Multilayered TiO2@SnO2 hollow nanostructures: facile synthesis and enhanced photocatalytic performance†
Jing Wanga,
Xiuying Wang*ab,
Xiaoli Dong*a,
Xiufang Zhanga,
Hongchao Maa and
Xu Feib
aSchool of Light Industry and Chemical Engineering, Dalian Polytechnic University, #1 Qinggongyuan, Dalian 116034, P. R. China. E-mail: wang_xy@dlpu.edu.cn; dongxl@dlpu.edu.cn; Fax: +86 411 86323736
bInstrumental Analysis Center, Dalian Polytechnic University, #1 Qinggongyuan, Dalian 116034, P. R. China
First published on 3rd November 2014
Abstract
Multilayered TiO2@SnO2 hollow nanostructures have been successfully synthesized via a simple approach employing the template-assisted and hydrothermal methods. The results showed that the molar ratio of Ti(SO4)2 to SnSO4 played important roles in the composition and morphology of the nanostructures. The as-prepared TiO2@SnO2 hollow nanostructures exhibited excellent photocatalytic activity when used as photocatalysts for the degradation of organic dyes. The mesoporous structure could facilitate the diffusion of dye molecules. In addition, the multilayered hollow nanostructure allows multiple reflections of incident light, making efficient utilization of light energy. Moreover, the coupling TiO2 with SnO2 could also effectively improve the charge separation efficiency.
1. Introduction
Since the discovery of the photoelectrochemical behaviors of TiO2, it has been recognized as one of the most promising photocatalysts and widely used in a variety of fields, including self-cleaning surfaces, water purification, sterilization, and water-splitting.1–4 However, the photocatalytic performance of TiO2 is greatly limited by fast recombination of photoinduced electron and hole pairs and low efficiency in light utilization. Up to now, a number of effective approaches have been explored to further improve the TiO2 properties, for instance coupling with other semiconductor materials,4–10 doping with metal and nonmetal elements,11–13 and surface modification with noble metals.14–16 Among these, fabrication of functional composites with other semiconductors, including CdS, Fe2O3, ZnO, Cu2O, SnS2, SnO2 and so on,4–10 presents one of the most commonly used methods. In these researches, the main purpose is to engineer the bandgap of TiO2 and enhance separation of photoinduced electron and hole pairs, leading to the increase of the efficiency of light absorption and utilization. Until now, many materials have been successfully synthesized and illustrated improved photocatalytic performance. Nevertheless, these materials with superior photocatalytic performance usually require complicated preparation process and/or sophisticated apparatus, inhibiting the practical application of these approaches.In the past few decades, the development of nanostructured materials has boosted the photocatalysis researches into a new era. A number of reports indicate that fabrication of photocatalysts with nanostructures can effectively improve the photocatalytic performance.17–19 Consequently, nanostructured photocatalyst materials have been paid a considerable amount of attention as a potential candidate for photocatalysis. Up to now, a series of nanostrucutred photocatalysts have been successfully prepared, such as nanowire, nanotube, porous materials and so on.20–23 Among these nanostructures, hollow spheres have been paid increasing attention owing to their better light harvesting and simple preparation.24–27 Recently, it has been confirmed that single-shelled and multilayered core–shell structures can give birth to an increase of the optical path length and enhancement of light absorption and utilization compared to the hollow spheres.28,29 Although a series of core–shell nanostructures have been prepared, the core and the shell of most materials are single phase, respectively. Therefore, it would be desirable to develop a simple synthesis process to fabricate the multilayered hollow nanostructures, in which both the core and the shell are composites.
In this paper, multilayered TiO2@SnO2 hollow nanostructures have been successfully synthesized by employing template-assisted and hydrothermal methods. Our results show that the hierarchical nanostructure and composition play important roles in photocatalytic behaviors of the samples. The multilayered hollow nanostructure allows multiple reflections of incident light, making efficient utilization of light energy. The mesoporous structure could facilitate the diffusion of dye molecules. In addition, the coupling TiO2 with SnO2 promotes the separation of photoinduced electron and hole pairs.
2. Experimental section
2.1. Preparation of multilayered TiO2@SnO2 hollow nanostructures
A strategy combining template-assisted and hydrothermal methods was adopted in the preparation of multilayered TiO2@SnO2 hollow nanostructures. At first, 1.6 g of titanium sulfate (Ti(SO4)2), 0.86 g of tin sulfate (SnSO4) and 0.64 g of resorcinol were dissolved in 12 mL of deionized water. Subsequently, 1.6 mL of 35 wt% formaldehyde was added to the solution under vigorous stirring and the mixture was transferred to a 20 mL Teflon-lined stainless steel autoclave. After heated in an electric oven at 85 °C for 48 h, the autoclave was cooled down naturally. The intermediate products were collected, then washed with deionized water several times, and then dried at 85 °C for a few hours. Finally, the intermediate products were calcined at elevated temperature for 3 h in air to produce the final products. The obtained products were denoted as S-T, where T is the calcination temperature. For photocatalytic performance comparison, TiO2 hollow microspheres were prepared under the same conditions in the absence of SnSO4, calcined at 450 °C for 3 h in air and denoted as TiO2-450.2.2. Characterization
The morphology of the samples was observed by scanning electron microscopy (SEM, JEOL JSM 4800F, JEOL 6610) and transmission electron microscopy (TEM, JEOL 2100 at 200 kV). Powder X-ray diffraction (XRD) measurements carried out in a Shimadzu XRD-6100 diffractometer with a Cu Kα line of 0.154 nm was used to determine the crystalline phase. The N2 adsorption–desorption was measured by JW-BK222 nitrogen adsorption analyzer. The chemical composition was analyzed by energy-dispersive X-ray spectroscopy (EDS, Oxford INCA). UV/Vis diffuse reflectance spectroscopy (UV/Vis-DRS) were recorded in the range of 200–800 nm using a CARY 100 CONC spectrophotometer with BaSO4 as a reference.2.3. Photocatalytic degradation of MO
The photoreactor was designed with an internal Hg lamp (350 W) surrounded by a water-cooling quartz jacket to cool the lamp. 100 mg of photocatalysts was added to 200 mL of methyl orange (MO) solution with an initial concentration of 30 mg L−1. Prior to photoreaction, the suspensions were magnetically stirred in darkness for 40 min to reach an adsorption–desorption equilibrium between the organic molecules and the catalyst surface. At an irradiation interval of every 15 min, the suspensions (3 mL) were collected and centrifuged to remove the photocatalyst particles. The MO concentration was analyzed by UV-visible spectrophotometer (Shimadzu, UV-1600 pc) monitoring the absorption maximum at λmax = 463 nm.3. Results and discussion
The morphology and texture of the as-synthesized products were investigated by SEM and TEM. Fig. 1 display typical SEM and TEM images of S-450. It is clear that the samples are mainly composed of uniform core–shell nanostructured microspheres with the diameter of around 3 μm (Fig. 1a). The SEM images with high magnification (Fig. 1b) reveal that the core of microsphere consists of lots of small-sized nanoparticles, whereas the shell contains lots of large nanoparticles, assembled irregularly, leaving a number of large channels. The texture of the samples is further studied by TEM images. It is interesting that the as-synthesized microspheres possess a multilayered hollow nanostructure (Fig. 1c). The structure of the exterior shell was also investigated by high-magnification TEM image (Fig. 1d). The large nanoparticles in the shell are composed of small-sized nanoparticles with the diameter of approximately 6 nm and a certain amount of small mesopores with the diameter of 3–4 nm. Based on the SEM and TEM studies, the as-synthesized microspheres are considered to be a hierarchical nanostructure. In addition, the products prepared under similar conditions without tin source are mainly composed of hollow microspheres (ESI, Fig. S1†), as previous report.30 | ||
| Fig. 1 (a and b) SEM and (c and d) TEM images of S-450. The inset is the TEM image of exterior shell with high magnification. | ||
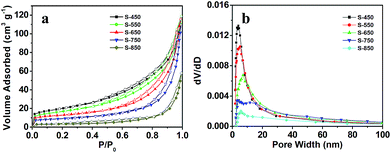
The mesoporous feature of S-450 was further confirmed via the N2 adsorption–desorption measurement (Fig. 2). The isotherm of S-450 presents a typical type IV curve, indicating the presence of mesopores in the microspheres (Fig. 2a). Fig. 2b shows the corresponding BJH pore size distributions (PSD) and it is clearly observed that the diameters of pores in S-450 are mainly in the range of 3–4 nm. In addition, the pores larger than 10 nm also exist in the as-synthesized product, which may be attributed to the accumulation of large particles. The surface area and pore volume of S-450 are 70.8 m2 g−1 and 0.189 cm3 g−1, respectively. On the basis of N2 adsorption–desorption studies, S-450 possess hierarchical porous structure, consistent with the TEM observation. It should be worth noting that the calcination temperature plays important roles in the porous structures of core–shell microspheres. As shown in Fig. 2b, mesopores are still present in the products, but the pore size increases with the calcination temperature. In addition, high calcination temperature makes the surface area and pore volume of the microspheres reduce (ESI, Table S1†). It may be attributed to the aggregation of smaller nanoparticles, resulting in the larger nanoparticles at high temperature (ESI, Fig. S2†).
The crystalline structures of core–shell microspheres were also investigated via XRD analysis. Fig. 3 displays the XRD patterns of the multilayered TiO2@SnO2 hollow nanostructures calcined at various temperatures. It is apparent that both anatase TiO2 (JCPDS-21-1272) and rutile SnO2 (JCPDS-41-1445) are observed in the XRD patterns of microspheres calcined below 750 °C, showing that the phase of these products is mainly composed of anatase TiO2 and rutile SnO2. Further increasing the calcination temperature to 850 °C, the diffraction peaks indexed to the rutile TiO2 (JCPDS 21-1276) appear, indicating that a considerable amount of anatase TiO2 transform into rutile TiO2. In addition, TiO2-450 prepared without Sn sources at 450 °C are mainly composed of anatase TiO2, similar to previous report.30
Aiming at investigating the formation mechanism of multilayered hollow nanostructures, XRD and SEM studies for intermediate product before calcination were performed. As shown in Fig. 4a, all the intermediate products are spheric in shape and coated with small nanoparticles. According to the previous report,30 the coated nanoparticles should be anatase TiO2, which is in agreement with the XRD pattern (Fig. 4b). In addition, Sn(II) species will be formed by the hydrolysis of SnSO4 under hydrothermal conditions and exist in the interior of intermediate products. Sequently, SnO2 nanoparticles are obtained via the oxidation of Sn(II) species during calcination.31 Based on the above studies, a general illustration for the formation of multilayered hollow nanostructures is proposed (Scheme 1). During hydrothermal reaction, Sn(II)–polymer spherical composites are formed via the hydrolysis of SnSO4 and polymerization reaction between resorcinol and formaldehyde. In the meantime, TiO2 nanoparticles obtained via the hydrolysis of Ti(SO4)2 are coated on the surface of Sn(II)–polymer spherical composites, forming the intermediate products. During calcination process, the polymer is removed and SnO2 nanoparticles are formed via the oxidation of Sn(II) species, aggregate to the centre of the spheres and form the interior shell and solid core. Meantime, TiO2 nanoparticles around the intermediate product mainly form the stable exterior shell structure and the multilayered hollow nanostructures are obtained. The EDS results of interior shell and exterior shell (ESI, Fig. S3†) demonstrate both of the shells consist of TiO2 and SnO2.
It is found that the molar ratio of Ti(SO4)2 to SnSO4 plays important roles in the composition and morphology of the products in this case. The XRD patterns of the products prepared with various Ti(SO4)2 to SnSO4 molar ratios show that all the samples are composed of anatase TiO2 and rutile SnO2 (Fig. 5a). The content of TiO2 increases with the molar ratio of Ti(SO4)2 to SnSO4. Fig. 5b–d display the SEM images of the products prepared with various Ti(SO4)2 to SnSO4 molar ratios. As shown in Fig. 5b, when Ti(SO4)2 to SnSO4 molar ratio is increased to 3.36![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the products are mainly composed of hollow microspheres. It may be attributed to that more Ti(SO4)2 could inhibit the formation of Sn(II) species via the hydrolysis of SnSO4, resulting in the decrease of SnO2 content and disappearing of the core structure. As the Ti(SO4)2 to SnSO4 molar ratio is decreased to 2.80
1, the products are mainly composed of hollow microspheres. It may be attributed to that more Ti(SO4)2 could inhibit the formation of Sn(II) species via the hydrolysis of SnSO4, resulting in the decrease of SnO2 content and disappearing of the core structure. As the Ti(SO4)2 to SnSO4 molar ratio is decreased to 2.80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, a certain amount of core–shell microspheres begin to appear in the products (Fig. 5c). Further decreasing the Ti(SO4)2 to SnSO4 molar ratio to 0.56
1, a certain amount of core–shell microspheres begin to appear in the products (Fig. 5c). Further decreasing the Ti(SO4)2 to SnSO4 molar ratio to 0.56![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the products mainly consist of broken hollow spheres (Fig. 5d). It may be reasonable that less TiO2 nanoparticles in the products could not form stable shell structure.
1, the products mainly consist of broken hollow spheres (Fig. 5d). It may be reasonable that less TiO2 nanoparticles in the products could not form stable shell structure.
 | ||
| Fig. 5 (a) XRD patterns and (b–d) SEM images of the products prepared with various Ti(SO4)2 to SnSO4 molar ratios. | ||
The photocatalytic performance of multilayered TiO2@SnO2 hollow nanostructures was evaluated via photodegradation of MO in aqueous solution under UV light irradiation. For comparison, the photocatalytic activities of both TiO2-450 and commercial P25 (Degussa AG, Frankfurt, Germany) were also measured under the same conditions. Fig. 6 shows the photodegradation of MO in the presence of various catalysts under UV light irradiation. It can be clearly seen that the photocatalytic activity of TiO2-450 is higher than that of P25. It may be attributed to the hollow microsphere nanostructure, which could allow reflection of UV light in the interior, improving the utilization of UV light and therefore possessing an improved photocatalytic performance.28 In addition, porous structure and higher specific surface area can also enhance the photocatalytic performance, facilitating the diffusion of dye molecules and possessing more active sites (ESI, Table S1†).32 It should be noting that S-450 exhibit higher photocatalytic activity than TiO2-450. It is ascribed to the introduction of SnO2, which can effectively suppress the charge recombination. According to the energy band theory and previous report,33 when the TiO2@SnO2 composites are excited by UV light with a photon energy higher than the band gaps of SnO2 and TiO2, the electrons in their valence band (VB) can be excited to their corresponding conduction band (CB) with simultaneous generation of the same amount of holes in their VB. Then, the photoinduced electrons and holes can be separated under the influence of the electrostatic field, the photoinduced electron transfer will occur from the CB of TiO2 to the CB of SnO2 and, conversely, the photoinduced hole transfer can take place from the VB of SnO2 to the VB of TiO2, as illustrated in Scheme 2a. In a word, composites with the matchable band potentials lead to improving separation efficiency of photoinduced electron and hole pairs. Furthermore, DRS results demonstrate that the higher light absorption of multilayered hollow nanostructure than that of hollow microsphere (ESI, Fig. S4†). It may be attributed to multiple reflections of incident light in the interior of hollow nanostructures (Scheme 2b), making more efficient utilization of light energy and giving birth to an improved catalytic activity. It can be seen that the calcination temperature plays important roles in photocatalytic behaviors for the as-synthesized microspheres. The photodegradation efficiency for the microspheres increases with the elevating of calcination temperature up to 750 °C. It may be attributed to that high calcination temperature leads to better crystal structure and less crystal defect for the microspheres, improving carrier transport efficiency and displaying higher activity. However, when the calcination temperature is further elevated to 850 °C, the photodegradation efficiency dramatically decreases. This is because a considerable amount of rutile TiO2 are present in the microspheres. According to previous report,34 the photocatalytic activity of rutile TiO2 is usually lower than that of anatase TiO2. Consequently, the photocatalytic activity of S-850 decreases.
 | ||
| Scheme 2 Schematic illustration of (a) electron transfer process in TiO2@SnO2 and (b) multiple reflections within the hollow sphere and multilayered core–shell sphere. | ||
4. Conclusion
Multilayered TiO2@SnO2 hollow nanostructures were successfully synthesized via a simple approach employing the template-assisted and hydrothermal methods. The molar ratio of Ti(SO4)2 to SnSO4 and calcination temperature play important roles in controlling the composition and morphology of the products. As-fabricated multilayered hollow nanostructures endow the microspheres with enhanced photocatalytic activity attributed to multiple reflections of incident light. Meanwhile, the TiO2@SnO2 composites effectively promote photoinduced carrier separation efficiency for their matchable band potentials. The multilayered TiO2@SnO2 hollow nanostructures could also be supposed to apply in high-efficiency dye sensitized solar cells due to superior light-scattering performance.Acknowledgements
The research was supported by Program for Key Science & Technology Platform ([2011]191) in Universities of Liaoning Province, the National Natural Science Foundation of China (Grant no. 21476033), Cultivation Program for Excellent Talents of Science and Technology Department of Liaoning Province (no. 201402610), Science and Technology Research Project of the Education Department of Liaoning Province (no. L2014225) and Open Project of State Key Laboratory for Supramolecular Structure and Materials (SKLSSM201425).References
- T. Kamegawa, Y. Shimizu and H. Yamashita, Adv. Mater., 2012, 24, 3697 CrossRef CAS PubMed.
- S. Izadyar and S. Fatemi, Ind. Eng. Chem. Res., 2013, 52, 10961 CrossRef CAS.
- W. L. Wang, Q. K. Shang, W. Zheng, H. Yu, X. J. Feng, Z. D. Wang, Y. B. Zhang and G. Q. Li, J. Phys. Chem. C, 2010, 114, 13663 CAS.
- K. Pan, Y. Z. Dong, W. Zhou, Q. J. Pan, Y. Xie, T. F. Xie, G. H. Tian and G. F. Wang, ACS Appl. Mater. Interfaces, 2013, 5, 8314 CAS.
- X. Lia, T. Xia, C. H. Xu, J. Murowchick and X. B. Chen, Catal. Today, 2014, 225, 64 CrossRef PubMed.
- L. Qin, X. X. Pan, L. Wang, X. P. Sun, G. L. Zhang and X. W. Guo, Appl. Catal., B, 2014, 150, 544 CrossRef PubMed.
- F. X. Xiao, ACS Appl. Mater. Interfaces, 2012, 4, 7055 CAS.
- S. S. Zhang, C. Liu, X. L. Liu, H. M. Zhang, P. R. Liu, S. Q. Zhang, F. Peng and H. J. Zhao, Appl. Microbiol. Biotechnol., 2012, 96, 1201 CrossRef CAS PubMed.
- Z. Y. Zhang, C. L. Shao, X. H. Li, Y. Y. Sun, M. Y. Zhang, J. B. Mu, P. Zhang, Z. C. Guo and Y. C. Liu, Nanoscale, 2013, 5, 606 RSC.
- A. Kusior, J. Klich-Kafel, A. Trenczek-Zajac, K. Swierczek, M. Radecka and K. Zakrzewska, J. Eur. Ceram. Soc., 2013, 33, 2285 CrossRef CAS PubMed.
- Y. P. Wang and J. Li, Appl. Surf. Sci., 2008, 254, 5276 CrossRef CAS PubMed.
- Y. H. Lin and T. K. Tseng, Appl. Catal., A, 2014, 469, 221 CrossRef CAS PubMed.
- Y. Ma and J. Zhang, J. Hazard. Mater., 2010, 182, 386 CrossRef CAS PubMed.
- J. Goebl, J. B. Joo, M. Dahl and Y. D. Yin, Catal. Today, 2014, 225, 90 CrossRef CAS PubMed.
- C. T. Yang, N. Balakrishnan and V. R. Bhethanabotla, J. Phys. Chem. C, 2014, 118, 4702 CAS.
- X. Y. Pan and Y. J. Xu, ACS Appl. Mater. Interfaces, 2014, 6, 1879 CAS.
- B. Weng, S. Q. Liu, Z. R. Tang and Y. J. Xu, RSC Adv., 2014, 4, 12685 RSC.
- S. A. Ansari, M. M. Khan, M. O. Ansari, S. Kalathil, J. Lee and M. H. Cho, RSC Adv., 2014, 4, 16782 RSC.
- J. Tian, Z. H. Zhao, A. Kumar, R. I. Boughton and H. Liu, Chem. Soc. Rev., 2014, 43, 6920 RSC.
- J. Pan, J. T. Li, Z. L. Yan, B. H. Zhou, H. S. Wu and X. Xiong, Nanoscale, 2013, 5, 3022 RSC.
- S. Q. Liu, M. Q. Yang and Y. J. Xu, J. Mater. Chem. A, 2014, 2, 430 CAS.
- M. Y. Wang, J. Ioccozia, L. Sun, C. J. Lin and Z. Q. Lin, Energy Environ. Sci., 2014, 7, 2182 CAS.
- J. B. Joo, M. Dahl, N. Li, F. Zaera and Y. D. Yin, Energy Environ. Sci., 2013, 6, 2082 CAS.
- J. B. Joo, I. Lee, M. Dahl, G. D. Moon, F. Zaera and Y. D. Yin, Adv. Funct. Mater., 2013, 23, 4246 CrossRef CAS.
- G. L. Li, H. Y. Zhang, J. Lan, J. Li, Q. W. Chen, J. Y. Liu and G. B. Jiang, Dalton Trans., 2013, 42, 8541 RSC.
- Y. Zheng, J. H. Cai, K. L. Lv, J. Sun, H. P. Ye and M. Li, Appl. Catal., B, 2014, 147, 789 CrossRef CAS PubMed.
- J. H. Cai, Z. Y. Wang, K. L Lv, Y. Zheng, J. G. Yu and M. Li, RSC Adv., 2013, 3, 15273 RSC.
- H. X. Li, Z. F. Bian, J. Zhu, D. Q. Zhang, G. S. Li, Y. N. Huo, H. Li and Y. F. Lu, J. Am. Chem. Soc., 2007, 129, 8406 CrossRef CAS PubMed.
- J. F. Qian, P. Liu, Y. Xiao, Y. Jiang, Y. L. Cao, X. P. Ai and H. X. Yang, Adv. Mater., 2009, 21, 3663 CrossRef CAS.
- F. Zhang, Yu. Zhang, S. Y. Song and H. J. Zhang, J. Power Sources, 2011, 196, 8618 CrossRef CAS PubMed.
- F. Zhang, K. X. Wang, X. Y. Wang, G. D. Li and J. S. Chen, Dalton Trans., 2011, 40, 8517 RSC.
- T. Leshuk, S. Linley, G. Baxter and F. Gu, ACS Appl. Mater. Interfaces, 2012, 4, 6062 CAS.
- M. F. Abdel-Messih, M. A. Ahmed and A. S. El-Sayed, J. Photochem. Photobiol., A, 2013, 260, 1 CrossRef CAS PubMed.
- M. Murdoch, G. I. N. Waterhouse, M. A. Nadeem, J. B. Metson, M. A. Keane, R. F. Howe, J. Llorca and H. Idriss, Nat. Chem., 2011, 3, 489 CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra12103a |
| This journal is © The Royal Society of Chemistry 2014 |