Recent mechanistic developments and next generation catalysts for the Sonogashira coupling reaction
Milandip Karak
a,
Luiz C. A. Barbosa
*ab and
Gráinne C. Hargaden
c
aDepartamento de Química, Universidade Federal de Viçosa, Av. Peter Henry Rolfs, s/n Campus Universitário, CEP 36570-900, Viçosa, MG, Brazil. E-mail: lcab@ufmg.br
bDepartamento de Química, Universidade Federal de Minas Gerais, Av. Pres. Antônio Carlos, 6627, Campus Pampulha, CEP 31270-901, Belo Horizonte, MG, Brazil
cSchool of Chemical and Pharmaceutical Sciences, Dublin Institute of Technology, Kevin Street, Dublin 8, Ireland
First published on 7th October 2014
Abstract
The Pd-catalyzed Sonogashira reaction is a powerful method for the formation of Csp2–Csp bonds and has found application in a wide variety of areas including medicinal chemistry, agrochemistry, materials and electronics. Development of competent catalysts for the Sonogashira reaction is a particular scientific challenge since it is traditionally a di-metallic-mediated homogeneous catalytic process including some major drawbacks. This review provides a concise overview of the mechanistic aspects of the Cu co-catalyzed, Cu-free and Au-catalyzed Sonogashira coupling processes. More recent developments and next generation catalysts for the Sonogashira reaction are also presented. These include non transition-metal catalysts, metal free couplings and photo-induced protocols. Finally, the application of metal nanoparticles in Sonogashira reactions is presented. These include Pd nanoparticles, Pd bi- and tri-metallic nanoparticles, magnetically separable Pd/Fe3O4 nanoparticles, Ru nanoparticles and Au nanoparticles.
1. Introduction
Over the past 15 years, there has been a growing interest in the Sonogashira coupling reaction1 which is one of the most powerful methods for the formation of Csp2–Csp bonds. The Pd catalyzed Csp2–Csp coupling reaction between aryl halides and terminal alkynes (Scheme 1) has become the most important method for preparing aryl alkynes and conjugated alkenynes, which are often intermediates or precursors in the synthesis of natural products,2,3 pharmaceuticals of biological activity,4,5 non-linear optical materials and molecular electronics,6–8 dendrimeric and polymeric materials,9–11 macrocycles with acetylene links12 and polyalkynylated molecules.13,14Although the Sonogashira reaction became the most popular route for carrying out alkynylation of aryl or alkenyl halides, the standard Pd catalyzed and Cu co-catalyzed Sonogashira reactions has some major drawbacks including the use of highly expensive Pd catalysts (sometimes required in high loadings), difficulties in recovering these catalysts, high reaction temperatures, air sensitivity of transition-metal complexes and the use of large amounts of ligands and amines. Another drawback of the Sonogashira reaction is the formation of homocoupled products upon exposure of the alkynes to oxidizing agents, Cu salts or air (Glaser coupling15 or Hay coupling16). The homocoupling products can be formed in considerable yields and tend to be difficult to separate from the desired products due to similar chromatographic mobility.17 With these significant drawbacks, a more efficient process is highly desirable. To date, several modifications including Pd-free,18 Cu-free,19 amine-free,20 ligand-free21 and solvent-free22 conditions and also variations of the ligands, Pd sources, solvents and bases with a view to performing the coupling reactions more efficiently have been reported. Several modifications, including the use of inexpensive metal catalysts, such as Cu,23,24 Fe,25 and Ni26 in place of Pd in the Sonogashira reaction have been successfully described, but there has been some concerns that the observed effects were due to trace amounts of Pd contamination present in the reagents used. Even now the exact mechanism of the homogeneous Pd catalyzed and Cu co-catalyzed Sonogashira reaction is not well understood and has remained a topic of debate.
In view of several remarkable improvements of the Sonogashira reactions in recent years, the focus of this review is the current development of the mechanism and next generation catalysts protocols for the Sonogashira reactions with their variety of scopes and limitations.
2. Mechanistic study: a concise overview
In recent years, there has been a significant effort in the development of experimental procedures which work in the absence of Cu salts, namely, Cu-free Sonogashira reactions. Although Cu(I) plays a crucial role in the Sonogashira reaction, the addition of a Cu(I) salt under the reaction conditions leads to some drawbacks, including the induction of a Glaser15 type oxidative homocoupling of the terminal alkyne to yield the corresponding diyne.27 In some cases it has been observed that Cu(I) salts have an inhibitory effect in the Sonogashira reaction.28,29 Several computational studies have focused on the mechanism of this Cu-free protocol.30–322.1. Copper co-catalyzed reaction mechanism
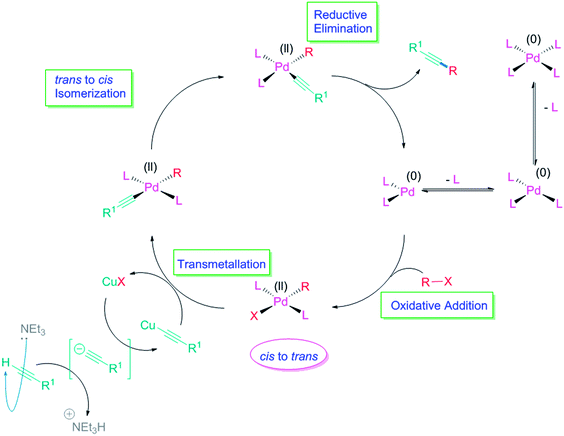
The general Sonogashira protocol for the reaction of terminal alkynes with aryl or alkenyl halides or triflates involves a Pd(0)/Cu(I) catalytic system and at least a stoichiometric amount of base.33,34 The presence of a Cu(I) salt is generally believed to facilitate the transfer of the alkynyl group to the Pd catalyst by the in situ generation of a Cu-acetylide species and the subsequent transmetallation of this group to Pd. The generally accepted Pd-catalyzed cycle is based on four steps: (A) oxidative addition (where Pd is oxidized from Pd(0) to Pd(II)) (cis–trans); (B) transmetallation (Pd remains as Pd(II)); (C) trans–cis isomerization (Pd remains as Pd(II)); and (D) reductive elimination (where Pd is reduced from Pd(II) to Pd(0)) (Fig. 1).Mechanistic studies of the oxidative addition step predict the formation of cis-Pd(II)L2RX complexes, which have rarely been isolated.35 Therefore, the isomerization of cis-Pd(II)L2RX to trans-Pd(II)L2RX complex is proposed (Fig. 1). This step was thoroughly investigated and four separate pathways were found (two of them autocatalytic and two solvent assisted).35 Computational studies concluded that the ligand assisted mechanism was energetically the most favored.36 The isomerization of the oxidative addition product cis-Pd(II)L2RX to trans-Pd(II)L2RX complex is usually very fast37 and therefore, the trans-complex is generally accepted as the starting point of the deprotonation step. The next step in the Pd-catalyzed cycle would connect with the cycle of the Cu co-catalyst. Careful analysis of the catalytic cycle reveals that the terminal alkyne is not the direct nucleophile in the Pd-catalyzed cycle. It is proposed that Cu-acetylide is the nucleophile for the Pd catalyzed cycle, and is also the catalytic species for the Cu-catalyzed cycle.38 Thus, a transmetallation from the Cu-acetylide formed in the Cu-cycle would generate a Pd-complex species, which gives the final coupled alkyne after trans–cis isomerization and reductive elimination, with regeneration of the catalyst. The reductive elimination step is usually irreversible. Recently, a report established that the transmetallation step of the Sonogashira reaction is the rate-limiting step39 which was demonstrated to be a Pd catalyzed and Cu co-catalyzed synergistic process and exhibits a first-order kinetic dependence on the Pd and Cu catalysts, respectively.
2.2. Copper-free reaction mechanism
Several studies have been carried out in an attempt to propose a reasonable mechanism for the Cu-free Sonogashira reaction by chemical kinetics experiments and DFT (density functional theory) calculations. The temperature dependent Sonogashira reactions for several different aryl halides, and the corresponding activation enthalpies and entropies, determined by means of Eyring plots, has established a linear correlation between ΔH≠ and the Kohn–Sham HOMO energies of aryl halides. Notably, in the same year, another study attempted to explain a sophisticated mechanism for the Sonogashira reaction based on theoretical study employing DFT calculations.40 Such studies demonstrated that there are two pathways in the oxidative addition step, the neutral and the anionic pathways, which could both give rise to cis-configured square planar Pd(II)–diphosphine intermediates. Initiated by this intermediate, reductive elimination of aryl acetylene is energetically favorable. More recent studies also revealed that the Pd catalyzed Cu-free Sonogashira reaction mechanism, explained by DFT calculations and theoretical mechanistic studies,41,42 goes via two most common routes: (a) carbopalladation43,44 and (b) deprotonation45 (Fig. 2). | ||
| Fig. 2 Catalytic cycles proposed for the Cu-free Sonogashira reaction involving two common routes designated as carbopalladation43 and deprotonation.45 | ||
Both mechanisms share the initial oxidative addition of the organohalide R1-X to the Pd(0)L2 complex giving intermediate 1 where the metal has been oxidized from Pd(0) to Pd(II). Subsequently the ligand is replaced by the alkyne, which results in the formation of complex 2, with Pd remaining as Pd(II) (Fig. 2). The theoretical results demonstrated that the proposed carbopalladation mechanism has a very high energy barrier,42 which indicates that this mechanism does not occur under the reaction conditions. In the deprotonation mechanism,46 the calculated Gibbs energy barriers indicate that both cationic and anionic mechanisms are feasible (Fig. 3).42 The cationic mechanism involves the L–X ligand substitution in complex 2 (Fig. 3) giving rise to the cationic Pd complex cis-[Pd(R1)(alkyne)(L)2]+ (Fig. 3; cationic pathway), which undergoes deprotonation of the alkyne by an external base and subsequent reductive elimination of Pd(II) to Pd(0). In contrast, in the anionic mechanism the deprotonation of the alkyne occurs first resulting in the anionic complex cis-[Pd(R1)-(acetylide)(X)(L)]− (Fig. 3; anionic pathway), in which the L–X ligand substitution takes place followed by the reductive elimination step to regenerate Pd(0). Besides, the stabilization of a negatively charged transition state for the coupling reactions of all aryl halides in Sonogashira reactions is well described by Hammett parameter σ−.47,48 Additionally, the electronic nature of the substituents directly linked to the terminal alkynes where the alkynes connected to electron withdrawing groups (EWG) favor the anionic mechanism and the alkynes bearing electron donating groups (EDG) favor the cationic pathway.46 In addition, mechanistic studies46 of the Cu-free version of the Sonogashira reaction have shown that the polarity and hydrogen bonding ability of the solvents are important in accelerating the reaction by stabilizing the ionic intermediates of the catalytic cycle, whereas steric bulk decreases the stabilizing ability of the solvent.49 The ligand size influences the catalytic activity of the Pd catalyst complexes. Increasing the reactivity of the catalytic system by using bulkier phosphine ligands has been shown to be a successful method of performing the Cu-free Sonogashira reaction.50
 | ||
| Fig. 3 Cationic and anionic pathways for the deprotonation mechanism.46,42 | ||
Recently, a study pointed out an alternative mechanism for the Sonogashira reaction named ‘ionic mechanism’. This new proposal helps to explain the role of additional ligands or bases in the reaction medium.42 In the ionic mechanism, halide ions are always replaced by the base in the first step and results in the formation cationic Pd complex, [Pd(R1) (base)(L)2]+. This mechanism simultaneously depends on the base and the acidity of the proton of the alkyne, which at the same time depends on the electron withdrawing ability of the group coordinated to the alkyne. Thus, the reaction through this mechanism is expected to be faster when alkynes bearing EWGs are used.
2.3. Gold-catalyzed reaction mechanism: a controversy
Recently, gold catalysts have excelled owing to their unique reactivity in Sonogashira reaction. Although a number of Au-catalyzed Sonogashira reactions have been reported, the mechanistic details are somewhat ambiguous. As Au(I) has the same d10 configuration as Pd(0) and Cu(I), it is thought to catalyze reactions typically promoted by Pd, that is, cross-coupling reactions. From this hypothesis, research has successfully advanced to enable cross-coupling reactions with Au catalysts.A combination of kinetic and theoretical studies51 concluded that Au is sufficiently active to promote the Sonogashira reaction. Initially, it is reasonable to consider that similar d10/d8 interplay might be operative for the two isoelectronic couples Pd(0)/Pd(II) and Au(I)/Au(III). Both d8 metal centers are not only isoelectronic, they also form square-planar complexes. For the d8 metal centers, Au(I) is usually coordinated in a linear manner. Although Pd(0) with common ligands is tetrahedral, it is known to dissociate in solution and it is believed that the reactive species are dicoordinated PdL2.52 However, there is an important difference in the lower oxidation state of the two systems; for PdL2 only the oxidative addition reaction is expected, but for AuXL both oxidative addition and transmetallation reactions are plausible.53,54 Therefore, two cycles were considered where these two steps are transposed (Fig. 4; cycles A and B).55 The feasibility of the two cycles and their steps as isolated processes was experimentally investigated.56 According to this experiment, Au was not capable to catalyze the Pd-free Sonogashira reaction as a sole catalyst. The investigation revealed that Au(I) is unable to activate the electrophile aryl halide by undergoing oxidative addition and the transmetallation reaction between an aryl–Au(III) complex and other alkynyl derivative was also overruled, thus both coupling cycle frustrated. Interestingly, the addition of a catalytic amount of Pd complex results in the coupling product which suggested that the Pd catalyst performs the oxidative addition, not the Au catalyst. In these conditions the reaction would be a common Sonogashira reaction catalyzed by Pd, with Au(I) playing the role classically played by Cu(I).57
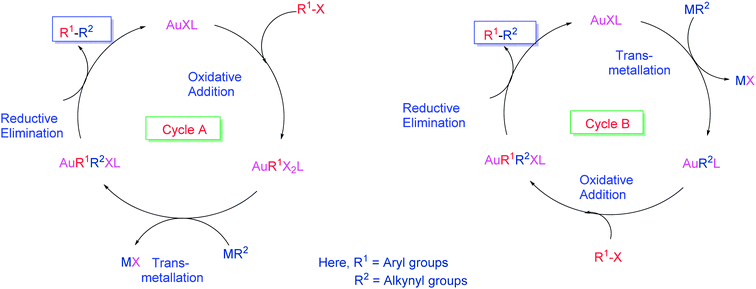 | ||
| Fig. 4 Hypothetical cycles for Au-catalyzed Sonogashira reaction.55,56 | ||
On the other hand, Corma and co-workers58,51 took on the challenge to promote Au catalysts as efficient alternatives to Pd catalysts. On the basis of their hypothesis, a kinetic study of the Sonogashira reaction between iodobenzene and phenylacetylene with a series of catalyst samples consisting of a constant amount of Au/CeO2 (99.999% Au, compared to the 99.9% Au used in the standard preparation of Au/CeO2) and increasing amounts of Pd was carried out and concluded that Au is intrinsically active for the Sonogashira reaction between iodobenzene and phenylacetylene, and that the presence of Pd is not strictly necessary for the reaction to occur.
Over the past number of years, there has been some discussion in the literature regarding these mechanisms. For the more traditional reaction conditions the mechanism is well established, however, the actual mechanism that operates under newly developed reaction conditions remains unclear. In view of this mechanistic complexity, further studies are needed to better understand the course of Sonogashira reactions with catalysts that are practically utilized.
3. Next generation catalysts: future of the Sonogashira reaction
The original Sonogashira reaction is usually performed using a Pd–phosphine ligand complex, e.g. Pd(PPh3)4, Pd(PPh3)2Cl2, Pd(dppe)Cl2, Pd(dppp)Cl2, Pd(dppf)Cl2 as the catalyst, in the presence of a catalytic amount of a Cu(I) salt as co-catalyst and an amine under homogeneous conditions. Pd(PPh3)4 and Pd(PPh3)2Cl2 are the palladium sources most frequently employed in a Cu co-catalyzed Sonogashira reaction due to their stability and solubility.59 In the case of deactivated bromoarenes or low reacting chloroarenes, more electron rich phosphine ligands have been employed to provide an easier oxidative insertion to aryl halides.60 In addition, sterically ligated Pd complexes are also crucial to oxidative addition which promotes an easier dissociation from the Pd(0)L2 resting state. In summary electron-richness favors the oxidative addition step, whereas steric bulkiness favors the formation of low coordinate and highly active Pd complexes.61 The required addition of Cu(I) halide in the standard Sonogashira reaction presents some drawbacks including environmental concerns, and the necessity to perform the coupling reaction in the absence of oxygen to minimize the occurrence of the Hay16 or Glaser15 Cu catalyzed homocoupling27 of the employed terminal alkynes. In some cases, Cu has been shown to inhibit the reaction.28,29 Reagent grade argon or nitrogen is typically used to provide the inert atmospheric conditions required to minimize this undesired reaction, although special equipment designed to avoid the presence of oxygen, such as two-chamber reaction systems, have been developed.62Currently the use of expensive Pd–phosphine complexes, high Pd loadings, and Pd contamination of the products has rendered Pd catalysts unpopular for some applications. This is a significant issue for large-scale industrial applications as27,63 the trace residues of transition metals are often difficult to remove from final products, which may be active pharmaceutical ingredients.64 Furthermore, the presence of the Cu salt can facilitate homocoupling of the terminal alkynes and the diyne by-product may be difficult to separate from the target product.65,66 Consequently, it is advantageous to develop transition-metal-free cross-coupling reaction conditions. Recently, UV/visible-light photocatalysis has entered the field of cross-coupling reactions. The photochemical protocols can easily overcome the problems associated with the conventional thermal processes, although the development of efficient methods to facilitate the recovery and reuse of metal complexes has remained an important goal. The immobilization of homogeneous/heterogeneous catalysts onto polymers,67 carbon nanofiber,68 clay,69 montmorillonite,70 silica and magnetic mesoporous silica,71 zeolite,72 metal oxides73 and silica–starch74 has been an expanding research area offering the advantages of easy product separation, catalyst recovery and recycling. From an economic and environmental standpoint, it is desirable to use the catalysts without employing hazardous additives as a greener alternative to conventional homogeneous or heterogeneous processes.
3.1. Non-transition metal based and/or metal free Sonogashira reaction
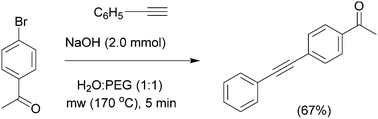
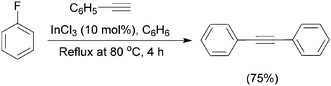
In 2003, the first example of a transition-metal free microwave assisted Sonogashira type reaction using water as the solvent, poly(ethylene glycol) (PEG) as the phase-transfer agent, and sodium hydroxide as the base was reported. The protocol worked well for a range of aryl iodides and with some aryl bromides (Scheme 2).75 Changing the phase-transfer agent from tetrabutylammonium bromide (TBAB) to PEG had a significant effect in increasing the reaction yields.In the same year, another study demonstrated the efficiency of microwave-assisted transition-metal free Sonogashira-type reactions76 using water as the sole solvent, without the need for Cu(I) or any transition-metal–phosphane complex. Surprisingly, TBAB acted as the phase transfer catalyst in this protocol while PEG was not effective. In both cases inorganic bases were shown to be more efficient than organic bases. The scope of this methodology was investigated with a wide variety of well tolerated functional groups. The aryl chlorides proved to be inert under these reaction conditions, and complete regioselectivity was reported for the reaction of 4-bromo-1-chlorobenzene. In 2005, another study reported a simple transition-metal free Sonogashira-type reaction using indium-(III) trichloride as the catalyst,77 (Scheme 3) which has been shown to promote the cross-coupling of activated and unactivated aryl iodides, bromides, chlorides, and even fluorobenzene to produce phenylacetylene, in dry benzene as the solvent.
Subsequently, another transition-metal free formal Sonogashira-type reaction has been developed through nucleophilic aromatic substitution of β-carbonyl sulfones to electron-deficient aryl fluorides by using K2CO3 as base and acetone as the solvent (Scheme 4).78 The scope of these reactions is demonstrated for the synthesis of Sonogashira products from a range of electron-deficient aryl fluorides with a variety of functional groups and aryl, heteroaryl, alkyl, and alkoxy substituted sulfone nucleophiles. The alkyne formation process in this formal Sonogashira reaction is affected by the steric bulk of the substituents on the nucleophile. Increased steric hindrance of the nucleophile would slow down the intermolecular SNAr step, but speed up the intramolecular Smiles rearrangement. When the adduct is treated with base, Sonogashira product arise through α-deprotonation of the adduct where the intermediate Z-enolate subsequently rearrange and eliminate SO2 and BTO – (BT = benzothiazol-2-yl) to yield alkynes (Scheme 5).
In 2010, another study reported a transition-metal free Sonogashira-type reaction between aryl and alkynyl Grignard reagents by using 2,2,6,6-tetramethylpiperidine-N-oxide radical (TEMPO) as a mild organic oxidant (Scheme 6).79 Although, the practical protocol tolerates versatile functional groups, installation of substituents at the ortho position of the aryl–Mg derivative to suppress the undesired oxidative homocoupling reaction of aryl Grignard reagents is a major limitation of this type of oxidative cross coupling.
More recently, another novel Sonogashira-type reaction of terminal alkynes with unreactive aryl fluorides in the presence of sodium, sodium methoxide, and calcium hydroxide in the presence of a Grignard reagent has been developed (Scheme 7).80 Although efficient in many cases, these protocols are limited by the narrow choice of unreactive arylfluorides and the requirement of a strong EWG in fluoroarenes for activation of the C–F bond. Mechanistic studies (Scheme 8) demonstrated electron transfer from an electron-donor species, sodium, to aryl fluoride to get free aryl radical and the fluoride anion. The fluoride anion reacts with the calcium ion to form insoluble calcium fluoride which is favorable for the cleavage of the C–F bond. The other crucial intermediate alkynylmagnesium chloride, is generated by the reaction of aryl acetylene with butylmagnesium chloride with the assistance of sodium methoxide. In the next step, the phenyl acetylide anion attacks aryl radical in an SRN1 manner, forming the required product and also resulting in the formation of undesired biphenyl derivatives through aryl radicals.
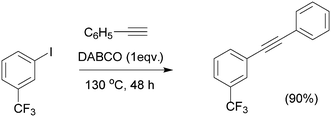
A metal-free Sonogashira coupling protocol between phenylacetylene and electron-deficient aryl halides catalyzed by 1,4-diazabicyclo[2.2.2]octane (DABCO) was reported.81 The reaction can be carried out under relatively mild conditions. The nucleophilicity and steric constraints of the base used are critical parameters to be considered (Scheme 9). In the proposed reaction mechanism (Scheme 10), DABCO acts as a nucleophile attacking the electrophilic carbon from the triple bond in the alkyne (Scheme 10, path a). The relatively stabilized intermediate attacks the aryl halide to yield the cross-coupled product. An alternative pathway (Scheme 10, path b) involving nucleophilic substitution of the aryl halide through the iodine, followed by attack of the newly generated nucleophilic carbon in the aromatic ring to another molecule of alkyne, to give the Sonogashira coupling product. The rate determining step is much faster in path a due to nucleophilic substitution in the aryl halide being favored as the iodine has a large electronic cloud that facilitates the nucleophilic attack leaving as hydrogen iodide. EWGs in the aromatic ring reduce electron density in the aromatic ring, thus destabilizing the positive charge generated in the C–I bond and driving the attack of the nucleophile.
In another study, two sets of conditions for base-mediated, transition-metal free alkynylation of aryl chlorides which proceed via a benzyne intermediate have been developed. In this case no mechanism has been suggested (Scheme 11).82 The first set of conditions involves the use of the hindered lithium 2,2,6,6-tetramethylpiperidide (TMPLi) base in a pentane–THF mixture at room temperature. The second set of conditions involves use of a metal alkoxide base in dioxane. The method also tolerates different EWGs and EDGs.
Although studies reported that only trace amounts of Pd as impurities in basic salts were able to catalyze C–C bond forming reactions, yielding the cross-coupled product in a transition-metal free reaction,83 control experiments have completely ruled out the possibility of Pd contamination being responsible for the observed high yields.76,84
3.2. Photo induced Sonogashira reaction
UV/Vis light-induced photocatalysis offers the possibility of initiating organic transformations with high selectivity under mild conditions. Recently, UV/Vis light-induced photocatalysis has been applied to Sonogashira reactions. A radically different approach for the synthesis of aryl acetylenes is based on the use of phenyl cations (Scheme 12). The triplet state intermediates are obtained by photolysis of aryl halides substituted with EDGs85,86 and addition to alkenes to form an aryl–C bond via a phenonium ion.85,87 The phenyl cations react with triple bonds to yield β-phenylvinyl cations, or more probably cyclic vinylenebenzenium ions with a nucleophilic solvent. Under these conditions, addition to form an alkoxystyryl derivative occurred (Scheme 12, path a),88 whereas the proton elimination competed to a variable extent and led either to an aryl alkyne (Scheme 12, path b) or an aryl allene (Scheme 12, path c). Thus, the choice of reaction conditions is critical so that the desired elimination occurs to form an aryl alkyne. | ||
| Scheme 12 Proposed intermediates in the photo generation and reaction of the vinylene benzenium ion. | ||
A metal-free protocol for the mild alkynylation of aromatic compounds substituted with EDG has been developed which could be achieved under longer UV irradiation times at room temperature (Scheme 13).89 In this case, UV irradiation of an aryl chloride leads to the formation of a phenyl cation, which further reacts with alkynes. As noted by the authors, this photo-initiated Sonogashira reaction has some limitations, which includes UV light absorption by solvents, aromatic alkyne reactants and products. Low reaction yields were also observed when the starting aromatic halides absorb poorly at 254/310 nm.
Further investigations in this area reported that activated aryl bromides efficiently couple with aryl or trimethylsilyl alkynes under visible light irradiation in the presence of a tris(bipyridine)ruthenium(II) complex (TB) at room temperature (Scheme 14).90 The photo-excited TB complex serves as an electron donor to reduce the Pd(II) precursor, without forming Pd(0)–phenylacetylide to promote the catalytic reaction. It should be noted that under the same conditions, the reaction of 4-chloroacetophenone and phenylacetylene afforded 4-acetylphenyl phenyl acetylene in 36% yield.
 | ||
| Scheme 14 Sonogashira reaction in the presence of tris(bipyridine)ruthenium(II) complex (TB), under irradiation of visible light. | ||
Another Sonogashira-type photocatalytic reaction involved the arylation of alkynes with diazonium salts by using 2,2′-bipyridine ruthenium complexes, [Ru(bpy)3]2+ has been reported (Scheme 15).91 As observed from Scheme 15, the yield is moderated and in the original paper only two examples are reported, since the method was primarily developed for coupling diazonium salts with phenyl acetylene and its derivatives. The scope and possible application of this method is still to be developed.
Although the Sonogashira reaction is mainly associated with the coupling between a terminal alkyne and an aryl or vinyl halide, a three-component carbonylative Sonogashira reaction has recently been developed. In this procedure, an alkyl iodide, carbon monoxide (CO), and a range of alkynes are reacted in the presence of a catalytic amount of Pd in the presence of light, resulting in the formation of alkynyl ketones (Scheme 16).92 A variety of functional groups are tolerated in this reaction; however in this proposed methodology the reaction of aryl bromides and chlorides were not discussed. Mechanistic studies demonstrated that alkyl iodide reacts with Pd(0) under irradiation to afford alkyl radical and unfamiliar Pd(I)I93–95 via a one electron transfer process (Scheme 17). The resulting alkyl radical traps CO to form an acyl radical which then affords an alkyl radical through 5-exo-cyclization of the acyl radical. The acyl radical species generated from CO and the alkyl radical couples with Pd(I) iodide to lead to acylpalladium intermediate and gives the alkynyl ketone.
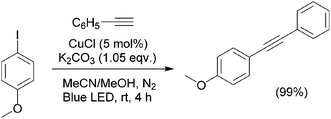
Recently, another study reported that simple Cu(I) chloride salt, in the absence of Pd and ligands, can catalyze the Sonogashira reaction under blue LED light irradiation at room temperature (Scheme 18).84 In the process it is thought that Cu-acetylide is the key light absorbing species, and is the driving force for photo-induced Sonogashira reactions. The deprotonation by base and subsequent coordination by CuCl, results in in situ formation of Cu-phenylacetylide (1) (Scheme 19). As Cu-phenylacetylide can exist in a polymeric form,96 the structure of (1) may be either a monomeric or a polymeric form or both. The direct photoexcitation of Cu-phenylacetylide (λabs = 476 nm) leads to the formation of electron-deficient acetylene moiety via ligand to metal charge transfer (LMCT), which generates a partially positive charge at the acetylene site, and thus favors nucleophilic attack of electron rich substituted phenyl halides to form transition state intermediate (3) (Scheme 19), which leads to the direct formation of the C–C coupling products. It was observed that electron-rich aryl halides reacted significantly faster than the electron-deficient examples and aryl chlorides were unreactive in this process.
In contrast, another study demonstrated a simple way to utilize sunlight as a renewable energy source for Sonogashira reactions, without the need for any special apparatus or reaction conditions. The process involves using Pd-catalysts under irradiation with sunlight in the absence of Cu salt (Scheme 20).97 Only three examples were reported, as the method was primarily developed for Mizoroki–Heck and Suzuki–Miyaura coupling reactions. Considering the high yields obtained for the three reported examples, this protocol should be more thoroughly investigated in order to uncover its full potential application and scope.
Although, UV/Vis light induced cross coupling works well for aryl bromides/iodides, in many cases it is ineffective for aryl chlorides. Despite some limitations, these protocols have distinct advantages over the thermal alternatives, since the reactions are carried out at room temperature. Mild conditions such as low catalyst loading and high reaction yields at room temperature make these current approaches a very promising, industrially scalable green process as an alternative to the conventional high-temperature Sonogashira reactions, for the synthesis of natural products and medicinal compounds.
3.3. Nanoparticles as catalysts in Sonogashira reaction
Although, homogeneous transition metal catalysts remain the most active catalysts for Sonogashira reactions, efficient separation and recycling are still scientific challenges, because catalyst deactivation often occurs during the workup.98 Additionally, homogeneous catalysts have a greater tendency to lead to metal contamination in the target products. To overcome these problems, metal nanoparticles (MNPs) have attracted much attention in the last several years. MNPs exhibit distinct physicochemical properties compared to their bulk counterparts due to their large surface-to-volume ratio, thus providing many highly active metal uncoordinated sites.99 Furthermore, immobilizing MNPs onto solid supports can minimize atom/ion leaching from the particles.100 Research into the preparation and use of supported MNPs as a catalyst in processes such as the Sonogashira reaction has been reported in recent years, which are particularly characterized by their catalytic activity, stability, recovery and reusability.Activity: Generally, the performance of MNP catalysts for many different reactions is strongly dependent on the shape, size, dispersion and support and, consequently, on the synthesis methods, reducing agents, and stabilizers employed to make them. Transition MNPs are especially active catalytic systems due to their large surface area-to-volume ratios.101,102 It was observed that poly(vinylpyrrolidone) (PVP)-stabilized spherical-shaped palladium nanoparticles (Pd–NPs) with 2.1 nm in size were more active catalysts than PVP-stabilized tetrahedral-shaped platinum nanoparticles (Pt–NPs) with 5.0 nm in size.103 As the nanoparticle size is associated with reactivity, small nanoparticles would allow more favorable oxidative addition of the metal to the carbon–halogen bond at the rim of the nanoparticle.104
Stability: The most important aspect to discuss about the stability of metal NPs in the C–C cross-coupling reaction is associated with the ‘Ostwald ripening’ process,98 which is the main reason behind cluster growth. This mechanism involves the detachment of atoms from smaller clusters and the attachment of these atoms to the larger clusters that have lower surface energy. The high temperature reaction conditions can promote the increase of the nanoparticle size by Ostwald ripening process and lower the catalytic activity of the MNPs. Although the increase in size of the MNPs can be avoided by adding excess of stabilizers, such as trialkylammonium salts, ionic liquids, or poly(ethylene glycol) (PEG), which can act as ligands surrounding the dispersed nanoparticles and therefore minimizing their tendency to undergo agglomeration103–105 but the addition of excess stabilizers can make the catalyst less active. Furthermore, when excess of the reaction product is added into the reaction medium, the catalytic activity also decreases.
Recovery and Reusability: The recovery and reusability of the MNP catalysts are important issues in C–C cross-coupling reactions. The immobilization of colloidal MNPs on a solid support is a useful alternative as the catalyst can be recycled by simple filtration.106 Recent findings revealed some nano-level and metalodendritic Pd-catalysts can be recovered by precipitation, ultrafiltration, or ultracentrifugation.107,108 As the reaction conditions for C–C cross-coupling usually involve high temperature for a long period of time, the growth of the MNPs by Ostwald ripening can be expected to continue during and after recycling of the catalysts. As a result, the catalytic activity of MNPs gradually decreases after each recycle.
A particular consideration concerning the mechanism of Pd–NPs catalyzed C–C coupling reaction when Pd–NPs are involved is whether the coupling is catalyzed by the nanoparticles themselves or by the molecular species. Thus, when Pd–NPs are used as catalysts, the leaching and the formation of catalytically active molecular species must be considered. According to this hypothesis, three possible mechanism have been proposed (Fig. 6): (a) Pd–NPs can act as a genuine heterogeneous catalyst, with the reaction occurring on the surface; (b) Pd–NPs can act as a reservoir of molecular species, and Pd(0) atoms can leach from the nanoparticles and enter into a homogeneous catalytic cycle; (c) the oxidative addition can occur at the nanoparticle surface followed by leaching of the formed [Pd(Ar)X] complexes, which then initiates another homogeneous catalytic cycle.
 | ||
| Fig. 6 The three possible mechanisms for the Pd–NPs catalyzed C–C coupling reaction.100 | ||
3.3.1.1. Palladium nanoparticles with copper co-catalysts. Although one of the main interests in using Pd–NPs in the Sonogashira process lies in their high reactivity, and therefore the suitability of performing Cu and ligand-free couplings, there are examples where Cu(I) and a phosphine ligand have been added to a homogeneous reaction catalyzed by Pd–NPs. The Sonogashira reaction was efficiently catalyzed with receptor-modified β-cyclodextrin-capped Pd–NPs (β-CD/Pd)110 and co-catalyzed with CuI in aqueous solution at room temperature without phosphine ligand through host–guest recognized catalytic process (Scheme 21). The Sonogashira reaction of aryl bromides was relatively slow with lower yields than those reported for the corresponding aryl iodides under the same conditions; however, eliminating the organic solvent in the Sonogashira reaction represents a significant advancement in the development of a greener process.
Recently it has been demonstrated that nano-sized MCM-41-Pd catalyst111 effectively catalyze the Sonogashira reaction of various aryl and heteroaryl halides with phenyl acetylene (Scheme 22) and alkynols. Reactions of aryl iodides with several EWGs and EDGs proceeded well with the use of 0.01–0.1 mol% of NS-MCM-41-Pd, and the Sonogashira reaction of aryl bromides under the same conditions did not afford any product. Replacing the TEA solvent by NMP resulted in product formation with a yield of 30% at an elevated temperature. A yield of up to 56% could be achieved by performing the reaction in toluene at 100 °C for 24 h. Aryl chlorides are completely inert under the aforementioned conditions. However, the major advantage of this catalyst is that the short and highly-connective wormhole-like channels of nanosized MCM-41 lead to facile exchange of reactants, salts and products throughout the nanochannels, avoiding saturation of activity. In addition, the nanosized low Pd loadings (0.01 mol%) MCM-41-Pd catalyst was recovered by centrifugation of the reaction solution and re-used in further runs without significant loss of reactivity.
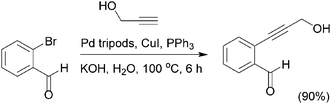
Further studies have shown that palladium–polypyrrole (Pd–PPy) nanoparticles112 in combination with a Cu(I) salt, can be efficiently used as a catalyst for the Sonogashira reaction of iodobenzene with phenylacetylene, in the presence of sodium carbonate with NMP as the solvent. In this procedure the addition of DMF or DMSO facilitates the use of lipophilic organic substrates and inorganic salts used as bases. This typical Pd–NC is an active catalyst for the Sonogashira coupling of iodo- and bromoarenes but not for the chloroarenes. Another study demonstrated that recyclable and highly active Pd tripods nanoparticles113 catalyzed the Sonogashira reaction in water as a sole solvent (Scheme 23). Their use as recyclable catalysts was investigated in the reaction between iodobenzene and phenyl acetylene to form diphenyl acetylene. The product yield was 93% for the first run after 6 h of reaction at 100 °C, and 91% and 90% for the second and third cycles, respectively.
The reaction carried out with a variety of aryl iodides and bromides containing EDGs and EWGs in the presence of CuI as a co-catalyst, triphenyl phosphine as the ligand and potassium hydroxide as the base. In general, the Pd tripods were successfully catalyzed the Sonogashira reaction with excellent product yields ranging for 85–93%. The versatility of the Pd tripod nanocrystals for a broad scope of Sonogashira reactions and their easy separation from the products implies that this protocol have great potential to be explored in synthetic work and they may be employed for producing many important bioactive molecules.
3.3.1.2. Palladium nanoparticles in copper-free reaction conditions. Cu-free and ligand-free Sonogashira reactions have been carried out using different procedures in order to stabilize the Pd–NPs. In 2002, the first example of Cu and ligand-free heterogeneous immobilized layered double hydroxide and Merrifield resin supported nanopalladium [LDH–Pd(0)]106 catalysts exhibited higher activity and selectivity in the Sonogashira reaction of electron-poor and electron-rich chloroarenes over the homogeneous PdCl2 system. The catalyst is quantitatively recovered from the reaction by a simple filtration and reused for a number of cycles with almost consistent activity in the coupling reactions (Scheme 24).
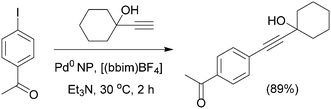
Stable and crystalline clusters of Pd(0)–NPs114 can also catalyze the Sonogashira reaction at room temperature in the presence of the ionic liquid 1,3-dibutylimidazolium tetrafluoroborate [(bbim)BF4] (Scheme 25). The reaction is carried out under ultrasound irradiation with enhanced reaction rates and excellent chemoselectivity. In this procedure neither Cu co-catalyst nor phosphine ligands are required. It was observed that the process tolerates both EDGs and EWGs in the aryl halides as well as terminal acetylenes. However, in the case of less reactive bromobenzenes, the homocoupled product was observed to an extent of 6–7% and aryl chlorides are inert in this catalytic system.
Notably, in the absence of ligand, Cu and amine, a recyclable poly(vinylpyrrolidone) (PVP) supported nanosized Pd(0) metal colloids catalyzed Sonogashira reaction of aryl iodides and bromides with terminal alkynes was developed without significant loss of the catalytic activity for eight consecutive cycles (Scheme 26) although, decreases in reaction yields were observed after eight cycles. The electron-neutral, electron-rich, and electron-poor aryl iodides reacted with terminal alkynes smoothly. Regardless of their electronic character, both of the aromatic terminal alkynes and aliphatic terminal alkynes components coupled with aryl iodides. For electron-rich aryl bromides, relatively low yield was obtained under the reaction conditions.115
Recently, PVC (polyvinyl chloride) supported Pd–NPs116 were also explored for the Sonogashira reaction in water as a solvent. In this case the catalyst only works for the aryl iodides substituted with several EWGs and EDGs. The catalyst can be used in five successive runs with no loss in its reactivity in Sonogashira reactions which confirm the stability of Pd–PVC composite. The slight decrease observed in the yield during the recycling experiment can be accounted to agglomeration of metal nanoparticles within the PVC matrix during the course of the reaction. In the same year, another recent study revealed, PVC supported Pd–NPs [PVC–dtz–Pd(II)]117 (Scheme 27) are highly functionalized for Sonogashira reaction of versatile aryl halides including aryl chlorides with different EDGs and EWGs in aerobic conditions. However, in the case of aryl chlorides the product yield is low to moderate, 43–64%. The solid catalyst was recovered by separation of the organic compounds from the reaction mixture by extraction, and the recovered catalyst was repeated for five cycles; some decrease in the catalytic activity was observed with metal leaching.
High leaching-resistant Pd–NPs on carbonized electrospun polyacrylonitrile (PAN) nanofibers (Pd–NPs/CENFs)118 showed good catalytic activity in the Sonogashira reaction of iodobenzene and phenylacetylene in liquid-phase. The catalyst was retrieved after each run by filtration, washing and drying under vacuum and repeatedly used for ten times without significant loss of reactivity. It was also reported that Pd nanoclusters,119 synthesized by a simple method in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixed solvent system of MeCN–MeOH without any stabilizer were found to catalyze the Cu, amine and ligand free Sonogashira reactions in open air; however this catalytic system only works for aryl iodides (Scheme 28). In this case, the material existed as leached Pd nanoclusters during the reactions but it was unclear if Pd was redeposited at the end of the reaction.
1 mixed solvent system of MeCN–MeOH without any stabilizer were found to catalyze the Cu, amine and ligand free Sonogashira reactions in open air; however this catalytic system only works for aryl iodides (Scheme 28). In this case, the material existed as leached Pd nanoclusters during the reactions but it was unclear if Pd was redeposited at the end of the reaction.
Another study illustrated that PEG stabilized Pd–NPs,120 synthesized from the reaction of a Fischer carbene complex of tungsten with K2PdCl4 at room temperature, displayed high catalytic activities in the Sonogashira reaction. Under the conditions described, water is the sole solvent and no Cu(I) salt, phosphine ligand or amine are required. Although the reaction proceeded well with aryl iodides, no coupling product was observed with activated bromides even at elevated temperatures under a wide range of conditions. It was also reported the application of Pd–NPs supported on MOF-5121 in the Cu and amine-free Sonogashira reaction in the presence inorganic base in methanol. Recently, the immobilized, homogeneous and super paramagnetic Pd–NPs122 (Scheme 29) was used as an efficient catalyst for the Cu-free Sonogashira reaction without added phosphine ligands. The catalyst was recovered by simple magnetic decantation and reused several times without significant degradation in catalytic activity. The presence of EWGs on the benzene ring significantly accelerated the Sonogashira reaction. Although, this catalytic system successful for activated aryl bromides, chlorobenzene was inactive in the reaction with terminal alkynes.
It has been further demonstrated that organosilica xerogels doped with nanostructured Pd(0) can be used as stable heterogeneous and reusable catalysts,123 suitable for performing Sonogashira reactions in high selectivity without the need to exclude air or moisture; however the catalyst was successful only for aryl iodide. Reusing the catalyst in seven consecutive cycles did not result in any loss in catalytic activity with negligible (less than 0.2 ppm) leaching of Pd and Si. Complete substrate conversion was obtained even after the seventh cycle, with 99.5% selectivity. Notably, another study developed an air and moisture-stable and easily recoverable silica-coated magnetic nanoparticle-supported Pd catalyst (SiO2@Fe3O4–Pd)124 (Scheme 30) for the Sonogashira reaction in Cu-free condition (Scheme 31) but the catalytic system was no longer effective for the couplings of aryl chlorides. More recently, superparamagnetic nanoparticles functionalized with Schiff base immobilized Pd(II) complex [Fe3O4@SiO2/Schiff base–Pd(II)]125 were used as the catalyst for Cu and phosphine ligand-free Sonogashira reaction. Notably, this new catalyst also works for the coupling of aryl chlorides with excellent yield (79–92%). In another study, [Fe3O4@SiO2–polymer-imid–Pd] nanocatalyst126 has been efficiently applied for the Sonogashira reactions. The coupling reaction of phenylacetylene with both electron-releasing and electron-withdrawing aryl iodides and bromides afforded the coupling products in good yields, although aryl chlorides are less reactive. In contrast, a recent research developed a super paramagnetic nanocatalyst for Sonogashira reaction where palladium supported on nano-magnetite (Pd@Fe3O4) without SiO2 support.127 The catalyst was separated by a simple external magnetic field and the recycled catalyst was reused six times with no loss of activity. The electron-neutral, electron-rich and electron-poor aryl halides reacted with phenylacetylene smoothly to generate the corresponding cross-coupling products, although, aryl chlorides were not successful in this protocol.
Recently, agarose-supported reusable Pd–NPs128 have been developed for the Sonogashira reaction. In this protocol, PEG-400 is used as a green solvent under Cu and phosphine-free conditions, which caused the regular dispersion of the Pd–NPs on the surface of the agarose (Scheme 32). Herein, a range of aryl iodides, bromides and chlorides, bearing EWGs and EDGs, reacted with phenyl acetylene successfully. The recovered catalyst was recycled for five successive runs with a rather sharp decrease in the catalytic activity from the fourth run. The leaching of the catalyst into the reaction mixture was also determined by ICP analysis for the first run of recycling to be less than 2% after cooling the reaction mixture to room temperature.
The Pd nanoparticle catalysts supported on partially reduced graphene nanosheets (Pd/PRGO),129 synthesized by pulsed laser irradiation of aqueous solutions of graphene oxide and palladium ions, was successfully utilized in the Sonogashira reaction under ligand free microwave irradiation conditions. With more challenging substrate such as electron poor aryl chlorides, conversions of 10–15% were reported. Another recoverable and reusable high TON catalytic system of bisphosphinite PCP pincer Pd complex (Scheme 33) based on Merrifield resin130 was developed. This method exhibited good catalytic activity in Sonogashira reactions with different EWGs and EDGs connected aryl halides including inactive aryl chlorides under Cu and amine-free condition (Scheme 34). The recovery and reusability of the supported catalyst was investigated using iodobenzene and butyl acrylate as model substrates. The recovered catalyst was reused in ten subsequent reaction cycles and the catalyst retained its catalytic activity. A recent report demonstrated that the amido pincer complex is a competent catalyst precursor for the Sonogashira reactions of aryl halides with terminal alkynes under mild conditions.131 This catalysis is compatible with a variety of functional groups including alkyl, alkenyl, (hetero)aryl, silyl, fluoro, alkoxy, ketone, nitro, etc.
 | ||
| Scheme 33 Synthetic strategy for the preparation of modified Merrifield resin-supported PCP pincer palladium nanoparticles. | ||
Another heterogeneous, recyclable, high TON and TOF catalytic system based on Pd–NPs supported on polymeric N-heterocyclic carbene grafted silica (Si–PNHC–Pd) has also been developed.132 This catalyst was reused in twelve subsequent reactions and the catalyst retained its catalytic activity. Under such conditions excellent results in the Sonogashira reaction with various aryl halides including some aryl chlorides in the presence of a catalytic amount of TBAB were achieved. A heterogeneous stabilized retrievable Pd catalyst system has been developed based on immobilization of Pd–NPs on silica-bonded N-propylpiperazine sodium N-propionate (PNP–SBPPSP) substrate133 (Scheme 35). The catalyst can be recovered by simple filtration. The recycled catalyst was reused four times without loss of activity. This system efficiently catalyzes the Sonogashira reactions in Cu-free conditions including a range of aryl iodides, bromides and chlorides, bearing EWGs and EDGs (Scheme 36).
Recently a new method involving in situ generation of recyclable Pd–NPs into the nanopores of modified montmorillonite134 was developed. The catalytic activity of such nanoparticles in the Sonogashira reaction was high and 100% selectivity was observed when MeCN was the solvent in the presence of triethylamine and under Cu-free conditions. In another study, the use heterogeneous Pd/graphene (Pd/G) nanocomposites135 was reported. In this method, the catalyst showed good efficiency in Sonogashira reactions and the ability to recover and recycle the catalyst was an advantage. The activity of the catalyst was attributed to surface defects on graphene that act as nucleation and growth sites for Pd–NPs and facilitate higher dispersions of Pd–NPs. Notably, in another study, a novel Pd-containing 2D-hexagonal periodic mesoporous organosilica material (Pd–LHMS-3)136 showed good catalytic activity in Sonogashira reaction in the presence of water as the sole solvent and hexamine as base without Cu co-catalyst (Scheme 37). The main advantages of this catalyst is the presence of many donor sites, which serve as better chelators for the Pd-sites and thereby minimizes its possible leaching into solution. Upon completion of the reaction, the catalyst can be recovered by simple filtration and reused up to four times without any significant loss of reactivity, although conversion levels decreased significantly after the fourth catalytic cycle. EWGs and EDGs containing aryl iodides and sterically hindered ortho-substituted aryl iodides and heteroaryl iodides are well tolerated; although the Sonogashira reaction of aryl bromides did not occur in water and in the presence of hexamine, aryl bromide react with phenyl acetylene in DMF under nitrogen atmosphere.
Interestingly, the air–moisture stable and recyclable Pd(II)–Schiff base complex anchored to multi-walled carbon nanotubes (Pd–Schiff base@MWCNTs)137 behaves as a very efficient heterogeneous catalyst in the Sonogashira reaction in aqueous media. The coupling reaction of bromobenzene with phenylacetylene were very slow under the same reaction conditions giving trace amount of cross-coupled product after 24 h of reaction time and the coupling reaction of chlorobenzene with phenylacetylene did not occur at all. A new class of Pd–NPs,138 stabilized by Pd–C(binaphthyl) (Scheme 38) covalent bonds which helped the catalyst to retain its activity and particle size and restricts agglomeration, was efficiently applied as reusable catalysts for Sonogashira reactions with high turnover. After completion of the reaction, the catalyst was successfully recovered and reused for the next 2–3 cycles. EWGs and EDGs containing aryl iodides are well tolerated and provided excellent yields; although the Sonogashira reaction of aryl bromides and chlorides did not occur.
The application of Pd–NPs (2–6 nm) supported on pectin139 exhibited high catalytic efficiency and air stability in the Sonogashira reaction without Cu, amine and phosphine was also reported (Scheme 39). Although in the case of aryl iodides it was found that the activity of the catalyst was completely retained after two recycled runs, a gradual decrease in catalytic activity was observed in some cases. In the case of less reactive electron-rich aryl bromides such as 4-bromotoluene and 4-bromoanisole, the coupling reaction carried out over a longer time period. Very strong EWG (such as NO2) on the aromatic ring led to a high yield after shorter reaction time. Heterocyclic aryl bromides are also suitable substrates for this reaction. The coupling of aryl chlorides could be performing at 100 °C with good yields.
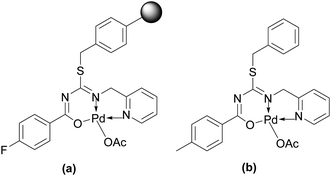
Very recently, a new polystyrene supported thiopseudourea–Pd(II) complex (Fig. 7) was found to be an active catalyst for the Cu-free Sonogashira reaction of different aryl halides.140 The catalyst was easily recoverable from the reaction mixture by simple filtration and reusable up to ten times without significant loss of activity. However, the catalyst only works for EDGs and EWGs containing aryl iodides.
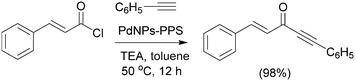
The Sonogashira coupling reaction between a range of aryl halides and phenylacetylene were carried out in the presence of a new Pd supported on ZnO nanoparticles (nano Pd/ZnO).141 It has been found that aryl iodides with a variety of EWGs and EDGs reacts well affording coupling products. On the other hand the reactivity of aryl bromides and chlorides with EWGs was higher than those bearing EDGs. Catalytic recycling experiments were carried out using a Sonogashira reaction of 1-(4-bromophenyl)ethanone with phenyl acetylene as a model reaction. The catalyst could be recovered from the reaction mixture by diluting with EtOAc and centrifugation to separate the catalyst. Only 0.01% of the Pd metal after is leached out from the catalyst surface after five cycles. Recently, a tris-imidazolium iodide stabilized Pd–NPs catalyzed the Sonogashira reaction with activated and deactivated iodoarenes has been described.142 The reaction is also successful for activated aryl bromides, whereas nonactivated aryl bromides don't react. The catalyst proved to be tolerant to a range of functional groups, with the expected products being obtained in moderate-to-good yields. A convenient method for the synthesis of PdNPs–PPS [PPS = poly(1,4-phenylene sulfide)]143 embedded into a polymer matrix via the thermolysis of palladium acetate is described which can be utilized as an efficient heterogeneous nanocatalyst for the acyl Sonogashira reaction under Cu-free reaction conditions (Scheme 40). The catalyst was recycled up to four times consecutively without any substantial change. The acyl Sonogashira reaction was examined through a variety of aryl/aliphatic acid chlorides with different aromatic/aliphatic terminal alkynes and was obtained in good yield.
Significantly, another novel Pd–NPs supported on two Smopex® metal scavengers (Pd/Smopex®-234 & Pd/Smopex®-111) can be successfully used for the three component carbonylative Sonogashira reaction affording the corresponding α,β-alkynyl ketones with high chemoselectivity.144 It has been reported that Pd/Smopex®-234 is a good catalyst for the reactions of phenylacetylene with aryl iodides bearing both EDGs (Me, OMe, Ph) and EWGs (Cl) in ortho or para positions. All reactions yielded the carbonylated coupled products with high chemoselectivity (82–93%). However, no conversion towards the alkynyl ketones was observed in the case of aryl bromides. The results obtained in the hot filtration test, the very low value (0.8%) of the Pd leached into solution during the reaction and the possibility of recycling the catalyst with no loss of activity seem to indicate heterogeneous behavior of Pd/Smopex®-234. Very recently, a new class of Pd–NPs grafted at the surface of Co-containing metal–organic framework material MCoS-1 (Pd(0)/MCoS-1)145 has been developed which revealed good catalytic activity for Sonogashira reactions in water without Cu co-catalyst (Scheme 41). The catalyst is easily recoverable by simple filtration and can be reused for six times without appreciable loss of catalytic activity. Importantly, results of the hot filtration test suggested that Pd did not leach out from the solid catalyst during the reaction. The aryl halides with EWGs gave excellent yields, while aryl halides with EDGs gave the coupling products in slightly lower yield. The ortho-substituted aryl iodides gave lower yield than the para-substituted ones, presumably due to steric effects. The coupling reaction of aryl bromides required longer reaction times and chlorobenzene gave no desired product.
3.3.1.3. Palladium containing bi-/trimetallic nanoparticles. The search for more efficient catalytic systems that combine the advantages of both homogeneous and heterogeneous catalysts is one of the most exciting challenges in catalysis research. In this regard, the Ni/Pd dual core nanoparticle146 catalyst, synthesized from the consecutive thermal decomposition of metal–surfactant complexes was applied to various Sonogashira reactions including sila-Sonogashira protocol (Scheme 42) and showed significantly enhanced catalytic activity when compared to normal Pd–NPs. Although, this semi-heterogeneous nanoparticle catalyst was recycled and reused five times without loss of catalytic activity for active bromoaryl substrates, it was inactive for chloroaryl substrates. In another aspect, a Pd/Cu co-catalyzed sila-Sonogashira reaction of (hetero)arylethynylsilanes with a variety of activated and deactivated (hetero)aryl bromides in the presence of a phase-transfer catalyst (benzyl(tri-n-butyl)ammonium chloride) has been developed.147
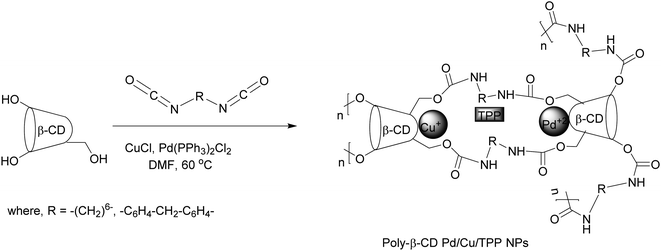
Pd containing Au–Ag–Pd triple core trimetallic nanoparticles148 were synthesized in the presence of cetyltrimethylammonium bromide as the capping agent. The material exhibited better catalyst activity in Sonogashira reactions compared to Pd–NPs under aerobic conditions. This activity is possibly due to the concerted electronic effects of the Au–Ag core onto the Pd shell atoms. The catalyst can be recovered by precipitation and ultracentrifugation and the recovered catalyst reused for the next reaction cycle. Notably, another study reported a sustainable protocol to synthesize the poly-βCD Pd/Cu/TPP nanoparticles149 (Scheme 43) which has moderate catalytic activity in Sonogashira reactions with very low metal leaching. The use of water or glycerol as a solvent rendered product purification easier and also allowed the catalyst to be recycled via simple filtration and washing. Another Cu-supported bimetallic nanopalladium catalyst (nano-Pd/PdO/Cu) was shown to be a selective catalyst for the Sonogashira reaction of different aryl halides.150 However, electron-deficient aryl halides favor this reaction, while electron-rich aryl halides are less prone to the process. Up to two cycles of full catalyst performance (100% conversion) can be attained, and a very high deactivation in a third cycle (5% conversion) was reported. Very recently, another Pd/Cu bimetallic nanoparticle embedded in macroporous ion-exchange resins has been developed and successfully applied for the Sonogashira reaction although it exhibited poor catalytic activity with deactivated aryl bromides.151 The recovered catalyst by simple filtration was reused up to four times without losing its activity.
In a recent study, an efficient heterogeneous catalyst based on Pd–Co alloy nanoparticles152 supported on polypropylenimine dendrimers was developed. The nanoparticles are grown on graphene nanosheets, and can be efficiently used for Sonogashira reactions under Cu and solvent-free conditions using ultrasound irradiation at room temperature. The catalyst can be easily recovered by simple filtration and reused six times without significant loss of activity (Scheme 44). From the reported results, the Sonogashira reaction of terminal alkyne with aryl halides including aryl chlorides possessing both EDGs and EWGs proceeded in high yields. It has been very recently shown that graphene (G) supported Pd–Co bimetallic NPs (Pd–Co/G) can successfully catalyze the Sonogashira reaction.153 Although, Pd–Co/G exhibited high selectivity, the catalyst is limited to aryl iodide and bromide. The catalyst can be easily recovered by a magnet due to their magnetic nature and reused five times without loss of significant activity.
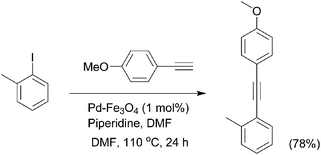
A magnetically separable and recyclable bimetallic heterogeneous Pd/Fe3O4 catalyst154 showed highly efficient catalytic activity for the carbonylative Sonogashira reaction under phosphine-free conditions. During the reaction between aryl iodides and terminal alkynes, the Pd is leached and served as the active catalyst, and at the end of the reaction, Pd is redeposited onto the support. Recently, a convenient bimetallic Pd/Fe3O4 heterodimeric nanocrystal155 catalyst system for Sonogashira reaction was developed (Scheme 45). In this process the catalyst could be easily separated by an external magnet and recycled six times without losing its catalytic activity. The results showed good yields of the Sonogashira products from the reactions with aryl iodides having EDGs and EWGs, regardless of the position of the substituent. However, reactions with aryl bromides gave lower yields leaving some unreacted starting materials.
A new approach to bimetallic Pd–Au/C nanoparticles (Scheme 46) for the Sonogashira reaction has been developed.156 In this procedure Au atoms stabilize catalytically active Pd–NPs when engaged in an alloy heterogenized on carbon. The increased durability makes the Pd–Au/C catalyst more recyclable than the Au-free Pd/C catalyst. However, the substrate scope is limited to aryl iodides with different functional groups.
The reports in the literature applying Pd–NPs as catalysts can be regarded as an important step towards a simple system with the potential for commercial exploitation of the nanocatalysts. It has already been demonstrated that leached Pd(0) species from the catalyst surface are the true catalytic species in various cross coupling reactions catalyzed by Pd–NPs.157,158 Due to the operational simplicity and robustness of these nanocatalysts we envisage that they can be further developed to be applied in other Pd promoted transformations in the future.
The Sonogashira reaction has also been studied in the presence of metallic Rh nanoparticle160 catalysts through surface-mediated heterogeneous catalysis. In this study the larger (8 nm) nanoparticles were found to be much better catalysts than small ones (2 nm), which is consistent with the hypothesis that steric limitations adversely affect the efficiency of the latter. However, the substrate scope is limited to aryl iodides. The paramagnetic magnetite (Fe3O4) nanoparticles (<30 nm)161 have been shown to be efficient as catalysts for the Sonogashira reaction under heterogeneous ligand-free conditions in ethylene glycol (Scheme 48). In this case, the catalyst can be easily separated by an external magnetic field and recycled for several consecutive runs without appreciable loss of its activity. But, the reaction of aryl bromides with terminal alkynes was disappointing and unreacted substrates were observed. However, it was also observed that activated aryl bromides such as 5-bromopyrimidine and 3-bromopyridine reacted smoothly in the presence of this catalyst and the desired alkynes were obtained in 76% and 80%, respectively.
It was reported that Ag nanocomposites162 can act as a catalyst in the Sonogashira reaction (Scheme 49). The activity of such material can be superior when compared to similar sized Pd nanocomposites, due to the unpaired electron of Ag in 5s orbital level which can easily be lost to form Ag+, which is beneficial to the key oxidative addition step in the Sonogashira reaction. This method exhibited good catalytic activity in Sonogashira reactions with different EWGs and EDGs connected to aryl halides, however in case of aryl chlorides only trace amount of coupling product was detected. Also, the Ag–NCs can be recycled and reused three times without loss of catalytic activity.
Currently, gold nanoparticles (Au–NPs) (Fig. 8) have received significant attention in the Sonogashira reactions due to their immense activity and selectivity. In 2007, a solid and stable Au supported on nanocrystalline CeO2 (Au/CeO2)58 heterogeneous catalyst which is active for performing the Cu-free Sonogashira reaction has been developed. Although the catalyst has some significant advantages, including no Au leaching and good recyclability, it produces the homocoupled products.
Notably, in the same issue, another study reported that Au nanoparticles supported on CeO2 or La2O3163 catalysts exhibit strongly enhanced selectivity toward Sonogashira reactions. Very recently, it was reported that thiolate-protected Au25(SCH2–CH2Ph)18 nanoclusters164 supported on oxides (such as CeO2, TiO2, MgO, and SiO2) able to catalyze Sonogashira reaction with high selectivity. DFT calculations of the co-adsorption of phenylacetylene and iodobenzene on the surface of the nanocluster found that the open facets of the nanocluster can strongly attract both reactants into a configuration ready for coupling. Research into the preparation and use of supported Au–NPs as a catalyst in processes such as the Sonogashira reaction has been reported in recent years, although in most cases only aryl iodides have been reported as coupling partners.
4. Outlook and summary
Among the newly developed catalytic systems, Pd–NPs possess a noteworthy position in the Sonogashira coupling reaction, although their catalytic activity and recyclability results are sometimes limited. In some cases, Cu, Fe, Ni, Ag or Au nanoparticles have been reported to act as reasonable catalysts in this protocol. Non-transition metal catalyzed and photo-induced Sonogashira reactions have opened a new window in the cross-coupling methodology. Although aryl iodides and bromides remain the most commonly used substrates, newer approaches, including supported nanoparticles and transition metal free systems can activate the more challenging aryl chlorides and aryl fluoride substrates.Mechanistic studies have also provided useful information for the development and detailed understanding of conventional Sonogashira reactions. We envisage that in the near future new, more efficient and green protocols for Sonogashira reactions will become available, enabling the production of a large array of compounds from the most diverse starting materials.
Acknowledgements
We are greateful to Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) for research fellowships (LCAB, MK), and Fundação de Amparo à Pesquisa de Minas Gerais (FAPEMIG), for financial support. GH thanks DIT for research support.References
- K. Sonogashira, Y. Tohda and N. Hagihara, Tetrahedron Lett., 1975, 16, 4467–4470 CrossRef.
- M. Toyota, C. Komori and M. Ihara, J. Org. Chem., 2000, 65, 7110–7113 CrossRef CAS PubMed.
- I. I. Paterson, R. D. Davies and R. Marquez, Angew. Chem., Int. Ed., 2001, 40, 603–607 CrossRef CAS.
- N. D. Cosford, L. Tehrani, J. Roppe, E. Schweiger, N. D. Smith, J. Anderson, L. Bristow, J. Brodkin, X. Jiang, I. McDonald, S. Rao, M. Washburn and M. A. Varney, J. Med. Chem., 2003, 46, 204–206 CrossRef CAS PubMed.
- M. C. Bagley, J. W. Dale, E. A. Merritt and X. Xiong, Chem. Rev., 2005, 105, 685–714 CrossRef CAS PubMed.
- L. Brunsveld, E. W. Meijer, R. B. Prince and J. S. Moore, J. Am. Chem. Soc., 2001, 123, 7978–7984 CrossRef CAS PubMed.
- M. Sonoda, A. Inaba, K. Itahashi and Y. Tobe, Org. Lett., 2001, 3, 2419–2421 CrossRef CAS PubMed.
- O. Mongin, L. Porres, L. Moreaux, J. Mertz and M. Blanchard-Desce, Org. Lett., 2002, 4, 719–722 CrossRef CAS PubMed.
- B. Li, Y. Fu, Y. Han and Z. Bo, Macromol. Rapid Commun., 2006, 27, 1355–1361 CrossRef CAS.
- D.-J. Bak, S.-C. Han, S.-H. Jin and J.-W. Lee, Bull. Korean Chem. Soc., 2011, 32, 3211–3212 CrossRef CAS.
- T. Hagiwara, Y. Murano, Y. Watanabe, T. Hoshi and T. Sawaguchi, Tetrahedron Lett., 2012, 53, 2805–2808 CrossRef CAS.
- S. Hoger, S. Rosselli, A. D. Ramminger and V. Enkelmann, Org. Lett., 2002, 4, 4269–4272 CrossRef PubMed.
- Y. Tobe, N. Utsumi, A. Nagano and K. Naemura, Angew. Chem., Int. Ed., 1998, 37, 1285–1287 CrossRef CAS.
- K. Onitsuka, M. Fujimoto, N. Ohshiro and S. Takahashi, Angew. Chem., Int. Ed., 1999, 38, 689–692 CrossRef CAS.
- C. Glaser, Ber. Dtsch. Chem. Ges., 1869, 2, 422–424 CrossRef.
- A. S. Hay, J. Org. Chem., 1962, 27, 3320–3321 CrossRef CAS.
- R. Chinchilla and C. Najera, Chem. Rev., 2007, 107, 874–922 CrossRef CAS PubMed.
- J. H. Li, J. L. Li, D. P. Wang, S. F. Pi, Y. X. Xie, M. B. Zhang and X. C. Hu, J. Org. Chem., 2007, 72, 2053–2057 CrossRef CAS PubMed.
- T. Fukuyama, M. Shinmen, S. Nishitani, M. Sato and I. Ryu, Org. Lett., 2002, 4, 1691–1694 CrossRef CAS PubMed.
- M. Beaupérin, E. Fayad, R. Amardeil, H. Cattey, P. Richard, S. Brandès, P. Meunier and J.-C. Hierso, Organometallics, 2008, 27, 1506–1513 CrossRef.
- J. Gil-Moltó and C. Nájera, Eur. J. Org. Chem., 2005, 2005, 4073–4081 CrossRef.
- Y. Liang, Y. X. Xie and J. H. Li, J. Org. Chem., 2006, 71, 379–381 CrossRef CAS PubMed.
- K. G. Thakur, E. A. Jaseer, A. B. Naidu and G. Sekar, Tetrahedron Lett., 2009, 50, 2865–2869 CrossRef CAS.
- J. T. Guan, G.-A. Yu, L. Chen, T. Qing Weng, J. J. Yuan and S. H. Liu, Appl. Organomet. Chem., 2009, 23, 75–77 CrossRef CAS.
- M. Carril, A. Correa and C. Bolm, Angew. Chem., Int. Ed., 2008, 47, 4862–4865 CrossRef CAS PubMed.
- I. P. Beletskaya, G. V. Latyshev, A. V. Tsvetkov and N. V. Lukashev, Tetrahedron Lett., 2003, 44, 5011–5013 CrossRef CAS.
- P. Siemsen, R. C. Livingston and F. Diederich, Angew. Chem., Int. Ed., 2000, 39, 2632–2657 CrossRef CAS.
- D. Gelman and S. Buchwald, Angew. Chem., Int. Ed., 2003, 42, 5993–5996 CrossRef CAS PubMed.
- M. Aufiero, F. Proutiere and F. Schoenebeck, Angew. Chem., Int. Ed., 2012, 51, 7226–7230 CrossRef CAS PubMed.
- L. Sikk, J. Tammiku-Taul, P. Burk and A. Kotschy, J. Mol. Model., 2012, 18, 3025–3033 CrossRef CAS PubMed.
- M. García-Melchor, A. A. C. Braga, A. Lledós, G. Ujaque and F. Maseras, Acc. Chem. Res., 2013, 46, 2626–2634 CrossRef PubMed.
- P. Burk, J. Tammiku-Taul and L. Sikk, Proc. Est. Acad. Sci., 2013, 62, 133–140 CrossRef.
- P. V. D. Weghe, Lett. Org. Chem., 2005, 2, 113–117 CrossRef.
- K. Sonogashira, in Metal-Catalyzed Cross-Coupling Reactions, Wiley-VCH, Weinheim, 2004 Search PubMed.
- A. L. Casado and P. Espinet, Organometallics, 1998, 17, 954–959 CrossRef CAS.
- R. Á. O. N. Faza, A. R. de Lera and D. J. Cárdenas, Adv. Synth. Catal., 2007, 349, 887–906 CrossRef.
- P. Fitton and E. A. Rick, J. Organomet. Chem., 1971, 28, 287–291 CrossRef CAS.
- P. Bertus, F. Fecourt, C. Bauder and P. Pale, New J. Chem., 2004, 28, 12–14 RSC.
- C. He, J. Ke, H. Xu and A. Lei, Angew. Chem., Int. Ed., 2013, 52, 1527–1530 CrossRef CAS PubMed.
- L.-P. Chen, S.-G. Hong and H.-Q. Hou, Chin. J. Struct. Chem., 2008, 27, 1404–1411 CAS.
- L. Sikk, J. Tammiku-Taul and P. Burk, Organometallics, 2011, 30, 5656–5664 CrossRef CAS.
- M. García-Melchor, M. C. Pacheco, C. Nájera, A. Lledós and G. Ujaque, ACS Catal., 2012, 2, 135–144 CrossRef.
- H. A. Dieck and F. R. Heck, J. Organomet. Chem., 1975, 93, 259–263 CrossRef CAS.
- C. Amatore, S. Bensalem, S. Ghalem and A. Jutand, J. Organomet. Chem., 2004, 689, 4642–4646 CrossRef CAS.
- A. Soheili, J. Albaneze-Walker, J. A. Murry, P. G. Dormer and D. L. Hughes, Org. Lett., 2003, 5, 4191–4194 CrossRef CAS PubMed.
- T. Ljungdahl, T. Bennur, A. Dallas, H. Emtenäs and J. Mårtensson, Organometallics, 2008, 27, 2490–2498 CrossRef CAS.
- C. Hansch, A. Leo and R. W. Taft, Chem. Rev., 1991, 91, 165–195 CrossRef CAS.
- S. Ehrenson, R. T. C. Brownlee and R. W. Taft, in Progress in Physical Organic Chemistry, John Wiley & Sons, Inc., 2007, pp. 1–80 Search PubMed.
- T. Ljungdahl, K. Pettersson, B. Albinsson and J. Martensson, J. Org. Chem., 2006, 71, 1677–1687 CrossRef CAS PubMed.
- K. H. Shaughnessy, P. Kim and J. F. Hartwig, J. Am. Chem. Soc., 1999, 121, 2123–2132 CrossRef CAS.
- A. Corma, R. Juarez, M. Boronat, F. Sanchez, M. Iglesias and H. Garcia, Chem. Commun., 2011, 47, 1446–1448 RSC.
- F. Barrios-Landeros, B. P. Carrow and J. F. Hartwig, J. Am. Chem. Soc., 2009, 131, 8141–8154 CrossRef CAS PubMed.
- D. V. Partyka, M. Zeller, A. D. Hunter and T. G. Gray, Angew. Chem., Int. Ed., 2006, 45, 8188–8191 CrossRef CAS PubMed.
- A. S. Hashmi, C. Lothschutz, R. Dopp, M. Rudolph, T. D. Ramamurthi and F. Rominger, Angew. Chem., Int. Ed., 2009, 48, 8243–8246 CrossRef CAS PubMed.
- A. L. Casado and P. Espinet, Organometallics, 1998, 17, 3677–3683 CrossRef CAS.
- T. Lauterbach, M. Livendahl, A. Rosellón, P. Espinet and A. M. Echavarren, Org. Lett., 2010, 12, 3006–3009 CrossRef CAS PubMed.
- B. Panda and T. K. Sarkar, Chem. Commun., 2010, 46, 3131–3133 RSC.
- C. Gonzalez-Arellano, A. Abad, A. Corma, H. Garcia, M. Iglesias and F. Sanchez, Angew. Chem., Int. Ed., 2007, 46, 1536–1538 CrossRef CAS PubMed.
- C. Torborg and M. Beller, Adv. Synth. Catal., 2009, 351, 3027–3043 CrossRef CAS.
- I. P. Beletskaya and A. V. Cheprakov, J. Organomet. Chem., 2004, 689, 4055–4082 CrossRef CAS.
- C. A. Fleckenstein and H. Plenio, Organometallics, 2008, 27, 3924–3932 CrossRef CAS.
- X.-Y. Chen, C. Barnes, J. R. Dias and T. C. Sandreczki, Chem.–Eur. J., 2009, 15, 2041–2044 CrossRef CAS PubMed.
- I. Thome, A. Nijs and C. Bolm, Chem. Soc. Rev., 2012, 41, 979–987 RSC.
- J. Magano and J. Dunetz, Chem. Rev., 2011, 111, 2177–2250 CrossRef CAS PubMed.
- K. Heuze, D. Mery, D. Gauss and D. Astruc, Chem. Commun., 2003, 2274–2275 RSC.
- L. Yang, P. Guan, P. He, Q. Chen, C. Cao, Y. Peng, Z. Shi, G. Pang and Y. Shi, Dalton Trans., 2012, 41, 5020–5025 RSC.
- P. M. Price, J. H. Clark and D. J. Macquarrie, J. Chem. Soc., Dalton Trans., 2000, 101–110 RSC.
- J. Zhu, J. Zhou, T. Zhao, X. Zhou, D. Chen and W. Yuan, Appl. Catal., A, 2009, 352, 243–250 CrossRef CAS.
- K. K. R. Datta, M. Eswaramoorthy and C. N. R. Rao, J. Mater. Chem., 2007, 17, 613–615 RSC.
- K. B. Sidhpuria, H. A. Patel, P. A. Parikh, P. Bahadur, H. C. Bajaj and R. V. Jasra, Appl. Clay Sci., 2009, 42, 386–390 CrossRef CAS.
- B. Karimi and D. Enders, Org. Lett., 2006, 8, 1237–1240 CrossRef CAS PubMed.
- M. Choi, D. H. Lee, K. Na, B. W. Yu and R. Ryoo, Angew. Chem., Int. Ed., 2009, 48, 3673–3676 CrossRef CAS PubMed.
- F. Z. Su, Y. M. Liu, L. C. Wang, Y. Cao, H. Y. He and K. N. Fan, Angew. Chem., Int. Ed., 2008, 47, 334–337 CrossRef CAS PubMed.
- A. Khalafi-Nezhad and F. Panahi, Green Chem., 2011, 13, 2408–2415 RSC.
- N. Leadbeater, M. Marco and B. Tominack, Org. Lett., 2003, 5, 3919–3922 CrossRef CAS PubMed.
- P. Appukkuttan, W. Dehaen and E. Van der Eycken, Eur. J. Org. Chem., 2003, 2003, 4713–4716 CrossRef.
- D. Prajapati, H. N. Borah and R. C. Boruah, Synlett, 2005, 2823–2825 CrossRef.
- B. Prüger, G. Hofmeister, C. Jacobsen, D. Alberg, M. Nielsen and K. Jørgensen, Chemistry, 2010, 16, 3783–3790 CrossRef PubMed.
- M. Maji, S. Murarka and A. Studer, Org. Lett., 2010, 12, 3878–3881 CrossRef CAS PubMed.
- G. Jin, X. Zhang and S. Cao, Org. Lett., 2013, 15, 3114–3117 CrossRef CAS PubMed.
- R. Luque and D. Macquarrie, Org. Biomol. Chem., 2009, 7, 1627–1632 CAS.
- T. Truong and O. Daugulis, Org. Lett., 2011, 13, 4172–4175 CrossRef CAS PubMed.
- R. K. Arvela, N. E. Leadbeater, M. S. Sangi, V. A. Williams, P. Granados and R. D. Singer, J. Org. Chem., 2005, 70, 161–168 CrossRef CAS PubMed.
- A. Sagadevan and K. C. Hwang, Adv. Synth. Catal., 2012, 354, 3421–3427 CrossRef CAS.
- S. Protti, M. Fagnoni, M. Mella and A. Albini, J. Org. Chem., 2004, 69, 3465–3473 CrossRef CAS PubMed.
- B. Guizzardi, M. Mella, M. Fagnoni and A. Albini, J. Org. Chem., 2003, 68, 1067–1074 CrossRef CAS PubMed.
- S. Milanesi, M. Fagnoni and A. Albini, J. Org. Chem., 2005, 70, 603–610 CrossRef CAS PubMed.
- T. Okuyama, Acc. Chem. Res., 2001, 35, 12–18 CrossRef.
- S. Protti, M. Fagnoni and A. Albini, Angew. Chem., Int. Ed., 2005, 44, 5675–5678 CrossRef CAS PubMed.
- M. Osawa, H. Nagai and M. Akita, Dalton Trans., 2007, 827–829 RSC.
- P. Schroll, D. P. Hari and B. König, ChemistryOpen, 2012, 1, 130–133 CrossRef CAS PubMed.
- A. Fusano, T. Fukuyama, S. Nishitani, T. Inouye and I. Ryu, Org. Lett., 2010, 12, 2410–2413 CrossRef CAS PubMed.
- F. R. Lemke and C. P. Kubiak, J. Organomet. Chem., 1989, 373, 391–400 CrossRef CAS.
- H. Fischer, Chem. Rev., 2001, 101, 3581–3610 CrossRef CAS PubMed.
- K.-S. Focsaneanu, C. Aliaga and J. C. Scaiano, Org. Lett., 2005, 7, 4979–4982 CrossRef CAS PubMed.
- B. R. Buckley, S. E. Dann, D. P. Harris, H. Heaney and E. C. Stubbs, Chem. Commun., 2010, 46, 2274–2276 RSC.
- A. R. Chaudhary and A. V. Bedekar, Tetrahedron Lett., 2012, 53, 6100–6103 CrossRef CAS.
- L. M. Rossi, N. J. S. Costa, J. Limberger and A. L. Monteiro, in Nanocatalysis Synthesis and Applications, John Wiley & Sons, Inc., 2013, pp. 51–88 Search PubMed.
- R. Chinchilla and C. Najera, Chem. Soc. Rev., 2011, 40, 5084–5121 RSC.
- A. Balanta, C. Godard and C. Claver, Chem. Soc. Rev., 2011, 40, 4973–4985 RSC.
- M. Moreno-Mañas and R. Pleixats, Acc. Chem. Res., 2003, 36, 638–643 CrossRef PubMed.
- B. G. Johnson, Top. Catal., 2003, 24, 147–159 CrossRef CAS.
- R. Narayanan and M. A. El-Sayed, Langmuir, 2005, 21, 2027–2033 CrossRef CAS PubMed.
- C. S. Consorti, F. R. Flores and J. Dupont, J. Am. Chem. Soc., 2005, 127, 12054–12065 CrossRef CAS PubMed.
- R. Chinchilla and C. Nájera, in Nanocatalysis Synthesis and Applications, John Wiley & Sons, Inc., 2013, pp. 89–131 Search PubMed.
- B. M. Choudary, S. Madhi, N. S. Chowdari, M. L. Kantam and B. Sreedhar, J. Am. Chem. Soc., 2002, 124, 14127–14136 CrossRef CAS PubMed.
- D. Astruc, K. Heuzé, S. Gatard, D. Méry, S. Nlate and L. Plault, Adv. Synth. Catal., 2005, 347, 329–338 CrossRef CAS.
- D. Astruc and V. Martinez, in Metathesis Chemistry, ed. Y. Imamoglu, V. Dragutan and S. Karabulut, Springer, Netherlands, 2007, vol. 243, pp. 223–236 Search PubMed.
- A. Roucoux, in Surface and Interfacial Organometallic Chemistry and Catalysis, ed. C. Copéret and B. Chaudret, Springer, Berlin Heidelberg, 2005, vol. 16, pp. 261–279 Search PubMed.
- C. Xue, K. Palaniappan, G. Arumugam, S. A. Hackney, J. Liu and H. Liu, Catal. Lett., 2007, 116, 94–100 CrossRef CAS.
- B. N. Lin, S. H. Huang, W. Y. Wu, C. Y. Mou and F. Y. Tsai, Molecules, 2010, 15, 9157–9173 CrossRef CAS PubMed.
- T. V. Magdesieva, O. M. Nikitin, E. V. Zolotukhin, V. A. Zinovieva and M. A. Vorotyntsev, Mendeleev Commun., 2012, 22, 305–306 CrossRef CAS.
- Y.-T. Chu, K. Chanda, P.-H. Lin and M. H. Huang, Langmuir, 2012, 28, 11258–11264 CrossRef CAS PubMed.
- A. R. Gholap, K. Venkatesan, R. Pasricha, T. Daniel, R. J. Lahoti and K. V. Srinivasan, J. Org. Chem., 2005, 70, 4869–4872 CrossRef CAS PubMed.
- P. Li, L. Wang and H. Li, Tetrahedron, 2005, 61, 8633–8640 CrossRef CAS.
- B. M. Samarasimhareddy, G. Prabhu and V. V. Sureshbabu, Tetrahedron Lett., 2014, 55, 2256–2260 CrossRef.
- M. Bakherad, A. Keivanloo and S. Samangooei, Chin. J. Catal., 2014, 35, 324–328 CrossRef CAS.
- L. Chen, S. Hong, X. Zhou, Z. Zhou and H. Hou, Catal. Commun., 2008, 9, 2221–2225 CrossRef CAS.
- J. Athilakshmi, S. Ramanathan and D. K. Chand, Tetrahedron Lett., 2008, 49, 5286–5288 CrossRef CAS.
- S. Sawoo, D. Srimani, P. Dutta, R. Lahiri and A. Sarkar, Tetrahedron, 2009, 65, 4367–4374 CrossRef CAS.
- S. Gao, N. Zhao, M. Shu and S. Che, Appl. Catal., A, 2010, 388, 196–201 CrossRef CAS.
- N. T. S. Phan and H. V. Le, J. Mol. Catal. A: Chem., 2011, 334, 130–138 CrossRef CAS.
- M. Pagliaro, V. Pandarus, F. Beland, R. Ciriminna, G. Palmisano and P. D. Cara, Catal. Sci. Technol., 2011, 1, 736–739 Search PubMed.
- P. Li, L. Wang, L. Zhang and G.-W. Wang, Adv. Synth. Catal., 2012, 354, 1307–1318 CrossRef CAS.
- M. Esmaeilpour, A. R. Sardarian and J. Javidi, J. Organomet. Chem., 2014, 749, 233–240 CrossRef CAS.
- E. Mohsen, J. Jaber, M. A. Mehdi and N. D. Fatemeh, J. Iran. Chem. Soc., 2013, 11, 499–510 CrossRef.
- M. Nasrollahzadeh, M. Maham, A. Ehsani and M. Khalaj, RSC Adv., 2014, 4, 19731 RSC.
- H. Firouzabadi, N. Iranpoor, F. Kazemi and M. Gholinejad, J. Mol. Catal. A: Chem., 2012, 357, 154–161 CrossRef CAS.
- S. Moussa, A. R. Siamaki, B. F. Gupton and M. S. El-Shall, ACS Catal., 2011, 2, 145–154 CrossRef.
- B. Tamami, M. M. Nezhad, S. Ghasemi and F. Farjadian, J. Organomet. Chem., 2013, 743, 10–16 CrossRef CAS.
- Y.-T. Hung, M.-T. Chen, M.-H. Huang, T.-Y. Kao, Y.-S. Liu and L.-C. Liang, Inorg. Chem. Front., 2014, 1, 405 RSC.
- B. Tamami, F. Farjadian, S. Ghasemi and H. Allahyari, New J. Chem., 2013, 37, 2011–2018 RSC.
- K. Niknam, A. Deris, F. Panahi and M. Reza Hormozi Nezhad, J. Iran. Chem. Soc., 2013, 10, 1291–1296 CrossRef CAS.
- B. J. Borah and D. K. Dutta, J. Mol. Catal. A: Chem., 2013, 366, 202–209 CrossRef CAS.
- K. H. Lee, S.-W. Han, K.-Y. Kwon and J. B. Park, J. Colloid Interface Sci., 2013, 403, 127–133 CrossRef CAS PubMed.
- A. Modak, J. Mondal and A. Bhaumik, Green Chem., 2012, 14, 2840–2855 RSC.
- M. Navidi, N. Rezaei and B. Movassagh, J. Organomet. Chem., 2013, 743, 63–69 CrossRef CAS.
- D. Ganapathy and G. Sekar, Catal. Commun., 2013, 39, 50–54 CrossRef CAS.
- A. Khazaei, S. Rahmati and S. Saednia, Catal. Commun., 2013, 37, 9–13 CrossRef CAS.
- S. Keesara, S. Parvathaneni, G. Dussa and M. R. Mandapati, J. Organomet. Chem., 2014, 765, 31–38 CrossRef CAS.
- M. Hosseini-Sarvari, Z. Razmi and M. M. Doroodmand, Appl. Catal., A, 2014, 475, 477–486 CrossRef CAS.
- M. Planellas, Y. Moglie, F. Alonso, M. Yus, R. Pleixats and A. Shafir, Eur. J. Org. Chem., 2014, 2014, 3001–3008 CrossRef CAS.
- S. Santra, K. Dhara, P. Ranjan, P. Bera, J. Dash and S. K. Mandal, Green Chem., 2011, 13, 3238–3247 RSC.
- L. A. Aronica, A. M. Caporusso, G. Tuci, C. Evangelisti, M. Manzoli, M. Botavina and G. Martra, Appl. Catal., A, 2014, 480, 1–9 CrossRef CAS.
- A. S. Roy, J. Mondal, B. Banerjee, P. Mondal, A. Bhaumik and S. M. Islam, Appl. Catal., A, 2014, 469, 320–327 CrossRef CAS.
- S. U. Son, Y. Jang, J. Park, H. B. Na, H. M. Park, H. J. Yun, J. Lee and T. Hyeon, J. Am. Chem. Soc., 2004, 126, 5026–5027 CrossRef CAS PubMed.
- F. Bellina and M. Lessi, Synlett, 2012, 23, 773–777 CrossRef CAS.
- P. Venkatesan and J. Santhanalakshmi, Langmuir, 2010, 26, 12225–12229 CrossRef CAS PubMed.
- P. Cintas, G. Cravotto, E. C. Gaudino, L. Orio and L. Boffa, Catal. Sci. Technol., 2012, 2, 85–87 CAS.
- M. Korzec, P. Bartczak, A. Niemczyk, J. Szade, M. Kapkowski, P. Zenderowska, K. Balin, J. Lelątko and J. Polanski, J. Catal., 2014, 313, 1–8 CrossRef CAS.
- D. Sengupta, J. Saha, G. De and B. Basu, J. Mater. Chem. A, 2014, 2, 3986 CAS.
- A. Shaabani and M. Mahyari, J. Mater. Chem. A, 2013, 1, 9303–9311 CAS.
- Y.-S. Feng, X.-Y. Lin, J. Hao and H.-J. Xu, Tetrahedron, 2014, 70, 5249–5253 CrossRef CAS.
- J. Liu, X. Peng, W. Sun, Y. Zhao and C. Xia, Org. Lett., 2008, 10, 3933–3936 CrossRef CAS PubMed.
- J. Chung, J. Kim, Y. Jang, S. Byun, T. Hyeon and B. M. Kim, Tetrahedron Lett., 2013, 54, 5192–5196 CrossRef CAS.
- C. Rossy, J. Majimel, E. Fouquet, C. Delacôte, M. Boujtita, C. Labrugère, M. Tréguer-Delapierre and F.-X. Felpin, Chem.–Eur. J., 2013, 19, 14024–14029 CrossRef CAS PubMed.
- F. Zhao, B. M. Bhanage, M. Shirai and M. Arai, Chem.–Eur. J., 2000, 6, 843–848 CrossRef CAS.
- A. Biffis, M. Zecca and M. Basato, Eur. J. Inorg. Chem., 2001, 2001, 1131–1133 CrossRef.
- S. Park, M. Kim, D. H. Koo and S. Chang, Adv. Synth. Catal., 2004, 346, 1638–1640 CrossRef CAS.
- V. K. Kanuru, S. M. Humphrey, J. M. W. Kyffin, D. A. Jefferson, J. W. Burton, M. Armbruster and R. M. Lambert, Dalton Trans., 2009, 7602–7605 RSC.
- H. Firouzabadi, N. Iranpoor, M. Gholinejad and J. Hoseini, Adv. Synth. Catal., 2011, 353, 125–132 CrossRef CAS.
- M. Han, S. Liu, X. Nie, D. Yuan, P. Sun, Z. Dai and J. Bao, RSC Adv., 2012, 2, 6061–6067 RSC.
- S. K. Beaumont, G. Kyriakou and R. M. Lambert, J. Am. Chem. Soc., 2010, 132, 12246–12248 CrossRef CAS PubMed.
- G. Li, D.-E. Jiang, C. Liu, C. Yu and R. Jin, J. Catal., 2013, 306, 177–183 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2014 |