Construction of a fluorescence turn-on probe for highly discriminating detection of cysteine†
Abstract
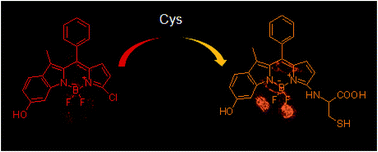
It is still a challenge to construct probes for discriminating thiols due to the similar structure and reactivities of thiol-containing molecules. We have here developed a Cys specific probe by utilizing the remarkable difference in reactivity toward Cys, Hcy and GSH. The reaction between the designed probe and Cys produces an amino-substituted BODIPY, giving a yellow fluorescence turn-on response. The response to Hcy or GSH shows a red fluorescence turn-on signal, due to the formation of sulfenyl-substituted BODIPY. These distinct fluorescence turn-on responses allow Cys to be distinguished from Hcy and GSH. This probe was also utilized for detection of Cys in living cells and monitoring cystathionine γ-lyase activity in vitro.

 Please wait while we load your content...
Please wait while we load your content...