palladium-catalyzed oxidative amination reaction displayed good functional group tolerance and proved to be a general method for facile construction of tertiary (E)-enamines. N-Methyl anilines with electron-donating groups, such as methyl, methoxyl, or electron-withdrawing groups, such as fluoro, chloro, bromo, nitro, formyl and ester on the aryl rings all gave the corresponding tertiary (E)-enamines 3aa–3qa in good to high yields, thus indicating that the electronic nature of the substrates has little influence on the oxidative amination reaction. Ortho-substituted anilines proceeded smoothly to give the corresponding tertiary enamines 3ba, 3ea, 3ga and 3ja in high yields. These results revealed that the steric features of the 2-position of the anilines is indeed beneficial to the oxidative amination reaction, which is consistent with the results reported by Obora.14 Expectedly, α- or β-naphthyl anilines were also tolerated to afford the corresponding enamines 3ra, 3sa in moderate yields.
| a Reaction conditions: 1 (0.2 mmol), 2a (0.3 mmol), PdCl2 (5 mol%), Cu(OAc)2 (1.2 equiv.), PivOH (1.0 equiv.) in CH3CN (2 mL) for 6–12 h at 100 °C in air; isolated yields. |
|---|
 |
In addition, anilines with different alkyl substituent group on the nitrogen atom, such as ethyl substituted aniline show good reactivity, producing the corresponding enamine 3ta in 72% yield. Notably, cyclic substrate, such as tetrahydroquinoline 1u, was also tolerated in the reaction to give the desired (E)-butyl 4-(3,4-dihydroquinolin-1-(2H)-yl)but-2-enoate 3ua in 55% yield. However, aliphatic secondary amines, such as diethylamine and piperidine were less reactive and gave only trace amounts of the desired enamines.
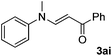
Next, various acrylates 2 were investigated to extend the substrate scope (Table 3). Acrylates, such as 2b–2e, showed good reactivity in the reaction to give the desired products 3ab–3ae in 60–84% yields (Table 3, entries 1–4). However, the (E)-3-(methyl(phenyl)amino)acrylonitrile 3ag was obtained in only 8% yield (Table 3, entry 6). And no reaction occurred when acrylamide 2f, ethyl 2-methylacylate 2h and 1-phenylprop-2-en-1-one 2i were used as the substrates (Table 3, entries 5, 7–8).
| Entry | Substrate | Product | Yield (%) |
|---|---|---|---|
| a Reaction conditions: 1a (0.2 mmol), 2 (0.3 mmol), PdCl2 (5 mol%), Cu(OAc)2 (1.2 equiv.), PivOH (1.0 equiv.) in CH3CN (2 mL) at 100 °C for 8–12 h in air; isolated yields. | |||
| 1 | 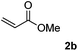 |
 |
75 |
| 2 |  |
 |
76 |
| 3 | 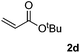 |
 |
60 |
| 4 |  |
 |
84 |
| 5 |  |
 |
0 |
| 6 |  |
 |
8 |
| 7 |  |
 |
0 |
| 8 |  |
 |
0 |
To demonstrate the synthetic utility of this reaction, a gram scale (10 mmol, 1.07 g) reaction was performed under the standard conditions. The desired (E)-enamine 3aa was obtained in 75% yield (Scheme 1).
To gain more insight into the mechanism of the reaction, the hydroamination product methyl 3-(methyl(phenyl)amino)propanoate 4 was conducted under the standard conditions (Scheme 2). No 3ab product was observed implying that the hydroamination/oxidation pathway is less likely.
On the basis of the aforementioned results and previous reports,9,14–16 a tentative mechanism for the reaction is proposed in Scheme 3. The reaction was initiated by the coordination of the olefin 2 to Pd(II) to give a palladium-olefin complex A, which undergoes nucleophilic attack by N-methyl aniline 1 to generate a σ-alkylpalladium complex B. We assumed that PivOH plays a role in the protonation of N-methyl aniline to prevent the competing coordination of N-methyl aniline with Pd(II),4a thus, promoting the coordination of acrylate 2 with Pd(II). Subsequently, β-hydride elimination of the intermediate B affords the (E)-enamine 3 and Pd(0) species. Finally, the Pd(0) species was reoxidized by Cu(OAc)2 to regenerate the active Pd(II) catalyst.
 | ||
| Scheme 3 Tentative mechanism for palladium-catalyzed oxidative amination of olefins with secondary anilines. | ||
In summary, we have developed an efficient palladium-catalyzed oxidative amination of olefins with secondary anilines for the synthesis of tertiary (E)-enamines. PivOH plays an important role in this transformation. This protocol tolerates a range of functional groups and is a reliable method for the rapid elaboration of readily available secondary anilines into a variety of tertiary (E)-enamines in high yields. Further study on the reaction scope is in progress.
Acknowledgements
This work was supported by generous grants from the National Natural Science Foundation of China (21272183, 21002077), and the Fund of the Rising Stars of Shanxi Province (2012KJXX-26).Notes and references
- (a) L. Yet, Chem. Rev., 2003, 103, 4283 CrossRef CAS PubMed; (b) C. Han, R. Shen, S. Su and J. A. Porco Jr, Org. Lett., 2004, 6, 27 CrossRef CAS PubMed; (c) D. J. Faulkner, Nat. Prod. Rep., 2000, 17, 7 RSC; (d) J. Kohno, Y. Koguchi, M. Nishio, K. Nakao, M. Kuroda, R. Shimizu, T. Ohnuki and S. Komatsubara, J. Org. Chem., 2000, 65, 990 CrossRef CAS PubMed.
- (a) J.-L. Li, T.-Y. Liu and Y.-C. Chen, Acc. Chem. Res., 2012, 45, 1491 CrossRef CAS PubMed; (b) K. L. Jensen, G. Dickmeiss, H. Jiang, Ł. Albrecht and K. A. Jørgensen, Acc. Chem. Res., 2012, 45, 248 CrossRef CAS PubMed; (c) G. Hou, W. Li, M. Ma, X. Zhang and X. Zhang, J. Am. Chem. Soc., 2010, 132, 12844 CrossRef CAS PubMed; (d) S. Mukherjee, J. W. Yang, S. Hoffmann and B. List, Chem. Rev., 2007, 107, 5471 CrossRef CAS PubMed.
- (a) A. V. Gulevich, A. S. Dudnik, N. Chernyak and V. Gevorgyan, Chem. Rev., 2013, 113, 3084 CrossRef CAS PubMed; (b) B. Stanovnik and J. Svete, Chem. Rev., 2004, 104, 2433 CrossRef CAS PubMed; (c) K. K. Toh, Y.-F. Wang, E. P. Jian Ng and S. Chiba, J. Am. Chem. Soc., 2011, 133, 13942 CrossRef CAS PubMed; (d) S. Rakshit, F. W. Patureau and F. Glorius, J. Am. Chem. Soc., 2010, 132, 9585 CrossRef CAS PubMed.
- (a) Z.-H. Guan, M. Chen and Z.-H. Ren, J. Am. Chem. Soc., 2012, 134, 17490 CrossRef CAS PubMed; (b) Z.-J. Zhang, X.-J. Quan, Z.-H. Ren, Y.-Y. Wang and Z.-H. Guan, Org. Lett., 2014, 16, 3292 CrossRef CAS PubMed; (c) L. Ran, Z.-H. Ren, Y.-Y. Wang and Z.-H. Guan, Chem.–Asian J., 2014, 9, 577 CrossRef CAS.
- (a) X.-L. Lian, Z.-H. Ren, Y.-Y. Wang and Z.-H. Guan, Org. Lett., 2014, 16, 3360 CrossRef CAS PubMed; (b) M. Chen, Z.-H. Ren, Y.-Y. Wang and Z.-H. Guan, Angew. Chem., Int. Ed., 2013, 52, 14196 CrossRef CAS PubMed; (c) Z.-J. Zhang, Z.-H. Ren, Y.-Y. Wang and Z.-H. Guan, Org. Lett., 2013, 15, 4822 CrossRef CAS PubMed; (d) M.-N. Zhao, H. Liang, Z.-H. Ren and Z.-H. Guan, Adv. Synth. Catal., 2013, 355, 221 CrossRef CAS; (e) L. Li, M.-N. Zhao, Z.-H. Ren, J. Li and Z.-H. Guan, Org. Lett., 2012, 14, 3506 CrossRef CAS PubMed; (f) Z.-H. Guan, L. Li, Z.-H. Ren, J. Li and M.-N. Zhao, Green Chem., 2011, 13, 1664 RSC.
- (a) J. R. Dehli, J. Legros and C. Bolm, Chem. Commun., 2005, 973 RSC; (b) A.-Z. A. Elassara and A. A. El-Khairb, Tetrahedron, 2003, 59, 8463 CrossRef.
- (a) A. Volkov, F. Tinnis and H. Adolfsson, Org. Lett., 2014, 16, 680 CrossRef CAS PubMed; (b) X. Yan, C. Chen, Y. Zhou and C. Xi, Org. Lett., 2012, 14, 4750 CrossRef CAS PubMed; (c) Z.-H. Guan, Z.-Y. Zhang, Z.-H. Ren, Y.-Y. Wang and X. Zhang, J. Org. Chem., 2011, 76, 339 CrossRef CAS PubMed; (d) S. Ueno, R. Shimizu and R. Kuwano, Angew. Chem., Int. Ed., 2009, 48, 4543 CrossRef CAS PubMed.
- (a) E. M. Beccalli, G. Broggini, M. Martinelli and S. Sottocornola, Chem. Rev., 2007, 107, 5318 CrossRef CAS PubMed; (b) M. Johannsen and K. A. Jørgensen, Chem. Rev., 1998, 98, 1689 CrossRef CAS PubMed.
- For selected examples of oxidative amination of amides, sulfonamides, or imides see: (a) N. Panda, A. K. Jena and M. Raghavender, ACS Catal., 2012, 2, 539 CrossRef CAS; (b) X. Liu and K. K. Hii, Eur. J. Org. Chem., 2010, 5181 CrossRef CAS; (c) J. M. Lee, D.-S. Ahn, D. Y. Jung, J. Lee, Y. Do, S. K. Kim and S. Chang, J. Am. Chem. Soc., 2006, 128, 12954 CrossRef CAS PubMed; (d) A. Klapars, K. R. Campos, C.-y. Chen and R. P. Volante, Org. Lett., 2005, 7, 1185 CrossRef CAS PubMed; (e) L. Wu, S. Qiu and G. Liu, Org. Lett., 2009, 11, 2707 CrossRef CAS PubMed; (f) C. C. Scarborough and S. S. Stahl, Org. Lett., 2006, 8, 3251 CrossRef CAS PubMed; (g) G. Liu and S. S. Stahl, J. Am. Chem. Soc., 2006, 128, 7179 CrossRef CAS PubMed; (h) M. M. Rogers, V. Kotov, J. Chatwichien and S. S. Stahl, Org. Lett., 2007, 9, 4331 CrossRef CAS PubMed; (i) J. L. Brice, J. E. Harang, V. I. Timokhin, N. R. Anastasi and S. S. Stahl, J. Am. Chem. Soc., 2005, 127, 2868 CrossRef CAS PubMed; (j) V. I. Timokhin, N. R. Anastasi and S. S. Stahl, J. Am. Chem. Soc., 2003, 125, 12996 CrossRef CAS PubMed; (k) Y. Bolshan and R. A. Batey, Angew. Chem., Int. Ed., 2008, 47, 2109 CrossRef CAS PubMed.
- (a) Y. Obora, Y. Shimizu and Y. Ishii, Org. Lett., 2009, 11, 5058 CrossRef CAS PubMed; (b) J. J. Bozell and L. S. Hegedus, J. Org. Chem., 1981, 46, 2561 CrossRef CAS.
- (a) T. E. Müller, K. C. Hultzsch, M. Yus, F. Foubelo and M. Tada, Chem. Rev., 2008, 108, 3795 CrossRef PubMed; (b) T. E. Müller and M. Beller, Chem. Rev., 1998, 98, 675 CrossRef PubMed; (c) M. Kawatsura and J. F. Hartwig, J. Am. Chem. Soc., 2000, 122, 9546 CrossRef CAS.
- (a) C. Zhang and N. Jiao, Angew. Chem., Int. Ed., 2010, 49, 6174 CrossRef CAS PubMed; (b) A. Grirrane, A. Corma and H. Garcia, Science, 2008, 322, 1661 CrossRef CAS PubMed; (c) A. Corma, P. Concepcin and P. Serna, Angew. Chem., Int. Ed., 2007, 46, 7266 CrossRef CAS PubMed.
- (a) R. I. McDonald, G. Liu and S. S. Stahl, Chem. Rev., 2011, 111, 2981 CrossRef CAS PubMed; (b) L. S. Boffa, Chem. Rev., 2000, 100, 1479 CrossRef CAS PubMed; (c) R. Jones, Chem. Rev., 1968, 68, 785 CrossRef CAS.
- Y. Mizuta, K. Yasuda and Y. Obora, J. Org. Chem., 2013, 78, 6332 CrossRef CAS PubMed.
- (a) X. Ji, H. Huang, W. Wu, X. Li and H. Jiang, J. Org. Chem., 2013, 78, 11155 CrossRef CAS PubMed; (b) X. Ji, H. Huang, W. Wu and H. Jiang, J. Am. Chem. Soc., 2013, 135, 5286 CrossRef CAS PubMed.
- (a) K. M. Gligorich and M. S. Sigman, Angew. Chem., Int. Ed., 2006, 45, 6612 CrossRef CAS PubMed; (b) L. S. Hegedus, B. Akermark, K. Zetterberg and L. F. Olsson, J. Am. Chem. Soc., 1984, 106, 7122 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra11543k |
| This journal is © The Royal Society of Chemistry 2014 |