Chromium terephthalate metal–organic framework MIL-101: synthesis, functionalization, and applications for adsorption and catalysis
Abstract
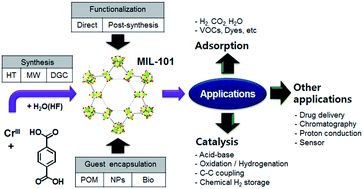
The chromium terephthalate metal–organic framework, MIL-101 (MIL, Matérial Institut Lavoisier), is comprised of trimeric chromium(III) octahedral clusters interconnected by 1,4-benzenedicarboxylates, resulting in a highly porous 3-dimentional structure. The large pores (29 and 34 Å) and high BET surface area (>3000 m2 g−1) with a huge cell volume (≈702 000 Å3) together with the coordinatively unsaturated open metal sites that can be subjected to diverse post-synthesis functionalization or guest encapsulation, and excellent hydrothermal/chemical stability, make MIL-101 particularly attractive for applications, such as selective gas adsorption/separation, energy storage and heterogeneous catalysis. This paper reviews the current status of research and development on the synthesis, functionalization and applications of MIL-101 for adsorption/catalytic reactions.

 Please wait while we load your content...
Please wait while we load your content...