Butterfly-scale architecture directed electrodeposition of Ag microband arrays for electrochemical detection†
Abstract
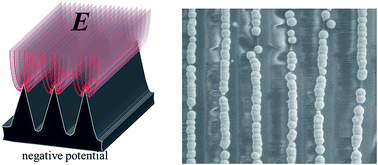
Butterfly scales with various elaborate architectures provide a large pool for structural design of microelectrode array arrangements. Here, the ridge array architecture of Troides aeacus butterfly scales was used as guidance to gather an electric field compactly around the ridge tips when a cathode potential was exerted. This tip effect directed the electrodeposition of Ag into microband arrays, on which effective electrochemical detection of hydrogen peroxide was achieved.

 Please wait while we load your content...
Please wait while we load your content...