Poly(γ-glutamic acid)–silica hybrids with fibrous structure: effect of cation and silica concentration on molecular structure, degradation rate and tensile properties
A. Obata*a,
S. Itoa,
N. Iwanagaa,
T. Mizunoa,
J. R. Jonesb and
T. Kasugaa
aGraduate School of Engineering, Nagoya Institute of Technology, Gokiso-cho, Showa-ku, Nagoya, 466-8555, Japan. E-mail: obata.akiko@nitech.ac.jp
bDepartment of Materials, Imperial College London, London, SW7 2AZ, UK
First published on 10th October 2014
Abstract
Biodegradable organic/inorganic hybrid fibremats were prepared by electrospinning using poly(γ-glutamic acid) (γ-PGA) and glycidoxypropyl trimethoxysilane (GPTMS) for application in bone tissue engineering. Ca(OH)2 was used for preparing the calcium salt form of γ-PGA (Ca-γPGA), which is water-soluble. Effects of the amount of Ca(OH)2 mixed with γ-PGA on the chemical structure and characteristics of resulting hybrid fibremats were studied. ATR-FTIR data indicated that cross-linking between γ-PGA chains was achieved due to the coupling reaction between the polymer side chain and epoxy terminated group of GPTMS and silica network formation between GPTMS molecules. 13C CP/MAS-NMR and 29Si MAS-NMR spectra indicated that open epoxy groups in GPTMS reacted with each other and the rate of condensation between its silanol groups was lower when the added amount of Ca(OH)2 was smaller. Tensile test data demonstrated that elongation to failure was improved for the hybrid fibremat in comparison with just Ca-γPGA fibremats.
Introduction
Tissue engineering using scaffold biomaterials is expected to be one of the useful techniques as an alternative approach to the use of autogeneic and allogeneic sources. One of the approaches currently in use in developing scaffold biomaterials is electrospinning (ES). It is a versatile method where nano and micro scale fibres can be fabricated cost-effectively from solutions of various substances by using high voltage and a grounded collector. Electrospun fibremats possess flexibility and a high interconnected porosity.1,2 The process of ES has been regarded to have great potential in the engineering of various types of tissues, such as vasculature, bone, neural and tendon/ligament. In addition, electrospun fibremats are useful for drug delivery system (DDS) applications, as ES affords great flexibility in selecting materials for the application.The main criteria required for the scaffolds are biocompatibility, biodegradability, flexibility, durability, and highly porous structure with an interconnected pore network for cell growth and flow transport of nutrients and metabolic waste.3–5 Aliphatic polyesters such as poly(lactic acid), poly(lactic-co-glycolic acid) and poly(caprolactone), have been investigated as scaffold materials due to their biocompatibility and biodegradability.6,7 These polymers also have excellent processability, so they can be fabricated into fibrous structure by ES method. Electrospun fibremats are highly porous and allows good cell adhesion and proliferation, therefore they are promising as the scaffolds.8,9 Such synthetic polyesters are, however, mostly hydrophobic, which can cause poor initial cell adhesion. In addition, organic solvents which are harmful to cells and natural tissues are used for processing them in most cases. Additional treatments for completely removing the solvents from the fabricated materials, such as heat-treatments, are needed, which causes the difficulty of safely inducing proteins, such as growth factors, into the materials to enhance tissue regeneration in body.10 Synthetic polyesters can also degrade rapidly due to autocatalytic hydrolysis.11 In order to overcome these difficulties, natural polymers, such as collagen and gelatin, which are hydrophilic and degrade by enzyme activity have been investigated for scaffolds.12,13
Poly(γ-glutamic acid), γ-PGA, is an anionic biopolymer contained in Japanese traditional food Natto, and high yields can be produced from a culture of Bacillus subtilis. γ-PGA is biodegradable and has adequate hydrophilicity, hence has been successfully utilized in surgical glue and recently investigated for scaffold application. γ-PGA is a poly(amino acid) formed by the amide bond linkage between the amino group on the α-carbon and the carboxyl group on the γ-carbon, and has reactive carboxyl groups on its side chain which allows chemical modification and covalent cross-linking. The polymer conformational changes in solution depending on ionic strength and pH, as with general peptides and proteins. The salt forms of Na+, Ca2+, and NH4+ of γ-polyglutamates are known to be soluble in water.14 The ionized salts of γ-PGA are no longer in helix structures due to the deprotonation of COOH groups to COO− groups,15 but behave like the open random coil state, which are soluble in water solution. Recent studies have shown that γ-PGA or γ-PGA-based composite nanofibres can be formed via the ES technique.16,17 However, in most cases, organic solvents such as trifluoroacetic acid (TFA) have been utilized. The salt forms of γ-PGA are more attractive for use in preparation of fibremats, as any organic solvent is not required. There is little report concerning the development of γ-PGA scaffolds using water as a solvent because of difficulty in obtaining fibremats with stability under physiological conditions.16
Poologasundarampillai et al. produced γ-PGA/bioactive silica hybrid porous scaffolds for bone regeneration by a sol–gel foaming process. The γ-PGA and bioactive silica were covalently bonded to each other (class II hybrid) through an organosilane coupling agent, glycidoxypropyl trimethoxysilane (GPTMS), with use of dimethyl sulfoxide (DMSO) as a solvent.18,19 This hybrid synthesis consisted of two reaction steps. In the first step γ-PGA was functionalised with GPTMS, with the hypothesis that the carboxylic acid of the polymer reacts with the epoxy group on GPTMS by ring opening esterification (nucleophilic attack). The second step was to add the functionalized polymer into the silica sol–gel process, with the hypothesis that the trimethoxysilane on GPTMS hydrolyses and undergoes condensation reactions with other silanol groups, e.g. those from hydrolysed TEOS or other hydrolysed GPTMS molecules, forming Si–O–Si bridging oxygen bonds. These hybrids had excellent stability in aqueous solution and it was possible to control mechanical and dissolution properties by varying the degree of GPTMS cross-linking. However the hybrids did not contain calcium. Calcium and silicate ions released from implant materials have been reported to have stimulatory effects on osteogenic cell functions and enhance bone formation.20 Therefore the presence of calcium in the hybrid is important. The conventional method for adding calcium into sol–gel glass is to use calcium nitrate as the calcium precursor, however the material must be heated to above 400 °C for the calcium to enter the silicate network,21 which is not possible in hybrids as they contain a polymer component. Valliant et al. produced calcium containing γ-PGA/bioactive silica hybrids (Ca-γPGA/silica) monoliths using the calcium salt form of the polymer and water as a solvent. The hybrid monoliths also exhibited excellent mechanical properties (non-brittle behaviour).22
In this work, Ca-γPGA/silica hybrid fibremats were fabricated using GPTMS and water as a solvent via the ES process. The calcium salt forms of γ-PGA was synthesized using calcium hydroxide (Ca(OH)2) in water. Ca-γPGA/silica hybrids made with GPTMS hybrids (Ca-γPGA/GPTMS) are expected to be promising candidates for the use in bone regeneration, especially the application for bone tissue engineering scaffolds. Incorporated cations have been known to influence structures of γ-PGA and silica networks.15,23 Here, Ca-γPGA/silica hybrids were synthesized and the effect of Ca(OH)2 and GPTMS concentrations on the molecular structure, degradation rate and tensile properties of the hybrids were investigated.
Experimental
Preparation of Ca-γPGA and Ca-γPGA/GPTMS fibremats
γ-PGA (Wako Pure Chemical Industries, Ltd., Japan, molecular weight (Mw): 200–500 kDa) and Ca(OH)2 (Kishida Chemical Co. Ltd., Japan) were mixed in Milli-Q water (Merck Millipore, USA) until it became transparent. The resulting solution is denoted by Ca-γPGA, hereafter. The polymer concentrations were set to be 16–22% (g ml−1 of water). GPTMS was added to Ca-γPGA solution and mixed for 3 h. The resulting solution is denoted by Ca-γPGA/GPTMS, hereafter. Table 1 shows sample codes and each chemical component. For Ca-γPGA, two types of solution with different concentrations of Ca(OH)2 were prepared. pH of the solutions was measured with a pH meter (F-51, Horiba Ltd., Japan). Viscosity of the solutions was measured at room temperature using a cone–plate type rotational viscometer (RE-80-L, Toki Sangyo, Japan).| Sample code | γ-PGA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1/2Ca(OH)2 (mol ratio) 1/2Ca(OH)2 (mol ratio) |
γ-PGA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) GPTMS (mol ratio) GPTMS (mol ratio) |
|
|---|---|---|---|
| Ca-γPGA | 6Ca | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.6 0.6 |
— |
| 9Ca | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.9 0.9 |
— | |
| Ca-γPGA/GPTMS | 6Ca2G | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.6 0.6 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.2 0.2 |
| 6Ca4G | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.4 0.4 |
||
| 9Ca2G | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.9 0.9 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.2 0.2 |
|
| 9Ca4G | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.4 0.4 |
||
Ca-γPGA and Ca-γPGA/GPTMS fibremats were fabricated with a Nanofiber Electrospinning Unit (Kato Tech Co, Japan). A high tension electric field of 20 kV was applied to the needle where the tip of the needle was positioned 150 mm from a grounded rotating drum (rotating speed: 200 mm min−1) that was used as the fibre collector. The electrospun fibremats were then placed in a drying oven (DX302, Yamato Scientific Co., Ltd., Japan) and dried at 50 °C for 24 h.
Structural analysis and morphological observation of fibremats
To investigate the chemical structure of the prepared hybrid fibremats, 13C cross-polarization and 29Si magic angle spinning nuclear magnetic resonance (13C CP and 29Si/MAS-NMR) (UNITY plus, Varian, USA) and attenuated-total reflection Fourier-transformed infrared spectroscopy (ATR-FTIR) (FT-IR-4000, JASCO, Japan) were utilized. 13C CP/MAS-NMR spectra were taken with 7.2 μs and 7.5 s recycle delays. 29Si MAS-NMR spectra were taken with 6.3 μs and 100 s recycle delays. Chemical shifts were measured relative to adamantine and poly(di-methyl silane) for 13C CP/MAS-NMR and 29Si MAS-NMR, respectively.The fibremats were coated with amorphous osmium by plasma chemical vapor deposition (CVD) using vaporized OsO4 and then morphologically observed by field-emission scanning electron microscopy (SEM) (JSM-6301F, JEOL, Japan). The average fibre diameter and standard deviation were measured from the SEM images (n = 30). Detailed morphology, crystal lattice and elemental composition of Ca-γPGA/GPTMS fibre were investigated by transmission electron microscopy (TEM) (JEM-z2500, JEOL, Japan) incorporating selected-area electron diffraction (SAED) and energy-dispersive X-ray spectroscopy (EDX).
Tensile test for fibremats
Tensile tests were performed with a universal testing machine (Autograph, AGS-G, Shimadzu Co., Japan) equipped with a 50 N load cell according to Japanese Industrial Standard (JIS L 1015). Rectangular samples with dimensions of 5 mm × 40 mm were cut from the fibremats. Test conditions were as follows: span length 20 mm, crosshead speed 1 mm min−1, n = 5.Calcium and silicate ion-releasing behaviors in buffer solution
50 mg of Ca-γPGA/GPTMS fibremats were immersed in 100 ml of Tris buffer solution (pH 7.4) and incubated at 37 °C for 528 h. At each time points (1, 2, 4, 8, 24, 72, 120, 168, 216, 264, 312, 360, 480, 528 h), a sample was removed (1 ml) and replenished with Tris buffer solution. Samples (1 ml) from each time point (n = 3) were analyzed for Si and Ca concentrations with an inductively coupled plasma atomic emission spectrometer (ICP-AES) (ICPS-7000, Shimadzu). Ratios of released elements to total amount of each element in the immersed fibremats were calculated assuming that Ca-γPGA/GPTMS fibremats consist of γ-PGA, Ca2+ ions, and completely condensed GPTMS (SiO3.5C6H11).Results and discussion
Hybrid solution and fibremat
Homogeneous and transparent solution was obtained when the mole ratio of γ-PGA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1/2Ca(OH)2 was 1
1/2Ca(OH)2 was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.6 or 1
0.6 or 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.9. γ-PGA exhibits helix-coil transition with the increase in pH of the solution,24 and is water-soluble when it becomes a random coil state.14 The pH of Ca-γPGA solution increased with increasing the amount of Ca(OH)2: pH 3.6 for 6Ca (γ-PGA
0.9. γ-PGA exhibits helix-coil transition with the increase in pH of the solution,24 and is water-soluble when it becomes a random coil state.14 The pH of Ca-γPGA solution increased with increasing the amount of Ca(OH)2: pH 3.6 for 6Ca (γ-PGA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1/2Ca(OH)2 of 1
1/2Ca(OH)2 of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.6, Table 1) and pH 4.5 for 9Ca (γ-PGA
0.6, Table 1) and pH 4.5 for 9Ca (γ-PGA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1/2Ca(OH)2 of 1
1/2Ca(OH)2 of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
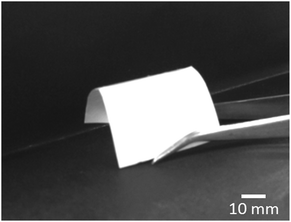
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.9, Table 1), respectively. Fig. 1 demonstrates the great flexibility of Ca-γPGA/GPTMS fibremat (9Ca4G), which could be easily folded using a pair of tweezers.
0.9, Table 1), respectively. Fig. 1 demonstrates the great flexibility of Ca-γPGA/GPTMS fibremat (9Ca4G), which could be easily folded using a pair of tweezers.
Molecular structure
Fig. 2(a) shows ATR-FTIR spectra of protonated γ-PGA (H-γPGA, raw material) and bulk powders and fibremats consisting of 6Ca and 9Ca. In the spectrum of H-γPGA, bands at 1700–1740 cm−1 were attributed to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O vibration of carboxyl groups.14 The band was lower in intensity in the spectrum of 6Ca compared with that of H-γPGA. Almost no bands in this position were found in the spectrum of 9Ca. Meanwhile, new bands were found at 1570 and 1410 cm−1 in 6Ca and 9Ca, which were assigned carboxylate group.25 These band intensities were higher in 9Ca than 6Ca.
O vibration of carboxyl groups.14 The band was lower in intensity in the spectrum of 6Ca compared with that of H-γPGA. Almost no bands in this position were found in the spectrum of 9Ca. Meanwhile, new bands were found at 1570 and 1410 cm−1 in 6Ca and 9Ca, which were assigned carboxylate group.25 These band intensities were higher in 9Ca than 6Ca.
An amide I band corresponding to the α-helical structure was observed at 1650 cm−1 in H-γPGA, while the band shifted to 1630 cm−1 in 6Ca and 9Ca. This means that β-sheet structure was formed in 6Ca and 9Ca.26 Carboxyl groups of γ-PGA were deprotonated due to coordination of ionized Ca2+ ions after mixing with Ca(OH)2 in water. The deprotonated groups electrostatically repelled each other leading to disruption of helical structure of γ-PGA. This contributed to the γ-PGA dissolution in water and allowed electrospinning (ES). No difference in the spectra between bulk powders and fibremats of Ca-γPGA (6Ca and 9Ca) was detected, hence further investigations would be needed for completely clarify effects of ES processing on the polymer structure of γ-PGA.
Fig. 2(b) shows ATR-FTIR spectra of 9Ca4G fibremats immediately after ES and after drying at 50 °C for 24 h. A new band appeared at 1730 cm−1 in the spectra of the both types of 9Ca4G sample, while no band at this wavenumber was found in 9Ca. This indicates that ester bonding formed between carboxyl group of γ-PGA and open epoxy ring of GPTMS.27 The band at 814 cm−1, attributed to the methoxy group of Si–OCH3,28 was observed in GPTMS without gelation, while no peak was observed in 9Ca4G, indicating complete hydrolysis of GPTMS in spinning solution. The bands at 1100 and 1025 cm−1 in 9Ca4G were attributed to the presence of Si–O–Si bonds (Si–O stretch) in cyclic and random network configuration, respectively.29 The intensity of band at 905 cm−1, which corresponds to Si–O− or Si–OH stretching30 (non-bridging oxygen bonds), decreased after the drying process. Higher band intensities assigned to Si–O–Si bonds were observed in 9Ca4G fibremat after drying compared with that immediately after ES. In particular, the absorbance of the band assigned to low-symmetry random silica network configurations (1025 cm−1) greatly increased after the drying process. These results suggest that the condensation reaction in GPTMS proceeded in the hybrid fibres after the solidification by ES. Therefore, GPTMS was successful in providing a cross-linking between γ-PGA polymer chains through the chemical reactions between carboxyl group on the side chain γ-PGA and epoxy terminated group of GPTMS and the silica formation by the condensation reaction in GPTMS.
Fig. 2(c) shows ATR-FTIR spectra of 6Ca4G and 9Ca4G fibremats. Higher absorbance of bands assigned to carboxylate groups of γ-PGA side chains (1570, 1410 cm−1) were observed in 9Ca4G compared with 6Ca4G. This indicates that some coordinations between Ca2+ ions and carboxylate anions of γ-PGA remained in the Ca-γPGA structure even after the addition of GPTMS. In addition, the peak assigned to Si–O− or Si–OH was higher in 6Ca4G compared with 9Ca4G.
Fig. 3(a) shows 13C CP/MAS-NMR spectra of 6Ca4G and 9Ca4G. Fig. 3(c) and Table 2 show the assignments of the observed signals. The peaks corresponding to γ-PGA and GPTMS were observed in both spectra.31–35 The intensities of peaks from the epoxy ring carbons (C1, C2) were lower in 6Ca4G than 9Ca4G. Nucleophilic opening of the epoxide ring did not go to completion. In addition, a peak shoulder newly appeared around 74–80 ppm in 6Ca4G. This peak is characteristic of C atoms in dioxane and oligo- or polyether derivatives (–[–CH2–CH(R)–O]n–; R: organic functional group) formed from a ring-opening reaction of the epoxy groups of GPTMS.32,33
 | ||
| Fig. 3 (a) 13C CP/MAS-NMR and (b) 29Si MAS-NMR spectra of 6Ca4G and 9Ca4G. (c) Scheme of structure of each chemical. | ||
| δ (13C) (ppm) | Assignment |
|---|---|
| 10 | C6 (GPTMS) |
| 24 | C5 (GPTMS) |
| 30 | β-CH2 (γ-PGA) |
| 33 | γ-CH2 (γ-PGA) |
| 44 | C1 (GPTMS epoxy) |
| 51 | C2 (GPTMS epoxy) |
| 55 | α-CH (γ-PGA) |
| 72 | C3 (GPTMS) |
| 74 | C4 (GPTMS) |
| 69, 74 | Dioxane |
| 70–80 | Polyether |
Fig. 3(b) shows the 29Si MAS-NMR spectra of 6Ca4G and 9Ca4G fibremats. Tn groups (CSi(OSi)n(OR)3−n) which were derived from the condensation between GPTMS were present in both samples. Tn groups consist of a Si atom with an Si–C bond and n bridging Si–O bonds.36 The peak area of T2 was larger than those of the other peaks, T1 and T3, in 6Ca4G, while the area of T3 was the largest in 9Ca4G. No T1 peak was found in the spectrum of 9Ca4G. Therefore more the condensation between GPTMS took place in 9Ca4G than 6Ca4G. The polyether might affect the condensation and the silica formation in the case of 6Ca4G.
Fibre structure
Fig. 4 shows the SEM images with the average fibre diameter (inset) of the 9Ca, 9Ca2G and 9Ca4G fibremats which were prepared by ES for each solution with different γ-PGA concentrations. The diameter was larger for Ca-γPGA/GPTMS fibremats (9Ca2G and 9Ca4G) than Ca-γPGA fibremats (9Ca). In addition, the fibre diameter became larger when the γ-PGA concentration and the amount of GPTMS in the hybrid solution increased. The increase in fibre diameter depended on the γ-PGA concentration was higher in the case of Ca-γPGA/GPTMS containing larger amount of GPTMS; the diameter increased from 214 ± 22 nm (18 wt%) to 722 ± 289 nm (22 wt%) in the case of 9Ca2G, and from 221 ± 25 nm (16 wt%) to 1672 ± 980 nm (22 wt%) in the case of 9Ca4G. This might be due to differences in the viscosity of solution for ES. Table 3 shows the viscosity value of the 9Ca, 9Ca2G and 9Ca4G solutions used for ES. Diameter of electrospun fibres generally increased with the increase in the viscosity of solution used for ES. The viscosity of 9Ca2G was higher than that of 9Ca in the case of 22 and 24 w/v% of the γ-PGA concentration. However, the fibre diameter of 9Ca2G was larger than that of 9Ca in the case of 18 w/v%, while the viscosity of the two samples was almost the same. No precise reason for this is found, but electrical conductivity and surface tension37 may have been affected by differences in the chemical compositions of the solutions. The viscosity of 9Ca4G was higher than the others and that of 24 w/v% was unable to be measured because of heterogeneity of the sample solution. The results of ATR-FTIR analysis demonstrated that the Ca-γPGA/GPTMS fibremats immediately after ES contained silica phase derived from GPTMS (Fig. 2(b)). Therefore, the solution before ES is expected to contain some condensed silica networks, which induced the entanglement of γ-PGA chains. These caused the higher viscosity of the hybrid solution (9Ca2G and 9Ca4G) compared with Ca-γPGA solution (9Ca). In addition, the silica condensation rate must be affected by pH of the solution, which changed as Ca content changed. Thus, the fibre diameter was controllable by changing the γ-PGA concentration of solution. Fibremats with the same fibre diameter (about 200 nm) were fabricated using various solutions and used for the tensile test and the immersion test. | ||
| Fig. 4 SEM images and average fibre diameter of (a and b) 9Ca, (c and d) 9Ca2G and (e and f) 9Ca4G fibremats prepared with the polymer concentration of (e) 16%, (a and c) 18% and (b, d and f) 22%. | ||
| Sample code | Polymer concentration | |||
|---|---|---|---|---|
| 10 w/v% | 18 w/v% | 22 w/v% | 24 w/v% | |
| 9Ca | 115 | 463 | 998 | 1199 |
| 9Ca2G | 105 | 438 | 1509 | 2805 |
| 9Ca4G | 1395 | 1856 | 3149 | N. D. |
Fig. 5 shows a TEM image of 9Ca4G fibremat and its SAED pattern. To elucidate distributions of the three reagents, γ-PGA, GPTMS and Ca(OH)2, in a fibre, 9Ca4G fibremat was selected for these analysis as it was fabricated with the highest additive content of Ca(OH)2 and GPTMS. The TEM image demonstrated that fibres were comprised of fibril-like entities with uniaxial orientation along the long axis of the fibre. The SAED pattern showed no arc-shaped reflections. Yoshioka et al. reported that oriented fibril-like entities were observed inside the electrospun polyethylene fibres with ≤400 nm in diameter and they were attributed to the orientation of polymer chains and the subsequent crystallization which were induced by elongational force on ES.38 Their SAED patterns showed sharp crystalline reflections. In the case of 9Ca4G fibremat, the SAED patterns indicated that no crystals were formed in it, while the oriented fibril-like entities were observed in its TEM image. This might indicate that GPTMS and silica derived from GPTMS interacted with γ-PGA chains at the molecular scale. Results of elemental mapping followed this speculation. Fig. 6 shows STEM and elemental mapping image of a 9Ca4G fibremat. N, Ca and Si elements correspond to γ-PGA, Ca(OH)2 and GPTMS, respectively. All elements homogenously dispersed inside the fibre. Therefore γ-PGA, Ca(OH)2 and GPTMS were well integrated at the molecular scale. The formation of β-sheet in Ca-γPGA solution might contribute to the chemical reaction between PGA and GPTMS at the molecular scale, while further investigations would be needed for completely clarify effects of polymer chain conformation on the resulting hybrid structure.
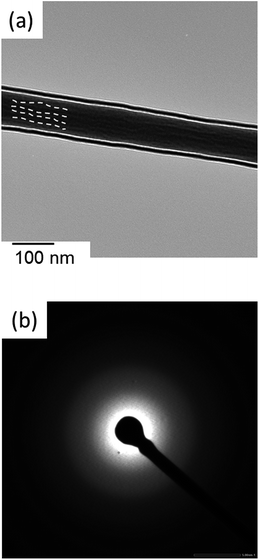 | ||
| Fig. 5 (a) TEM image and (b) SAED pattern of 9Ca4G fibremat. Dotted lines in TEM image indicate direction of the oriented fibril-like entities formed in the fibre. | ||
 | ||
| Fig. 6 (a) STEM and (b–d) elemental mapping image of 9Ca4G fibremat. (b) N, (c) Ca and (d) Si elements correspond to γ-PGA, Ca(OH)2 and GPTMS, respectively. | ||
Tensile property
Fig. 7 shows typical curves obtained from tensile test for the hybrid fibremats. Table 4 shows the maximum load and elongation to failure of the samples. Ca-γPGA fibremat (6Ca and 9Ca) possessed lower strength (∼0.26 N) and elongation (∼5%) compared with Ca-γPGA/GPTMS fibremats. Ca-γPGA/GPTMS fibremats showed higher load (∼0.46 N) and elongation (∼37%) and possessed plasticity.| Sample code | Maximum load/N | Elongation to failure (%) |
|---|---|---|
| 6Ca | 0.26 ± 0.04 | 5 ± 0.6 |
| 6Ca2G | 0.38 ± 0.03 | 37 ± 5 |
| 6Ca4G | 0.31 ± 0.1 | 22 ± 9 |
| 9Ca | 0.11 ± 0.03 | 4 ± 0.3 |
| 9Ca2G | 0.29 ± 0.02 | 35 ± 8 |
| 9Ca4G | 0.46 ± 0.09 | 30 ± 5 |
Ion release behaviour in Tris buffer solution
Fig. 8 shows the amounts of Ca and Si released from the fibremats in Tris buffer solution. 6Ca-series samples showed a rapid Ca release in 24 h after immersing and then a slow release after that. 40% of Ca in total amount contained in each sample was released in 528 h. 9Ca-series samples released larger amount of Ca in 24 h and then possessed slower release than 6Ca-series. Around 50% of Ca in total amount contained in each sample was released in 528 h. The released amounts of 9Ca-series samples were larger than those of 6Ca-series at all time points. The ATR-FTIR spectra (Fig. 2(a)) demonstrated that the carboxyl groups of γ-PGA formed coordination with ionized Ca2+ ions. Therefore Ca release might relate to the degradation of γ-PGA.In the case of Si, obvious differences in release behaviour were found between 6Ca-series and 9Ca-series samples. 6Ca-series samples showed a rapid release (initial burst) in 8 h and then a slow release until 528 h. 88% of Si in total amount contained in the samples was released in 528 h. On the other hand, 9Ca-series samples showed no initial burst and possessed a continuous release with time through the immersion. Although the amount of 9Ca4G reached about 60% after 528 h, the amount of 9Ca2G reached 100% after 480 h. The silica release decreased as calcium content increased. 13C CP/MAS-NMR spectra (Fig. 3(a)) indicated the formation of the dioxane, oligo- and polyether, which form no chemical bonding with γ-PGA, in 6Ca4G. This caused the initial burst of Si from 6Ca-series samples; 45% for 6Ca2G and 62% for 6Ca4G in 8 h.
The number of the reports on electrospun fibremats prepared using water as a solvent for the use in tissue engineering is few, while water must be one of the best solvents for the synthesis of biomaterials in terms of biocompatibility. To achieve the preparation of such fibremats, it is required to get a solution in which matrix materials, such as polymer and sol–gel glass, homogenously dissolve. Water-soluble materials should be the best candidate for the matrix. There is, however, difficulty in achieving development of fibremats that have stability under physiological conditions. In the present work, fibremats exhibiting stability in the buffer solution with pH = 7.4 for >528 h were successfully fabricated using γ-PGA and GPTMS by sol–gel method and ES technique.
It has been generally difficult to fabricate a fibremat using a solution containing network structure consisting of polymers and sol–gel glasses by ES. Although the cross-linking between γ-PGA chains formed by silica (GPTMS) might form a network structure in the hybrid solution, the content of formed structure should be small since the silica formation had not been completed until ES, as demonstrated in Fig. 2(b). More silica phase was formed in the fibremats by drying at 50 °C, which contributed to the improved stability in the buffer solution.
Tensile tests demonstrated that Ca-γPGA/GPTMS fibremats made from >20 ml% of GPTMS showed plastic deformation, while Ca-γPGA one did not show it. When comparing the curves of Ca-γPGA and Ca-γPGA/GPTMS fibremats (Fig. 7) there was a difference in the shapes of the curves as well as the ultimate load and strain values. Ca-γPGA/GPTMS fibremats had a flat curve, whereas Ca-γPGA curves showed a rapid increase and decrease in slope. The deformation of Ca-γPGA/GPTMS fibremats showed that incorporation of the silica phase derived from GPTMS into γ-PGA chain structure reduced the brittle nature of Ca-γPGA. When in tension, γ-PGA chains orient and become crystalline, which induces the brittle nature. The cross-linking by the silica derived from GPTMS intermediated between the polymer chains and inhibited the crystal formation. Thus the integration of interpenetrating networks of silica derived from GPTMS and the polymer at the molecular level (as shown in Fig. 6) resulted in the improved mechanical property. Gao et al. reported that gelatin/bioactive glass hybrid fibremats had higher ultimate stress and strain values compared with gelatin fibremats in the results of tensile test.39 Jiang et al. reported that PVA/GPTMS/TEOS hybrid films had improved tenacity in comparison with PVA films.40 In the case of γ-PGA, Poologasundarampillai et al. and Valliant et al. reported that γ-PGA/GPTMS/TEOS hybrid bulks had higher ultimate stress and strain values in the result of compression test than γ-PGA, especially strain to failure increased with the increase in cross-linking degree in the hybrid. Thus the hybrid between polymer and silica contribute to improved mechanical properties. The improved tensile property of Ca-γPGA/GPTMS fibremats could indicate cross-linking between GPTMS and γ-PGA chain.
The amount of Ca(OH)2 added influenced the chemical structure and subsequent degradation behaviour of Ca-γPGA/GPTMS fibremats. Disruption of glass networks is generally induced by mixed alkali and alkali-earth cations.23 However, in sol–gel processing, a temperature of more than 400 °C must be exceeded for calcium to enter the silicate network. Here, the rate of condensation in GPTMS was higher in 9Ca4G than 6Ca4G according to 29Si MAS-NMR and ATR-FTIR (Fig. 2 and 3(b)). Calcium ions form coordination with the polymer chains and do not accompany to the silica network derived from GPTMS in the case of Ca-γPGA/GPTMS, as shown in ATR-FTIR spectra (Fig. 2(a)). The pH of 6Ca solution was lower than that of 9Ca solution (pH 3.6 for 6Ca and pH 4.5 for 9Ca). Epoxy groups open more rapidly when the pH of the solution is lower. Studies of GPTMS in water show that the epoxy ring does not open spontaneously at a pH of 7 or higher, but does at lower pH.41 Therefore here, at pH 3.6, the epoxy ring is more likely to open by nucleophilic attack than at pH 4.5. Comparison of effectiveness of different nucleophiles in their effectiveness in opening the epoxy ring at pH 6 found –COOH groups to be more effective than –OH, –SH or –NH groups.42 The lower pH of 6Ca solution induced epoxy-opening in GPTMS prior to nucleophilic attack occurring and subsequent forming the diol of GPTMS. Formation of terminal methyl ether species can occur by reaction of an opened epoxy group with methanol and finally polyaddition reactions to form polyether chains.32 This could deteriorate the mobility of silanol groups and inhibit the silica network formation. Versace et al. reported that an increased steric bulk at the organic substituent in the epoxy cyclohexyl silane affected negatively the sol–gel polymerization.34 The dioxane and polyether formation resulted in steric hindrance, which inhibited the silica network formation. Although we have no evidence how the dioxane and polyether formation influence the property of the resulting hybrids, we expect that the influence is little. The dioxane and polyether would have no strong bonding with the matrix, since they rapidly released in Tris buffer solution. On the other hand, no peaks corresponding to dioxane and polyether were found in the 9Ca4G spectrum (Fig. 3(a)). The pH of 9Ca solution was higher than that of 6Ca solution, which reduced the amount of open epoxy. This might inhibit the dioxane and polyether formation in 9Ca4G and subsequently activate the condensation between the silanol groups. Therefore the amount of Ca(OH)2 added influenced the epoxy-opening in GPTMS and the subsequent condensation between the silanol groups rather than the scission of the silica network by the accommodation of calcium ions. Another consideration is that when Ca concentration was higher, (9Ca samples), more –COOH groups were occupied by Ca ions, reducing the number of nucleophilic groups on the polymer, which reduced nucleophilic opening of the epoxy ring and allowed more condensation of the silicate network. Increased pH also catalyses silicate condensation reactions.
The results of ion release test showed that 6Ca-series samples (6Ca2G and 6Ca4G) had a burst in silica release in 24 h after the immersion in Tris buffer solution, whereas 9Ca-series samples showed more sustained dissolution. The dioxane and polyether, which have no contribution to the cross-linking between the polymer chains, were expected to release from 6Ca-series samples immediately after the immersion, which resulted in the burst in silica release. The rate of condensation in GPTMS was lower in the 6Ca samples than the 9Ca-series. Thus the silica derived from GPTMS more effectively formed the cross-linking between the polymer chains in 9Ca-series samples in comparison with 6Ca-series. In the case of calcium release, although 9Ca-series samples had a gradually increasing slope, 6Ca-series samples had a burst in the release over the first 24 h. Increasing the Ca content decreased silica dissolution. This is most likely to be due to the increased Ca increasing silica network condensation during the synthesis, however it could also take part in ionic cross-linking.
Conclusions
γ-PGA-based fibremats were successfully prepared by ES using water as a solvent and found to exhibit the stability under physiological conditions, with controlled release of soluble silica and calcium which are known to stimulate osteogenic cells. Covalent bonding between the organic and inorganic components was shown by NMR and FTIR. The amount of Ca(OH)2 added influenced the chemical structure of the resulting hybrid fibres at the molecular level, increasing silica network connectivity, and subsequently related to their mechanical properties and degradation behaviour. The obtained fibremats maintained their shapes for more than 3 weeks in Tris buffer solution. The fibremats were fabricated in water and need no additional treatment for completely removing any solvents, which results in their high safety and must be one of the advantages for inducing proteins, growth factors etc., into them in future.Acknowledgements
This work was supported in part by JSPS KAKENHI Grant Number 26820304, the Naito Research Grant and Institute of Ceramics Research and Education (ICRE) of Nagoya Institute of Technology.Notes and references
- W. Cui, Y. Zhou and J. Chang, Sci. Technol. Adv. Mater., 2010, 11, 1–11 CrossRef.
- T. J. Sill and H. A. von Recum, Biomaterials, 2008, 29, 1989–2006 CrossRef CAS PubMed.
- D. W. Hutmacher, Biomaterials, 2000, 21, 2529–2543 CrossRef CAS PubMed.
- S. Yang, K.-F. Leong, Z. Du and C.-K. Chua, Tissue Eng., 2001, 7, 679–689 CrossRef CAS PubMed.
- S. F. Williams, D. P. Martin, D. M. Horowitz and O. P. Peoples, Int. J. Biol. Macromol., 1999, 25, 111–121 CrossRef CAS PubMed.
- J. J. Yoon, J. H. Kim and T. G. Park, Biomaterials, 2003, 24, 2323–2329 CrossRef CAS PubMed.
- S. Park, G. Kim, Y. C. Jeon, Y. Koh and W. Kim, J. Mater. Sci.: Mater. Med., 2009, 20, 229–234 CrossRef CAS PubMed.
- K. Fujikura, A. Obata and T. Kasuga, J. Biomater. Sci., Polym. Ed., 2012, 23, 1939–1950 CAS.
- I. K. Kwon, S. Kidoaki and T. Matsuda, Biomaterials, 2005, 26, 3929–3939 CrossRef CAS PubMed.
- K. Lee, E. A. Silva and D. J. Mooney, J. R. Soc., Interface, 2011, 8, 153–170 CrossRef CAS PubMed.
- R. R. M. Bos, F. B. Rozema, G. Boering, A. J. Nijenhius, A. J. Pennings, A. B. Verwey, P. Nieuwenhuis and H. W. B. Jansen, Biomaterials, 1991, 12, 32–36 CrossRef CAS PubMed.
- J. Venugopal, Y. Z. Zhang and S. Ramakrishna, Nanotechnology, 2005, 16, 2138–2142 CrossRef CAS PubMed.
- Y. Zhang, H. Ouyang, T. L. Chwee, S. Ramakrishna and Z. M. Huang, J. Biomed. Mater. Res., Part B, 2005, 72, 156–165 CrossRef PubMed.
- G. H. Ho, T. I. Ho, K. H. Hsieh, Y. C. Su, P. Y. Lin, J. Yang, K. H. Yang and S. C. Yang, J. Chin. Chem. Soc., 2006, 53, 1363–1384 CrossRef CAS.
- D. Bhattacharyya, J. A. Hestekin, P. Brushaber, L. Cullen, L. G. Bachas and S. K. Sikdar, J. Membr. Sci., 1998, 141, 121–135 CrossRef CAS.
- H. Yoshida, K. Klinkhammer, M. Masusaki, M. Möller, D. Klee and M. Akashi, Macromol. Biosci., 2009, 9, 568–574 CrossRef CAS PubMed.
- S. Wang, X. Cao, M. Shen, R. Guo, I. Bányai and X. Shi, Colloids Surf., B, 2012, 89, 254–264 CrossRef CAS PubMed.
- G. Poologasundarampillai, C. Ionescu, O. Tsigkou, M. Murugesan, R. G. Hill, M. M. Stevens, J. V. Hanna, M. E. Smith and J. R. Jones, J. Mater. Chem., 2010, 20, 8952–8961 RSC.
- G. Poologasundarampillai, B. Yu, O. Tsigkou, E. Valliant, S. Yue, P. D. Lee, R. W. Hamilton, M. M. Stevens, T. Kasuga and J. R. Jones, Soft Matter, 2012, 8, 4822–4832 RSC.
- I. D. Xynos, A. J. Edgar, L. D. K. Buttery, L. L. Hench and J. M. Polak, Biochem. Biophys. Res. Commun., 2000, 276, 461–465 CrossRef CAS PubMed.
- S. Lin, C. Ionescu, K. J. Pike, M. E. Smith and J. R. Jones, J. Mater. Chem., 2009, 19, 1276–1282 RSC.
- E. M. Valliant, F. Romer, D. Wang, D. S. McPhail, M. E. Smith, J. V. Hanna and J. R. Jones, Acta Biomater., 2013, 9, 7662–7671 CrossRef CAS PubMed.
- J. Serra, P. González, S. Liste, C. Serra, S. Chiussi, B. León, M. Pérez-Amor, H. O. Ylänen and M. Hupa, J. Non-Cryst. Solids, 2003, 332, 20–27 CrossRef CAS.
- F. Y. Siao, J. F. Lu, J. S. Wang, B. S. Inbaraj and B. H. Chen, J. Agric. Food Chem., 2009, 57, 777–784 CrossRef CAS PubMed.
- C. Picart, R. Elkaim, L. Richert, F. Audoin, Y. Arntz, M. D. S. Cardoso, P. Schaaf, J. C. Voegel and B. Frisch, Adv. Funct. Mater., 2005, 15, 83–94 CrossRef CAS.
- L. M. He, M. P. Neu and L. A. Vanderberg, Environ. Sci. Technol., 2000, 34, 1694–1701 CrossRef CAS.
- J. Zhu, J. Kim, H. Peng, J. L. Margrave, V. N. Khabashesku and E. V. Barrera, Nano Lett., 2003, 3, 1107–1113 CrossRef CAS.
- M. Kato, S. Katayama, W. Sakamoto and T. Yogo, Electrochim. Acta, 2007, 52, 5924–5931 CrossRef CAS.
- M. S. Oliver, K. Y. Blohowiak and R. H. Dauskardt, J. Sol–Gel Sci. Technol., 2010, 55, 360–368 CrossRef CAS.
- B. P. Mosher, C. Wu, T. Sun and T. Zeng, J. Non-Cryst. Solids, 2006, 352, 3295–3301 CrossRef CAS.
- M. Morikawa, S. Kagihiro, M. Haruki, K. Takano, S. Branda, R. Kolter and S. Kanaya, Microbiology, 2006, 152, 2801–2807 CrossRef CAS PubMed.
- P. Innocenzi, C. Figus, T. Kidchob, M. Valentini, B. Alonso and M. Takahashi, Dalton Trans., 2009, 9146–9152 RSC.
- A. Chemtob, F. Courtecuisse, C. Croutxé-Barghorn and S. Rigolet, New J. Chem., 2011, 35, 1803–1808 RSC.
- D.-L. Versace, A. Chemtob, C. Croutxé-Barghorn and S. Rigolet, Macromol. Mater. Eng., 2010, 295, 355–365 CrossRef CAS.
- B. Alonso, D. Massiot, F. Babonneau, G. Brusatin, G. Della Giustina, T. Kidchob and P. Innocenzi, Chem. Mater., 2005, 17, 3172–3180 CrossRef CAS.
- T. Yabuta, K. Tsuru, S. Hayakawa, C. Ohtsuki and A. Osaka, J. Sol–Gel Sci. Technol., 2000, 19, 745–748 CrossRef CAS.
- P. Lu and B. Ding, Recent Pat. Nanotechnol., 2008, 2, 169–182 CrossRef CAS PubMed.
- T. Yoshioka, R. Dersch, M. Tsuji and A. K. Schaper, Polymer, 2010, 51, 2383–2389 CrossRef CAS.
- C. Gao, Q. Gao, Y. Li, M. N. Rahaman, A. Teramoto and K. Abe, J. Appl. Polym. Sci., 2013, 127, 2588–2599 CrossRef CAS.
- R. Guo, C. Hu, F. Pan, H. Wu and Z. Jiang, J. Membr. Sci., 2006, 281, 454–462 CrossRef CAS.
- L. Gabrielli, L. Russo, A. Poveda, J. R. Jones, F. Nicotra, J. Jiménez-Barbero and L. Cipolla, Chem.–Eur. J., 2013, 19, 7856–7864 CrossRef CAS PubMed.
- L. Gabrielli, L. Connell, L. Russo, J. Jiménez-Barbero, F. Nicotra, L. Cipolla and J. R. Jones, RSC Adv., 2014, 4, 1841–1848 RSC.
| This journal is © The Royal Society of Chemistry 2014 |