Mercuration of apo-α-lactalbumin: binding of Hg2+ followed by protein-mediated nanoparticle formation†
Atul Gajanan Thawari,
Vijaya Kumar Hinge,
Mayur Temgire and
Chebrolu Pulla Rao*
Bioinorganic Laboratory, Department of Chemistry, Indian Institute of Technology Bombay, Powai, Mumbai-400076, India. E-mail: cprao@iitb.ac.in
First published on 30th September 2014
Abstract
Nanoparticles and nanocrystals of mercury are formed when Hg2+ salt reacts with apo-α-lactalbumin (apo-α-LA). Reduction followed by nanoparticle formation is further augmented by the protein, as it also acts as a coating agent. The initial interaction of Hg2+ with apo-α-LA was demonstrated by changes observed in absorption, emission, and CD spectroscopy, where the latter technique also detected structural changes in the protein. Such structural changes are expected when Hg2+ binds to the protein, and therefore, the binding was determined by isothermal titration calorimetry (ITC). The binding was further proven by MALDI, which showed mercurated species of protein with a Gaussian distribution exhibiting a weighted average of 6 and 9 Hg2+ ions bound to protein when apo-α-LA was treated with 10 and 100 equivalents, respectively. The molecular dynamics studies revealed the binding of Hg2+ ions followed by the structural changes that occurred in the protein. The reaction between Hg2+ and apo-α-LA yields non-crystalline nanoparticles at lower molar ratios of Hg2+ and crystalline ones at higher molar ratios. The existence of both of these nanoparticles was proven by extensive TEM studies, and the mercury nanocrystals were further studied using fluorescence microscopy. X-ray photoelectron spectroscopy demonstrated that the protein has the ability to convert Hg2+ to Hg0, and the resultant Hg0 cluster is known to be less harmful than Hg2+ to the organism. All of these studies support the use of apo-α-LA in the form of nanoparticles and nanocrystals to detoxify Hg2+.
Introduction
Since ancient days, metal nanoparticles have been of interest because of their technological applications.1 Several transition metals including silver and gold have been studied to discern their nanocharacteristics.2,3 Although mercury has special properties and characteristics among the metallic elements, its nanostudies are limited.1 The toxic properties of mercury are responsible for diseases such as acrodynia, Hunter-Russell syndrome, Minamata, and neurodegenerative disorders.4 Even a 5 μM Hg2+ solution can inhibit the elongation of Oryza sativa seedlings.5 The Hg2+ ion is hazardous because of its affinity to interact with protein residues such as cysteine, histidine, aspartic acid, and glutamic acid.6,7 The interaction of Hg2+ with free thiols of protein helps to decrease the toxicity of the metal ion by converting Hg2+ to Hg0. The development of techniques for easy and environmentally benign synthesis of stable mercury nanocrystals is currently a great challenge.1 While dental amalgams that contain mercury are well known in the literature, the generation of nanostructured mercury requires a harsh method.1,8In order to understand the effect of mercuration on proteins, apo-α-lactalbumin (apo-α-LA) was chosen because of its small size and its exposed residues of Glu, Asp, His, and Tyr, and also because of the prior knowledge gathered through studies carried out with Pb2+, Mg2+, Zn2+, Cu2+ and Al3+.9–11 It has been previously demonstrated that the side chains of Glu, Asp, His, and Tyr residues present in Aβ peptides and in proteins such as α-lactalbumin, human serum albumin, and β-lactoglobulin interact with various metal ions, resulting in aggregation and fiber formation.11–14 During the interaction of metal ions and protein, the protein often reduces the bound ions to form metallic species and/or nanocrystals.2 The interaction between α-LA and Hg2+ results in the formation of Hg0, which is less toxic and is well known for its ability to detoxify Hg2+ in some bacteria.6 Therefore, the aim of the present study is not only to demonstrate the binding of Hg2+ to apo-α-LA, but also to study the speciation and the ability of the protein to reduce the Hg2+ toxicity by converting this ion into a less toxic form, viz., Hg0, resulting in nanoparticles and nanocrystals. Different techniques were adopted, such as thermodynamics, spectroscopy, and microscopy coupled with molecular dynamics modeling to obtain the necessary data. Therefore, for the first time, we have demonstrated that mercury nanocrystals form under mild conditions wherein a protein plays an important role.
Results and discussion
Interaction between apo-α-LA and Hg2+
The interaction of Hg2+ and apo-α-LA was demonstrated based on absorption, emission, and CD spectroscopy. Apo-α-LA alone shows two characteristic absorption bands at 210 and 280 nm with a shoulder at 245 nm in 10 mM Tris buffer at pH = 7.4. At a low concentration (1–10 equivalents) of Hg2+, no significant changes were observed. However, upon addition of 100 equivalents, the absorbance of the 280 nm band increases from 0.3 to 1.4 and shows a prominent band at 300 nm along with a shoulder centered at approximately 350 nm (Fig. 1a), and such changes have been reported to be characteristic of metal ion binding.15,16 The mercury nanocrystal exhibits a characteristic surface plasmon resonance (SPR) band at 290 nm (Fig. 1b). The nanoparticle formation was also confirmed by the TEM studies. However, the absorbance of the 210 nm band decreases exponentially as a function of added [Hg2+] and is associated with a 25–30 nm redshift in the peak position that is attributable to the interaction of Hg2+ with the peptide bonds of α-LA. Indeed, such interactions have been reported in the literature.17The fluorescence in apo-α-LA arises from all the aromatic amino acids, viz., Trp, Tyr, and Phe. Most of these aromatic amino acids are exposed, and therefore, the fluorescence emission is primarily from Trp 104, which is buried in the protein. The fluorescence intensity of apo-α-LA observed at 335 nm decreases upon addition of Hg2+. An increase in the [Hg2+] up to 100 equivalents caused a decrease in the fluorescence intensity that was accompanied by a redshift of approximately 5 nm, resulting in the translocation of the Trp residue to the hydrophilic region due to the changes in the microenvironment (Fig. 1c).
Far UV CD is a well known tool in establishing protein conformation and nanoparticle formation.18 The far-UV CD spectra of apo-α-LA in the absence of Hg2+ shows two negative bands centered at 222 and 208 nm, which are characteristic of an α-helix. While the addition of [Hg2+] up to 10 equivalents brings only marginal changes, the addition of up to 100 equivalents brings a significant loss in the negative ellipticity of the band at 222 nm (Fig. 1d and e). This suggests that the addition of approximately 100 equivalents [Hg2+] destabilizes the secondary structure of apo-α-LA. Such structural changes favor the formation of nanoclusters of mercury-bound apo-α-LA, which is further supported by additional data in this study. Significant changes were noticed in the microenvironment of the aromatic amino acid residues, suggesting alterations in the tertiary structure of the protein in the presence of Hg2+ (Fig. 1e).
Binding of Hg2+ to apo-α-LA
The binding of mercury by apo-α-LA was demonstrated by ITC and MALDI mass spectrometry, and the species were modeled by MD computations. The binding of Hg2+ to α-LA was confirmed from ITC studies. Two moderate binding sites were observed by ITC with K1 = (3.5 ± 1.5) × 104 and K2 = (4.3 ± 2.6) × 104 M−1 when the titration data was fitted using a sequential binding model (Fig. 2). Both sites were exothermic and enthalpically favorable. The binding of Hg2+ was modeled based on MD computations.In order to understand the binding features associated with Hg2+ binding to α-LA, MD simulations were carried out for 25 ns at the conditions mentioned in the experimental section. At lower equivalents of Hg2+, the MD study resulted in α-LA bound by three mercury ions through carboxylate side chain moieties (SI 01†) with no significant changes in the conformation of the protein. This is also evident from the CD study carried out at this concentration. At higher equivalents, this resulted in the binding of six Hg2+ ions, viz., Hg1 to Hg6, to the surface of α-LA, as can be seen from Fig. 3.
At this concentration of Hg2+, the original Ca2+ site is disrupted due to the changes made to the structure of the protein, which were dependent on the number of equivalents of Hg2+ added; this was supported by CD studies. Significant structural changes were observed in the regions marked in Fig. 3g, wherein a striking variation is observed in the peptide region spanning from 100 to 111 amino acids, where the helical region is converted to a random coil. Thus, the MD results concur with those obtained from CD data, suggesting that although the protein conformation is unaltered at a low equivalent of Hg2+, the conformation is altered at a higher equivalent addition of Hg2+ due to the structural changes occurring in the protein.
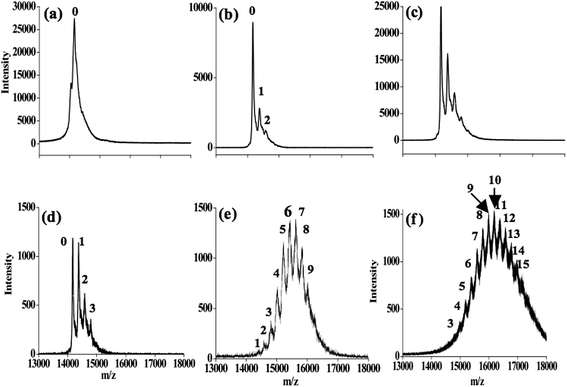
The Hg2+-bound species of α-LA were determined by MALDI-TOF MS (Fig. 4). These experiments were carried out for a solution of α-LA treated with up to 100 equivalents of Hg2+. As the number of added Hg2+ equivalents increases to 10, the peak corresponding to the unbound protein decreases and the protein bound to one, two, and three ions of Hg2+ increases, as can be seen from Fig. 4b–d. The initial result is similar to the ITC and the MD results, where two and three bindings were observed, respectively. However, when the solution of the 10 equivalents was incubated for one day, the peak corresponding to the unbound protein completely vanished and resulted in a complex spectrum where peaks corresponding to 1 to 9 ions of Hg2+-bound protein (Fig. 4e) are seen. The intensity profile of this complex spectrum follows a Gaussian distribution, and the maximum peak intensity was observed for 6 and 7 Hg2+-bound species (Fig. 4e). A weighted average shows the presence of approximately 6 Hg2+ per protein molecule.
In the case of 100 equivalents of Hg2+, the MALDI experiment yielded a complex spectrum that is similar, with a maximum intensity found for 10 Hg2+-bound proteins and the weighted average shifted to 9 Hg2+ per protein. A similar pattern was observed even after one day of incubation of the 100 equivalent solution. Because all these mercurated α-LA species were observable by MALDI, they must have moderate binding strengths, as also supported by the ITC. The current study originally suggested that 2–3 binding sites are available for Hg2+; however, upon addition of a higher equivalent of Hg2+, changes occur in the protein conformation and structure that further favor the binding of more Hg2+ ions to the protein. Secondary and tertiary structural changes caused by Hg2+ binding were already shown by the CD studies. All these studies support the formation of a statistical mixture of an Hg2+-bound α-LA species.
Formation of mercury nanoclusters covered with apo-α-LA
As Hg2+-induced structural changes of the protein were observed, the nanoparticles and nanocrystal formation was addressed by transmission electron microscopy (TEM) (Fig. 5, SI 02–05†). Apo-α-LA (10 μM), upon interaction with 10 equivalents of Hg2+, forms a non-crystalline type of nanoparticle that is 10–20 nm size (Fig. 5a–d). At low equivalents of Hg2+, apo-α-LA does not exhibit any significant change in its secondary structure based on CD studies, and hence no exposure of tyrosine and cysteine is expected to occur. Even upon increasing the equivalents to 100, the size of the nanoparticles that formed remained almost the same. However, several new characteristics of the nanocrystals were noticed due to secondary structural changes that are expected in the protein at this concentration of Hg2+, resulting in the exposure of the Tyr and Cys residues and thereby supporting the formation of crystalline nanoparticles of mercury (Fig. 5e–k, SI 02†). Similar particles were observed with a sample of apo-α-LA with 100 equivalents of Hg2+ that was dialyzed (SI 03†).Comparatively, large particles with a polycrystalline and/or amorphous nature were found with mercury perchlorate(II) salts used as a control for comparison (SI 04†). ANS study also supported the exposure of Tyr and Cys in the protein upon Hg2+ addition (SI 05†).
Exposure of Tyr and Cys followed by the reduction of Hg2+ to Hg0 by apo-α-LA
The treatment of apo-α-LA by Hg2+ resulted in mercury-bound species, as determined by MALDI-TOF, which indicated the formation of nanoparticles that were confirmed by TEM. The reduction of Hg2+ by the protein was studied by X-ray photoelectron spectroscopy (XPS) for the apo-α-LA that was treated with 100 equivalents of Hg2+ (Fig. 6a, SI 06†). The spectra showed peaks at 98.9 and 101.9 eV, respectively, for Hg0 and Hg2+, and the assignment of those peaks is in agreement with that given in the literature.19 This shows that the precursor Hg2+ is reduced to a less toxic Hg0 that is possible by the oxidation of the phenol moieties of four tyrosine residues in apo-α-LA. This is further supported by absorption studies (Fig. 6b) wherein the bands observed at 280 and 210 nm for α-LA were shifted to 285 and 235 nm, respectively, with a shoulder at approximately 245 nm; all this clearly suggests the exposure of tyrosine moieties to the environment.20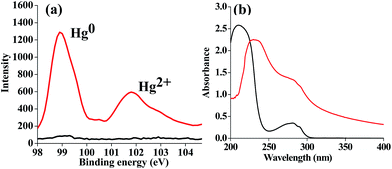 | ||
Fig. 6 (a) XPS spectra of Hg: α-LA ( ) and its mercury-bound species ( ) and its mercury-bound species ( ). (b) Absorption spectra of α-LA ( ). (b) Absorption spectra of α-LA ( ) and {α-LA + 100 equivalents of Hg2+} ( ) and {α-LA + 100 equivalents of Hg2+} ( ). ). | ||
Fluorescence microscopy studies of the Hg2+-bound apo-α-LA
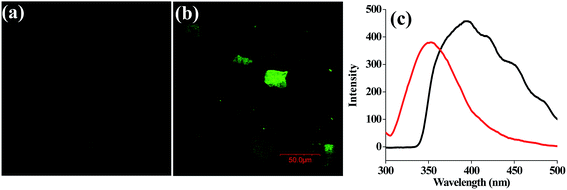
The laser confocal fluorescence microscopy image of {apo-α-LA + 100 equivalents of Hg2+} shows green fluorescence that is not found with the protein alone, thereby suggesting the formation of nanocrystals or nanoparticles of small size (5–10 nm) (Fig. 7a and b). However, the {apo-α-LA + 100 equivalents of Hg2+} exhibits a broad emission band centered at 400 nm (Fig. 7c).Conclusions and correlations
This study shows for the first time the utilization of protein, apo-α-LA, in the formation of mercury nanoparticles and nanocrystals. Although it is known that heavy metals such as Hg2+ and Pb2+ cause protein aggregation, the molecular details of detoxification of Hg2+ by proteins and in turn its effect on the protein structure and function, particularly the aggregational behavior, are not well understood.7 The aggregation is known to be the result of the exposure of hydrophobic residues. Controlling hydrophobic interactions is a means to control the size of nanoparticles. This, in turn, is regulated by changing the Hg2+ and apo-α-LA concentrations. Different concentrations of Hg2+ were used to study the effect on apo-α-LA conformation that results in nanocrystal formation. To our knowledge, this is the first example of green synthesis of mercury nanocrystals that is affected by the reducing action of the protein, apo-α-LA. Such nanocrystal formation is due to the specific and non-specific interaction of Hg2+ with α-LA followed by reduction. While the two specific binding sites were proven by ITC titrations, the specific as well as non-specific bindings were shown by MALDI mass spectrometry, where each peak of the complex spectrum observed corresponds to the protein bound to one or more Hg2+ ions, with a maximum number of 15 Hg2+ ions being bound. A weighted average of this shows binding by at least 10 Hg2+ ions. The binding of Hg2+ with carboxylate, histidine, and tyrosine as well as cysteine (disulfide form) residues results in the exposure of hydrophobic patches followed by the loss of secondary structure that induces the formation of nanoclusters. These findings were supported by MALDI and TEM studies.The nanocharacteristics of the protein-coated mercury depend on the concentration of Hg2+ ions. While non-crystalline nanoparticles were observed at lower molar ratios of Hg2+ ions, crystalline nanoparticles were observed at higher molar ratios. These nanocrystals were found to have the ability to convert highly toxic Hg2+ into the less toxic Hg0 form using the redox ability of different residues, including the tyrosine present in the protein. A recent report in the literature describes the synthesis of mercury nanocrystals carried out under harsh conditions consisting of nitric acid plus a polyvinyl alcohol mixture of mercury nitrate heated at 60–110 °C.1 In contrast, the present method utilizes a simple and environmentally friendly synthesis of the mercury nanocrystals, wherein the protein acts as a reducing as well as capping agent, and thus detoxifies mercury.
Experimental section
Materials
Bovine apo-α-LA and mercury perchlorate were procured from the Sigma-Aldrich Chemical Co., USA. All the other chemicals used were from E. Merck, Germany. An apoform of lactalbumin (apo-α-LA) was used in all experiments. The concentration of apo-α-LA was calculated from the absorbance at 280 nm using the molar extinction coefficient of 28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 M−1 cm−1.21
500 M−1 cm−1.21
Nanoparticle preparation
To a requisite volume of 10 μM of apo-α-LA solution in 1 ml Eppendorf tubes, 1 and 10 μl of 100 mM mercury perchlorate (Hg2+) was added to give 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 and 1
10 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 molar ratios of protein to metal ion, which were maintained throughout all the studies reported here. The 1 ml solutions thus prepared were incubated in a shaking stirrer for 2 h, and these were used for further studies.
100 molar ratios of protein to metal ion, which were maintained throughout all the studies reported here. The 1 ml solutions thus prepared were incubated in a shaking stirrer for 2 h, and these were used for further studies.
Absorption spectra
UV-visible absorption studies were performed using a Varian Cary 100 UV-spectrophotometer. A 10 μM cuvette concentration of protein was used for all experiments. The Hg2+ titrations of apo-α-LA were performed by continuous addition from 1 to 10 μl of the 10 mM and 100 mM Hg2+ stock solutions in such a way that the final volume of the solution was always maintained at 1 ml. A control was performed by titrating the Hg2+ with buffer and subtracting this from the {apo-α-LA + Hg2+} titrations. Only the subtracted data are shown as the final spectra in this study.Emission spectra
Fluorescence studies were performed on a PerkinElmer LS 55 fluorescence spectrometer. The samples were prepared in the same manner as that given for the absorption studies. These samples were diluted three-fold so that the final protein concentration was 3 μM. The metal ion concentration was adjusted so that the molar ratio for all the studies remained the same. The resultant solutions were used for the fluorescence studies by exciting at 280 nm and measuring the spectra from 290 to 500 nm using a 5 nm slit both for the excitation and emission gates, with a scan speed of 150 nm min−1. The control was performed with simple Hg2+ ion solution and was subtracted from the {apo-α-LA + Hg2+} titrations.Matrix assisted laser desorption ionization (MALDI) mass spectrometry
The mass spectra of native and metallated proteins were recorded on an Autoflex III TOF/TOF MS (MALDI-TOF mass spectrometer, Bruker Daltonics Co.). α-Cyano-hydroxy-trans-cinnamic acid was used as the matrix for the MALDI-TOF. The MALDI-TOF instrument was calibrated with horse heart myoglobin (m/z values, 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 952) prior to the measurements.
952) prior to the measurements.
XPS analysis
X-ray photoelectron spectroscopy (XPS) was performed in the analysis chamber of an Omicron XPS/UPS system. The base pressure of the chamber was 1.5 × 10−7 Pa. Aluminium K-alpha X-ray (1486.6 eV) was used as the excitation source. All the data were baseline corrected and plotted using Origin 7.5 software.Isothermal titration calorimetry (ITC)
Calorimetric titrations were performed at 25 °C with a microcal isothermal titration calorimeter from MicroCal ITC200 (Northampton, MA, USA) using 5 mM Hg2+. Successive additions of 2 μl of Hg2+ were separated by a 150 s interval to allow the peak resulting from the {apo-α-LA + Hg2+} interaction to return to the baseline. The ITC data were fitted with the Origin software package provided by MicroCal using curve fitting models of sequential sites.Sample preparation for FEG-TEM
In order to carry out the microscopy studies, 10 μM of apo-α-LA was incubated with 10 and 100 equivalents of Hg2+. Each sample was then sonicated for 5 min. The samples were spread over carbon-coated copper grids with a 200 mesh, dried, and then analyzed with a JEOL JEM-2100F (FEG-TEM) electron microscope operating at 200 kV.Binding by 8-anilinonaphthalene-1-sulfonate (ANS)
Fluorescence emission spectra for ANS were recorded from 400 to 600 nm by exciting the solutions at 365 nm. The excitation and emission slit widths were kept at 5 nm. 2–10 μl of a 2 mM ANS solution was added serially to a total volume of 1 ml, and the spectra were recorded. A blank titration was also performed with simple buffer as a control and was subtracted from the corresponding titration data.Circular dichroism (CD) spectroscopy
Far-UV CD spectra were collected using a Jasco J-815 CD spectrometer. The path length of the cuvette was 2 mm. Apo-α-LA samples with 1–10 equivalent of Hg2+ were studied at 10 μM concentration throughout the CD spectra. Three scans were recorded with a scan speed of 100 nm min−1 and were averaged.Molecular dynamics (MD) simulations
The initial coordinates of α-LA were downloaded from the X-ray crystal structure of α-LA (PDB code: 1HFZ).22 The two independent simulation studies were carried out with 10 and 50 Hg2+ ions. The initial models for both simulations were made from the protein crystal structure with the following modifications: (a) the unbound water molecules were removed, (b) Ca2+ was removed by retaining the two coordinated water molecules, (c) hydrogen atoms were added using the XLEaP program, (d) Hg2+ ions were manually placed around the protein randomly using the Accelrys DS Visualizer before performing the simulations study. All MD simulations were performed under periodic boundary conditions (PBC) using an explicit solvent (TIP3P water molecules) environment with the Amber 10 software package employing ff03 force field parameters.23,24 α-LA was solvated with 6760 and 7533 TIP3P25 water molecules for the 10 and 50 Hg2+ simulation models, respectively. A truncated octahedral box was used for the calculation with an initial volume of 264![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 257 and 298
257 and 298![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Å3 for the 10 and 50 Hg2+ simulation models, respectively. The charges for glutamate and aspartate residues were assigned as −1, lysine and arginine residues were set as +1, all Nε2-positions of histidines were protonated, and Cl− was added to neutralize the charge balance. The van der Waals radius (R, Å) and potential energy well depth (ε, kcal mol−1) parameters for Hg(II) were 1.60 and 1.0, respectively. The isothermal–isobaric (NPT) ensemble was employed with PBC. The trajectories were saved for every 1 ps interval for further analysis, and processing of the trajectories was carried out using the PTRAJ software package. The Particle Mesh Ewald (PME) summation was used to treat long-range electrostatic interactions, and a 12 Å cutoff was used for the van der Waals interactions.26 The SHAKE algorithm was used to constrain the bond lengths involving hydrogen atoms with a relative geometric tolerance of 0.0001. Constant pressure (1 atm) and temperature were maintained using the Berendsen weak-coupling algorithm27 with a relaxation time constant of 2 ps and Langevin thermostat with a time constant for heat bath coupling of 1 ps, respectively.28,29 All simulations were subjected to temperature, pressure, potential energy, and kinetic energy calculations as a function of time, and no significant changes were observed in these parameters.
000 Å3 for the 10 and 50 Hg2+ simulation models, respectively. The charges for glutamate and aspartate residues were assigned as −1, lysine and arginine residues were set as +1, all Nε2-positions of histidines were protonated, and Cl− was added to neutralize the charge balance. The van der Waals radius (R, Å) and potential energy well depth (ε, kcal mol−1) parameters for Hg(II) were 1.60 and 1.0, respectively. The isothermal–isobaric (NPT) ensemble was employed with PBC. The trajectories were saved for every 1 ps interval for further analysis, and processing of the trajectories was carried out using the PTRAJ software package. The Particle Mesh Ewald (PME) summation was used to treat long-range electrostatic interactions, and a 12 Å cutoff was used for the van der Waals interactions.26 The SHAKE algorithm was used to constrain the bond lengths involving hydrogen atoms with a relative geometric tolerance of 0.0001. Constant pressure (1 atm) and temperature were maintained using the Berendsen weak-coupling algorithm27 with a relaxation time constant of 2 ps and Langevin thermostat with a time constant for heat bath coupling of 1 ps, respectively.28,29 All simulations were subjected to temperature, pressure, potential energy, and kinetic energy calculations as a function of time, and no significant changes were observed in these parameters.
Confocal laser scanning microscopy (CLSM)
The samples for the CLSM were prepared on the coverslip and air dried, and were then scanned under the laser source having an excitation of 250–360 nm using an Olympus IX. The images were analyzed using Fluoview Viewer software.Acknowledgements
We dedicate this paper to Professor C.N.R. Rao on his 80th birthday. CPR acknowledges the financial support from DST, CSIR and DAE-BRNS. VKH acknowledges the IIT Bombay for RA ship. We thank IIT Bombay for TEM and MALDI-TOF. We thank BRAF (Bioinformatics Resources and Applications Facility) at Centre for Development of Advanced Computing (C-DAC), Pune, India for computational time.References
- G. V. Ramesh, M. D. Prasad and T. P. Radhakrishnan, Chem. Mater., 2011, 23, 5231–5236 CrossRef CAS.
- J. Xie, Y. Zheng and J. Y. Ying, J. Am. Chem. Soc., 2009, 131, 888–889 CrossRef CAS PubMed.
- A. K. Singh, R. Kanchanapally, Z. Fan, D. Senapati and P. C. Ray, Chem. Commun., 2012, 48, 9047–9049 RSC.
- P. S. Harikumar, A. Dhruvan, V. Sabna and A. Babitha, J. Toxicol. Environ. Health, 2011, 3, 008–013 CAS.
- M. Patra and A. Sharma, Bot. Rev., 2000, 66, 379–422 CrossRef.
- H. Qian, L. Sahlman, P. O. Eriksson, C. Hambraeus, U. Edlund and I. Sethson, Biochemistry, 1998, 37, 9316–9322 CrossRef CAS PubMed.
- D. B. Veprintsev, E. A. Permyako, L. A. Kalinichenko and L. J. Berliner, Biochem. Mol. Biol. Int., 1996, 39, 1255–1265 CAS.
- K. V. Katok, R. L. D. Whitby, T. Fukuda, T. Maekawa, I. Bezverkhyy, S. V. Mikhalovsky and A. B. Cundy, Angew. Chem., Int. Ed., 2012, 51, 2632–2635 CrossRef CAS PubMed.
- A. Saha and V. V. Yakovlev, J. Biophotonics, 2010, 3, 670–677 CrossRef CAS PubMed.
- P. A. Eugene and B. J. Lawrence, FEBS Lett., 2000, 473, 269–274 CrossRef.
- J. F. Graveland-Bikker, I. A. T. Schaap, C. F. Schmidt and C. G. de Kruif, Nano Lett., 2006, 6, 616–621 CrossRef CAS PubMed.
- P. Faller, C. Hureau and O. Berthoumieu, Inorg. Chem., 2013, 52, 12193–12206 CrossRef CAS PubMed.
- T. Dudev and C. Lim, Chem. Rev., 2013, 1, 538–560 Search PubMed.
- B. Zappone, M. P. De Santo, C. Labate, B. Rizzutia and R. Guzzi, Soft Matter, 2013, 9, 2412–3241 RSC.
- B. Roschitzki and M. Vasak, J. Biol. Inorg. Chem., 2002, 7, 6–616 CrossRef PubMed.
- G. Meloni, P. Faller and M. Vasak, J. Biol. Chem., 2007, 282, 16068–16078 CrossRef CAS PubMed.
- S. M. Kelly and N. C. Price, Curr. Protein Pept. Sci., 2000, 1, 349–384 CrossRef CAS.
- Y. Yu, Z. Luo, C. S. Teo, Y. N. Tan and J. Xie, Chem. Commun., 2013, 49, 9740 RSC.
- N. D. Hutson, B. C. Attwood and K. G. Scheckel, Environ. Sci. Technol., 2007, 41, 1747–1752 CrossRef CAS.
- E. P. Melo, M. R. Aires-Barros, S. M. B. Costa and J. M. S. Cabral, J. Biochem. Biophys. Methods, 1997, 34, 45–59 CrossRef CAS.
- A. Vanhooren, K. Vanhee, K. Noyelle, Z. Majer, M. Joniau and I. Hanssens, Biophys. J., 2002, 82, 407–417 CrossRef CAS.
- A. C. Pike, K. Brew and K. R. Acharya, Structure, 1996, 4, 691–703 CrossRef CAS.
- A. Case, T. A. Darden, T. E. Cheatham III, C. L. Simmerling, J. Wang, R. E. Duke, R. Luo, M. Crowley, R. C. Walker, W. Zhang, K. M. Merz, B. Wang, S. Hayik, A. Roitberg, G. Seabra, I. Kolossváry, K. F. Wong, F. Paesani, J. Vanicek, X. Wu, S. R. Brozell, T. Steinbrecher, H. Gohlke, L. Yang, C. Tan, J. Mongan, V. Hornak, G. Cui, D. H. Mathews, M. G. Seetin, C. Sagui, V. Babin and P. A. Kollman, AMBER 10, University of California, San Francisco, CA, 2008 Search PubMed.
- Y. Duan, C. Wu, S. Chowdhury, M. C. Lee, G. Xiong, W. Zhang, R. Yang, P. Cipelak, R. Luo and T. Lee, J. Comput. Chem., 2003, 24, 1999–2012 CrossRef CAS PubMed.
- W. L. Jorgensen, J. Chandrasekhar, J. D. Madura, R. W. Impey and M. L. Klein, J. Chem. Phys., 1983, 79, 926–935 CrossRef CAS PubMed.
- T. Darden, D. York and L. Pedersen, J. Chem. Phys., 1993, 98, 10089–10092 CrossRef CAS PubMed.
- H. J. C. Berendsen, J. P. M. Postma, W. F. van Gunsteren, A. DiNola and J. R. Haak, J. Chem. Phys., 1984, 81, 3684–3690 CrossRef CAS PubMed.
- B. P. Uberuaga, M. Anghel and A. F. Voter, J. Chem. Phys., 2004, 120, 6363–6374 CrossRef CAS PubMed.
- J. P. Ryckaert, G. Ciccotti and H. J. C. Berendsen, J. Comput. Phys., 1977, 23, 327–341 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra07156e |
| This journal is © The Royal Society of Chemistry 2014 |