Reversible mechanochromism in dipyridylamine-substituted unsymmetrical benzothiadiazoles†
Abstract
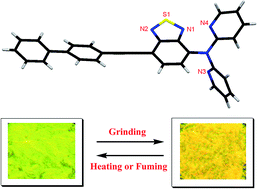
We report the design and synthesis of push–pull benzothiadiazoles (BTDs) of type D1–π–A–π–D2 and D1–π–A–D2. These BTDs show strong charge transfer interaction. BTD 3 shows reversible mechanochromism with color contrast between yellow (crystalline state) and orange (amorphous state). Photophysical and computational studies reveal that the planar orientation of the pyridyl and BTD unit in 2 results in no change in solid state emission whereas non-planar orientation of the dipyridylamine and BTD unit in 3 results in efficient mechanochromism.

 Please wait while we load your content...
Please wait while we load your content...