Fabrication of carbon nitride nanotubes by a simple water-induced morphological transformation process and their efficient visible-light photocatalytic activity
Zhenxing Zenga,
Kexin Li*a,
Liushui Yana,
Yuhua Daia,
Huiqin Guoa,
Mingxin Huob and
Yihang Guob
aKey Laboratory of Jiangxi Province for Persistent Pollutants Control and Resources Recycle, Nanchang Hangkong University, Nanchang 330063, PR China. E-mail: likx880@hotmail.com; Fax: +86 791 83953373; Tel: +86 791 83953373
bJilin Engineering Research Centre for Municipal Wastewater Treatment and Water Quality Protection, School of Environment, Northeast Normal University, Changchun 130117, PR China
First published on 4th November 2014
Abstract
Carbon nitride nanotubes (C3N4 NTs) were synthesized based on the nanosheets roll-up mechanism by a simple water-induced morphological transformation process using graphitic carbon nitride (g-C3N4) as a precursor. Water was used as the phase-transfer reagent, making the preparation process environmentally friendly. The visible-light photocatalytic activity of the as-prepared C3N4 NTs significantly increased compared to bulk g-C3N4 and g-C3N4 nanosheets toward rhodamine B degradation and hydrogen evolution from water-splitting. This result can be attributed to the high photogenerated carrier transfer efficiency, excellent mass transfer capability, sufficient active sites, and enhanced light utilization efficiency of C3N4 NTs.
1. Introduction
The visible-light photocatalytic activity of g-C3N4 for hydrogen production was discovered by Wang et al.1 Since then, the application of g-C3N4 as an efficient organic semiconductor photocatalyst or chemical catalyst for hydrogen evolution, organic pollutant degradation, and oxidation reaction has been widely developed.2,3 The g-C3N4 can be prepared through the polycondensation of various organic precursors, such as urea, cyanamide, and melamine, at high temperatures and exhibits a graphite-like interlayer structure.4–6 High-temperature calcination determines the layered bulk structure of g-C3N4. The bulk structure limits the catalytic activity of g-C3N4 because of the small number of active sites, rapid recombination of photogenerated electrons and holes, and high mass transfer resistance. The fabrication of g-C3N4-based heterostructured photocatalysts is an effective strategy to further improve the photocatalytic performance of bulk g-C3N4. However, the interaction of bulk g-C3N4 and the dopant through the interfacial reaction in these heterostructured photocatalysts only is limited to the exposed surface of bulk g-C3N4. Adjusting the morphology of bulk g-C3N4 is an important strategy to improve its photocatalytic performance. Yang et al.7 fabricated g-C3N4 nanosheets using a liquid exfoliation method to enhance the hydrogen evolution activity of bulk g-C3N4 under visible-light irradiation. Tubular nanostructure photocatalytic materials (e.g., TiO2 nanotubes) with open mesoporous morphology can efficiently transfer the photogenerated carriers along the 1D path, whereas their exposed inner and outer surface can lead to a high surface photocatalytic reaction rate.8,9 Therefore, the development of C3N4 NTs photocatalysts has gained increasing attention. However, controlling the morphology of bulk g-C3N4 to form C3N4 NTs during the high-temperature polycondensation process of organic precursors is difficult because of its layered structural tendency. Some scholars attempted to prepare C3N4 NTs using catalytic self-assembly, templating, or chemical vapor deposition method.10–12 However, the aforementioned processes suffer from some drawbacks, including harsh preparation conditions, operational complexity, toxicity, and high cost, which limit the large-scale preparation of C3N4 NTs.In the present study, C3N4 NTs were successfully demonstrated for the first time through a simple water-induced morphological transformation process from g-C3N4 nanosheets, and g-C3N4 nanosheets were obtained by ultrasonic exfoliation of bulk g-C3N4. Photocatalytic tests showed that the visible-light photocatalytic activity of C3N4 NTs significantly increased compared with that of bulk g-C3N4 and g-C3N4 nanosheets toward rhodamine B degradation and hydrogen evolution from water-splitting. Water was used as the “phase-transfer reagent” in this study, making the preparation process environmental friendly.
2. Experimental
2.1. Preparation of C3N4 NTs
C3N4 NTs were fabricated from g-C3N4 nanosheets through water-induced morphological transformation process, and g-C3N4 nanosheets were obtained by ultrasonic exfoliation of bulk g-C3N4. In a typical synthesis, 5 g of melamine was calcined in air at 550 °C for 2 h, and the heat rate is 10 °C min−1 in the heating process. After exfoliating the bulk g-C3N4 powder by a 500 W ultrasonic crasher for 1 h in water, the dry g-C3N4 nanosheets powder was heated at 350 °C for 10 min (heating rate is 10 °C min−1). Then, the hot g-C3N4 nanosheets powder was rapidly transferred to 500 mL cool water. Finally, the C3N4 NTs sample was obtained by centrifugation and dried.2.2. Characterizations
Field emission scanning electron microscopy (FESEM) images were recorded using a JEOL JSM-6700F field emission scanning electron microscope. Transmission electron microscopy (TEM) and selected area electron diffraction (SAED) images were recorded on a JEOL JEM-2010 transmission electron microscope at an accelerating voltage of 200 kV. X-ray diffraction (XRD) patterns were obtained using a Panalytical X'Pert PRO diffractometer via Cu Kα radiation. Nitrogen gas porosimetry measurements were performed on a Quantachrome NOVA 2000e surface area and porosity analyzer after the samples were outgassed under a vacuum at 70 °C for 20 min and 150 °C for 6 h. Fourier transform infrared (FTIR) spectra were recorded on a Bruker VERTEX 70 FTIR apparatus. UV-visible/diffuse reflection spectra (UV-vis/DRS) were conducted using a Lambda 750S UV-vis-NIR spectrometer. Photoluminescence (PL) measurements were carried out on a HITACHI F-7000 fluorescence spectrophotometer. The incident photon to current conversion efficiency (IPCE) was measured with a QE/IPCE Measurement Kit (Newport, USA) in the wavelength range of 300–750 nm.2.3. Adsorption and photocatalytic tests
A PLS-SXE300 Xe lamp served as the light source, and the output wavelength λ > 320 nm. The visible light irradiation was obtained by removing the UV irradiation from the lamp using a 400 nm cut filter, which can control the output wavelength λ > 400 nm. For the rhodamine B adsorption and degradation, the catalyst amount is 100 mg, the initial concentration of rhodamine B is 10 mg L−1, and the initial volume is 100 mL. Changes in the rhodamine B concentrations were analyzed using an UNICO UV-2000 spectrophotometer at λ = 554 nm. For the hydrogen evolution reaction conditions: 100 mg of catalyst loaded with 3 wt% of Pt co-catalyst; 100 mL of H2O containing 10 vol% triethylamine. The generated hydrogen was in situ analyzed with a GC 7890-II TCD gas chromatograph (TECHCOMP) using an MS-5 A column, which was connected to the gas circulating line with argon carrier.2.4. Photocurrent measurements
The working electrode was prepared on the rectangle titanium (Ti) sheets (size 10 mm × 50 mm, thickness 140 μm, purity > 99.6%), which was cleaned by sonication in water and alcohol for 10 min, respectively. The cleaned Ti sheets were chemically etched in a mixture of HF, HNO3 and H2O for 30 s (HF–HNO3–H2O = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5; v/v/v) followed by rinsing with distilled water and kept in alcohol. The catalyst powder (10 mg) was mixed with alcohol (2 mL) under sonication for 30 min to get slurry. The obtained slurry was used for spin-coating on the Ti sheet at an initial spin rate of 500 rpm for 9 s and then 2000 rpm for 10 s. After air drying, the prepared working electrode was heated at 60 °C for 6 h in air to improve the adhesion of the catalyst. The cooled Ti sheets were washed with water for three times. Finally, the sheets were dried at 60 °C for 24 h.
5; v/v/v) followed by rinsing with distilled water and kept in alcohol. The catalyst powder (10 mg) was mixed with alcohol (2 mL) under sonication for 30 min to get slurry. The obtained slurry was used for spin-coating on the Ti sheet at an initial spin rate of 500 rpm for 9 s and then 2000 rpm for 10 s. After air drying, the prepared working electrode was heated at 60 °C for 6 h in air to improve the adhesion of the catalyst. The cooled Ti sheets were washed with water for three times. Finally, the sheets were dried at 60 °C for 24 h.
Photocurrent measurements were carried out using the conventional three electrode setup connected to an electrochemical station (CHI 630E, Shanghai Chenhua, China). In this electrochemical system, the prepared catalyst/Ti sheet was used as the working electrode; a Pt wire was used as the counter electrode and a Ag/AgCl electrode (saturated KCl) was used as the reference electrode. The electrolyte is 0.01 mol L−1 Na2SO4 aqueous solution (100 mL). A 300 W Xe lamp served as a light source. The measurements were carried out at a constant potential of +1.0 V to the working electrode.
3. Results and discussion
3.1. Fabrication of C3N4 NTs by water-induced morphological transformation process
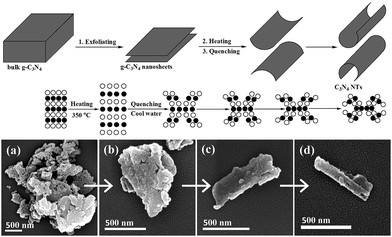
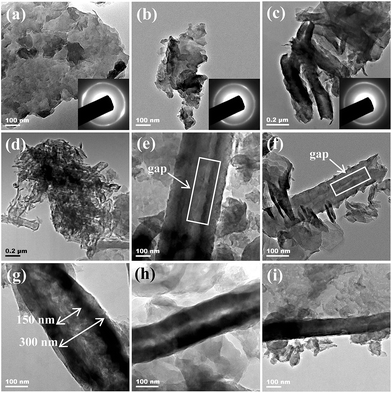
The fabrication of C3N4 NTs through water-induced morphological transformation is based on the nanosheets roll-up mechanism.13 Some scholars suggested that the tubular nanostructure reduces the charge imbalance caused by unsaturated or dangling bonds at the edges of the intermediate structure.14 Other studies justified nanotubes formation through an asymmetric chemical environment or mechanical stress.15 In the current preparation system, C3N4 NTs were formed through the crimping of g-C3N4 nanosheets after the sudden hot–cool process driven by the generated thermal stress. As shown in Fig. 1, the morphological transformation process was monitored via FESEM. First, bulk g-C3N4 was exfoliated to form g-C3N4 nanosheets with less than five layers after ultrasonic crashing for 1 h in water (Fig. 1a and b). The atomic spacing of the g-C3N4 nanosheets was then increased when the temperature increased from room temperature to 350 °C. In the subsequent cold water quenching process, the surface cooling rate of the g-C3N4 nanosheets was higher than the inner cooling rate. Therefore, the surface atoms in the g-C3N4 nanosheets rapidly returned to the initial position than the inner atoms. As a result, the thermal stress was generated in the g-C3N4 nanosheets, and the g-C3N4 nanosheets demonstrated inner stretching and surface compression. Finally, the g-C3N4 nanosheets formed a plastic deformation and then curled to form C3N4 NTs when the thermal stress exceeded the yield limit of the g-C3N4 nanosheets (Fig. 1c and d). In the C3N4 NTs preparation process, water was selected as the phase-transfer reagent because of its poor thermal conductivity. The poor thermal conductivity of water caused a relatively stable temperature difference at the interface of the g-C3N4 nanosheets and water, which resulted in a continuous thermal stress in the g-C3N4 nanosheets. Therefore, the morphological transformation process can occur under continuous thermal stress.3.2. Characterization
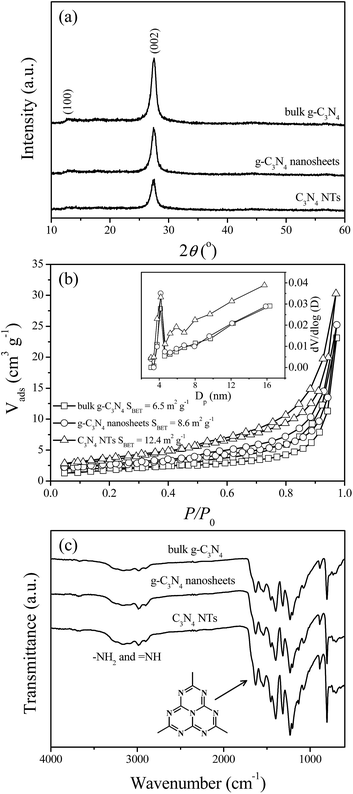
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N in the CN heterocycles. The sharp peak at 806.7 cm−1 is the typical bending vibration of s-triazine units. The broad absorption peaks located in the range of 3400 cm−1 to 2800 cm−1 originate from the stretching vibrational modes of the primary (−NH2) and secondary (
N in the CN heterocycles. The sharp peak at 806.7 cm−1 is the typical bending vibration of s-triazine units. The broad absorption peaks located in the range of 3400 cm−1 to 2800 cm−1 originate from the stretching vibrational modes of the primary (−NH2) and secondary (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–H) amines associated with uncondensed amino groups. The FTIR spectra of g-C3N4 nanosheets and C3N4 NTs are apparently similar to that of bulk g-C3N4, indicating that g-C3N4 nanosheets and C3N4 NTs maintain the same chemical structure as bulk g-C3N4. However, g-C3N4 nanosheets and C3N4 NTs demonstrate stronger FTIR modes than bulk g-C3N4 because of their more exposed surface functional groups. In addition, from the above XRD, BET surface area, and FTIR results it can be found that C3N4 NTs exhibit weaker XRD intensity, larger BET surface area, and stronger FTIR mode than g-C3N4 nanosheets. This result suggests that g-C3N4 nanosheets are exfoliated once again during the water-induced morphological transformation process.
N–H) amines associated with uncondensed amino groups. The FTIR spectra of g-C3N4 nanosheets and C3N4 NTs are apparently similar to that of bulk g-C3N4, indicating that g-C3N4 nanosheets and C3N4 NTs maintain the same chemical structure as bulk g-C3N4. However, g-C3N4 nanosheets and C3N4 NTs demonstrate stronger FTIR modes than bulk g-C3N4 because of their more exposed surface functional groups. In addition, from the above XRD, BET surface area, and FTIR results it can be found that C3N4 NTs exhibit weaker XRD intensity, larger BET surface area, and stronger FTIR mode than g-C3N4 nanosheets. This result suggests that g-C3N4 nanosheets are exfoliated once again during the water-induced morphological transformation process.
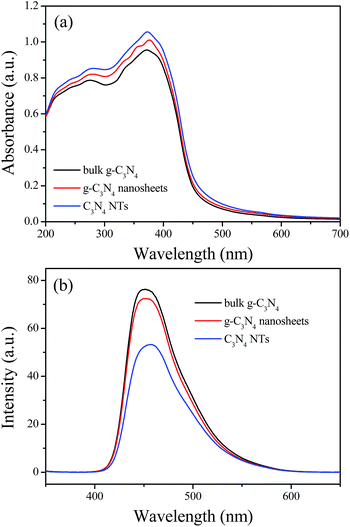
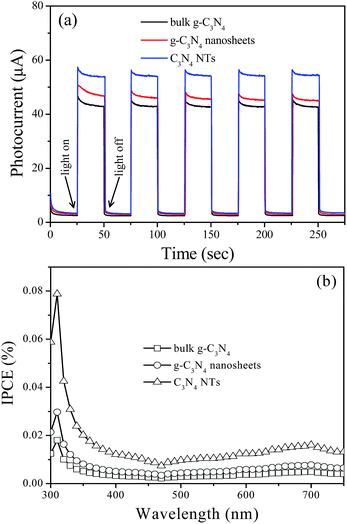
The photoelectric conversion efficiency of bulk g-C3N4, g-C3N4 nanosheets, and C3N4 NTs is studied by photocurrent and IPCE measurements (Fig. 5). As shown in Fig. 5a, sharp increases in the photocurrent responses are observed in all the tested working electrodes after pulse Xe lamp irradiation. The generated photocurrents are reproducible and stable during the five intermittent on–off irradiation cycles. The prompt increase in photocurrent response from light-off to light-on state is mainly ascribed to the fast separation and transportation of the photogenerated electron on the surface of the working electrodes. The C3N4 NTs exhibit a higher photocurrent response than bulk g-C3N4 and g-C3N4 nanosheets under the same conditions, indicating that tubular nanostructure can effectively delay the recombination rate of the photogenerated carriers. The IPCE measurements were conducted by directly coating the powder sample on the conductive surface of two pieces of overlapped FTO (fluorine-doped tin oxide) conductive glass. The result shows that C3N4 NTs have a higher IPCE value compared with bulk g-C3N4 and g-C3N4 nanosheets, implying that tubular nanostructure can effectively reduce the probability of electron–hole pair recombination by improving photogenerated carriers transfer capability, thereby prolonging the charge lifespan.
3.3. Adsorption kinetics, degradation kinetics, and hydrogen evolution activity
The mass transfer capacity of as-prepared materials is evaluated by adsorption kinetics study. The dynamic curves for the adsorption of rhodamine B on bulk g-C3N4, g-C3N4 nanosheets, and C3N4 NTs were shown in Fig. 6a. As shown in Fig. 6a, C3N4 NTs exhibit the highest adsorption amount and fastest adsorption rate among the tested materials. This result fully proves the excellent mass transfer capability of C3N4 NTs. The mass transfer capability has significant effect on the photocatalytic activity of as-prepared materials, therefore, C3N4 NTs sample is expected to exhibit high photocatalytic activity toward rhodamine B degradation and hydrogen evolution reaction. The photocatalytic activities of bulk g-C3N4, g-C3N4 nanosheets, and C3N4 NTs are evaluated by aqueous rhodamine B degradation and hydrogen evolution from water-splitting. The degradation kinetics curves of rhodamine B show that the degradation rate of C3N4 NTs sample is the fastest among the tested materials (Fig. 6b). Therefore, the K value (first-order rate constant) of C3N4 NTs sample is the largest among the tested materials. The photocatalytic activities of as-prepared materials for hydrogen evolution from water-splitting are evaluated in a 10 vol% triethylamine aqueous solution under λ > 400 nm visible-light irradiation in the presence of 3 wt% Pt nanoparticles co-catalyst. As shown in Fig. 6c, C3N4 NTs sample has the highest photocatalytic hydrogen evolution activity among the tested materials, and the hydrogen evolution efficiency of C3N4 NTs is 28.5 μmol h−1. Basing on the characterization results, we can attribute the superior visible-light photocatalytic activity of C3N4 NTs to their unique tubular nanostructure with high photogenerated carriers transfer efficiency, excellent mass transfer capability, sufficient active sites, and enhanced light utilization efficiency.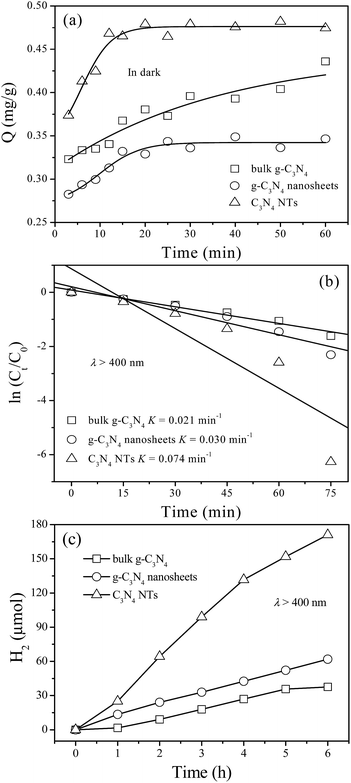 | ||
| Fig. 6 Kinetics curves of adsorption (a) and degradation (b) toward aqueous rhodamine B and hydrogen evolution activity (c) over bulk g-C3N4, g-C3N4 nanosheets, and C3N4 NTs photocatalysts. | ||
4. Conclusions
C3N4 NTs were successfully prepared based on the nanosheets roll-up mechanism via a simple water-induced morphological transformation process. The enhanced photocatalytic activity of as-prepared C3N4 NTs toward rhodamine B degradation and hydrogen evolution from water-splitting can be attributed to their unique tubular nanostructure with high photogenerated carriers transfer efficiency, excellent mass transfer capability, sufficient active sites, and enhanced light utilization efficiency. In addition to their valuable application as a photocatalyst, the as-prepared C3N4 NTs may provide broad applications in the fields of photoelectrochemistry, electronics, sensors, and energy storage.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (51208248, 21366024, 21165013, 51468043, 51238001, 51278092); Science and Technology Major Bidding Project of Jiangxi Province, China (no. Gan Ke Fa Ji Zi [2010] 217); Youth Science Foundation of Jiangxi Province, China (20114BAB213015); Natural Science Foundation of Jiangxi Provincial Department of Education, China (GJJ12456, GJJ14515).Notes and references
- X. Wang, K. Maeda, A. Thomas, K. Takanabe, G. Xin, J. M. Carlsson, K. Domen and M. Antonietti, Nat. Mater., 2009, 8, 76–80 CrossRef CAS PubMed.
- X. Wang, K. Maeda, X. Chen, K. Takanabe, K. Domen, Y. Hou, X. Fu and M. Antonietti, J. Am. Chem. Soc., 2009, 131, 1680–1681 CrossRef CAS PubMed.
- J. A. Singh, S. H. Overbury, N. J. Dudney, M. Li and G. M. Veith, ACS Catal., 2012, 2, 1138–1146 CrossRef CAS.
- Y. Zhang, J. Liu, G. Wu and W. Chen, Nanoscale, 2012, 4, 5300–5303 RSC.
- Y. Wang, J. Yao, H. Li, D. Su and M. Antonietti, J. Am. Chem. Soc., 2011, 133, 2362–2365 CrossRef CAS PubMed.
- Y. S. Jun, E. Z. Lee, X. Wang, W. H. Hong, G. D. Stucky and A. Thomas, Adv. Funct. Mater., 2013, 23, 3661–3667 CrossRef CAS.
- S. Yang, Y. Gong, J. Zhang, L. Zhan, L. Ma, Z. Fang, R. Vajtai, X. Wang and P. M. Ajayan, Adv. Mater., 2013, 25, 2452–2456 CrossRef CAS PubMed.
- W. H. Chiang and R. M. Sankaran, Nat. Mater., 2009, 8, 882–886 CrossRef CAS PubMed.
- O. K. Varghese, M. Paulose and C. A. Grimes, Nat. Nanotechnol., 2009, 4, 592–597 CrossRef CAS PubMed.
- J. Gao, Y. Zhou, Z. Li, S. Yan, N. Wang and Z. Zou, Nanoscale, 2012, 4, 3687–3692 RSC.
- S. W. Bian, Z. Ma and W. G. Song, J. Phys. Chem. C, 2009, 113, 8668–8672 CAS.
- J. W. Lee, R. Viswan, Y. J. Choi, Y. Lee, S. Y. Kim, J. Cho, Y. Jo and J. K. Kang, Adv. Funct. Mater., 2009, 19, 2213–2218 CrossRef CAS.
- P. Liu, H. Zhang, H. Liu, Y. Wang, X. Yao, G. Zhu, S. Zhang and H. Zhao, J. Am. Chem. Soc., 2011, 133, 19032–19035 CrossRef CAS PubMed.
- M. D. Hernández-Alonso, S. García-Rodríguez, B. Sánchez and J. M. Coronado, Nanoscale, 2011, 3, 2233–2240 RSC.
- A. Nakahira, T. Kubo and C. Numako, Inorg. Chem., 2010, 49, 5845–5852 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2014 |