Click chemistry route to tricyclic monosaccharide triazole hybrids: design and synthesis of substituted hexahydro-4H-pyrano[2,3-f][1,2,3]triazolo[5,1-c][1,4]oxazepines†
Saidulu Konda,
Pallavi Rao and
Srinivas Oruganti*
Dr Reddy's Institute of Life Sciences, University of Hyderabad Campus, Gachibowli, Hyderabad, 500 046, India. E-mail: soruganti@drils.org
First published on 19th November 2014
Abstract
A click chemistry approach to novel tricyclic monosaccharide triazole hybrids, namely, aryl substituted hexahydro-4H-pyrano[2,3-f][1,2,3]triazolo[5,1-c][1,4]oxazepine derivatives from an intramolecular 1,3-dipolar cycloaddition of 6-azido-4-O-propargyl glycopyranosides has been reported.
A carbohydrate inspired molecular hybridization approach incorporating natural chiral carbohydrate templates with heterocyclic rings offers an opportunity for the generation of an interesting array of structures and templates for drug discovery.1 For instance, small molecule carbohydrate-triazole hybrids have been utilized either as drug candidates or as mechanistic probes to unravel intricate biological pathways (Fig. 1).2
Against this background and given that the triazole moiety itself is present in many compounds exhibiting different biological properties such as antibacterial and anti-HIV,3 we were interested to design and synthesize a set of tailor made tricyclic monosaccharide-derived analogues incorporating a pharmacophoric 1,2,3-triazole ring (Fig. 2).
In this regard, construction of triazoles by Huisgen's 1,3-dipolar cycloaddition reaction4 between alkynes and alkyl azides, termed as ‘click-chemistry’ by Sharpless,5 appeared to us as a preferred means to rapidly assemble the tricyclic framework shown in 7 and 8. Though similar approaches have been described earlier for the synthesis of triazole-carbohydrate analogues,6,7 triazole moiety in these cases was invariably fused at the reducing end of the pentoses and hexoses. We were interested to synthesize fused carbohydrate aryl triazole hybrids, wherein the presence of aryl rings render the analogues drug-like with favourable C![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P values.
P values.
In the results described here-in, we have focussed on the introduction of a 4-aryl-triazole at the non-reducing end of the sugar by effecting an intramolecular [3 + 2] Huisgen's cycloaddition between a propargyl and an azide moiety anchored at the 4- and 6-OH groups of D-glucopyranoside derivative, to construct a triazole ring along with the concomitant formation of 1,4-oxazepine ring.
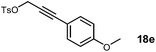
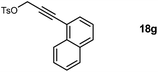
Starting from methyl α-D-glucopyranoside 9, benzylidene protection using benzaldehyde dimethyl acetal in the presence of catalytic I2 afforded 4,6-O-benzylidene derivative 10 (ref. 8) in good yield (Scheme 1). The 2- and 3-hydroxyl groups in 10 were protected as benzyl ethers using benzyl bromide/NaH milieu9 to obtain 11 in quantitative yield. Deprotection of the benzylidene acetal 11 by treatment with pTSA in MeOH afforded diol 12, which on regioselective tosylation of the primary hydroxyl using TsCl/Py gave 13 (ref. 11) in good yield. SN2 displacement of the tosylate moiety in 13 with sodium azide afforded the 6-azido compound 14 (ref. 10) in 92% yield. The methyl 6-azido-2,3-O-benzyl-α-D-glucopyranoside 14 obtained was chosen as the key carbohydrate precursor for further functionalization with the propargyl tosylate 18a and various substituted aryl-propargyl alcohol tosylates 18b–g (Schemes 2 and 3).
 | ||
| Scheme 2 Reagents and conditions: (a) Pd(PPh3)4, CuI (cat), K2CO3, DMF, 80 °C, 12 h, 80–90%; (b) TsCl, KOH, CH2Cl2, 0 °C, 1 h, 80–90%. | ||
Substituted aryl-propargyl tosylates 18b–g were synthesized from corresponding substituted aryl bromides and propargyl alcohol. Sonogashira coupling11 of aryl bromides with propargyl alcohol 15 in the presence of Pd(PPh3)4/CuI/K2CO3 milieu (Scheme 2) afforded 17b–g. Tosylation using TsCl/KOH12 on 17b–g gave the corresponding tosylates 18b–g in good yields. Various propargyl tosylates 18a–g synthesized are shown in Table 1 and were utilized for the proparglyation of the 4-OH in 14.
Alkylation of 14 with the tosylates 18a–g was achieved in the presence of NaH base to obtain azido–alkyne glucopyranosides 19a–g (Scheme 3). This set the stage for [3 + 2] cyclization13,14 and we initially attempted cycloaddition in 19a. One of the concerns was whether the orientation of the azide and alkyne moieties was suited for the intramolecular reaction and any competing intermolecular products would dominate affording dimeric or oligomeric mixtures. Satisfactorily, intramolecular [3 + 2] cycloaddition afforded the desired product 20a(ref. 6) in good yield and we did not observe products from intermolecular couplings when the reaction was monitored by LCMS. Subsequently debenzylation in 20a afforded compound 8a. The presence of a one proton singlet at δ 7.60 in 1H NMR and carbon signals at δ 136.1 and 132.9 in 13C NMR corresponding to the two carbon atoms of the triazole ring confirmed the formation of 8a. Encouraged by this result, cycloadditions were performed on 19b–g to obtain 20b–g in good yields (Table 2). Debenzylation of 20a–g furnished the diols 8a–g in excellent yields.
The pivotal ‘click-reaction’ was therefore a one-step process for the construction of fused tricyclic system from a differentially derivatized glycopyranoside. In conclusion, we have developed a efficient route for the synthesis of tricyclic monosaccharide-triazole hybrids by employing 1,3-dipolar cycloaddition reaction. These new chemical motifs can serve as promising leads as drug candidates or probes for metabolic pathways.
Acknowledgements
The authors thank Prof. Prabhat Arya for his valuable inputs. S.K. thanks UGC, New Delhi, for the award of research fellowship.Notes and references
- (a) B. Gabrielsen, M. J. Phelan, L. Barthel-Rosa, C. See, J. W. Huggins, D. F. Kefauver, T. P. Monath, M. A. Ussery, G. N. Chmurny, E. M. Schubert, K. Upadhya, C. Kwong, D. A. Carter, J. A. Secrist, III, J. J. Kirsi, W. M. Shannon, R. W. Sidwell, G. D. Kini and R. K. Robins, J. Med. Chem., 1992, 35(17), 3231 CrossRef CAS; (b) R. Alvarez, S. Velazquez, A. San-Felix, S. Aquaro, E. D. Clercq, C.-F. Perno, A. Karlsson, J. Balzarini and M. J. Camarasa, J. Med. Chem., 1994, 37, 4185 CrossRef CAS; (c) J. Li, M. Zheng, W. Tang, P.-L. He, W. Zhu, T. Li, J.-P. Zuo, H. Liu and H. Jiang, Bioorg. Med. Chem. Lett., 2006, 16(19), 5009 CrossRef CAS PubMed; (d) C. Hager, R. Miethchen and H. Reinke, J. Fluorine Chem., 2000, 104(2), 135 CrossRef CAS; (e) L. Diaz, J. Bujons, J. Casas, A. Llebaria and A. Delgado, J. Med. Chem., 2010, 53(14), 5248 CrossRef CAS PubMed.
- (a) G. O'Mahony, E. Ehrman and M. Grøtli, Tetrahedron Lett., 2005, 46, 6745 CrossRef PubMed; (b) K. P. Kaliappan, P. Kalanidhi and S. Mahapatra, Synlett, 2009, 13, 2162 CrossRef PubMed; (c) V. S. Sudhir, N. Y. P. Kumar, R. B. N. Baig and S. Chandrasekaran, J. Org. Chem., 2009, 74, 7588 CrossRef PubMed; (d) C. M. Klemm, A. Berthelmann, S. Neubacher and C. Arenz, Eur. J. Org. Chem., 2009, 17, 2788 CrossRef; (e) B. L. Wilkinson, H. Long, E. Sim and A. J. Fairbanks, Bioorg. Med. Chem. Lett., 2008, 18(23), 6265 CrossRef CAS PubMed; (f) M. Singer, M. Lopez, L. F. Bornaghi, A. Innocenti, D. Vullo, C. T. Supuranand and S. A. Poulsen, Bioorg. Med. Chem. Lett., 2009, 19(8), 2273 CrossRef CAS PubMed; (g) B. K. Singh, A. K. Yadav, B. Kumar, A. Gaikwad, S. K. Sinha, V. Chaturvedi and R. P. Tripathi, Carbohydr. Res., 2008, 343(7), 1153 CrossRef CAS PubMed; (h) L. Diaz, J. Casas, J. Bujons, A. Llebaria and A. Delgado, J. Med. Chem., 2011, 54, 2069 CrossRef CAS PubMed; (i) R. I. Anegundi, V. G. Puranik and S. Hotha, Org. Biomol. Chem., 2008, 6, 779 RSC.
- (a) C. H. Zhou and Y. Wang, Curr. Med. Chem., 2012, 19(2), 239 CrossRef CAS; (b) R. Kharb, P. C. Sharma and M. S. Yar, J. Enzyme Inhib. Med. Chem., 2011, 26(1), 1 CrossRef CAS PubMed; (c) R. Kharb, M. S. Yar and P. C. Sharma, Mini-Rev. Med. Chem., 2011, 11(1), 8496 CrossRef.
- R. Huisgen, R. Grashey and J. Sauer, Chemistry of Alkenes, Interscience, New York, 1964, sp. 806 Search PubMed.
- V. V. Rostovtsev, L. G. Green, V. V. Fokin and K. B. Sharpless, Angew. Chem., Int. Ed., 2002, 41, 2596 CrossRef CAS.
- A similar strategy has been reported by Hotha and co-workers for the synthesis of 1,2,3-triazole and 1,2,3,4-tetrazole fused polycyclic compounds from carbohydrate derived azido–alkyne and azido–cyanide precursors. See: S. Hotha, R. I. Anegundi and A. A. Natu, Tetrahedron Lett., 2005, 46, 4585 CrossRef CAS PubMed.
- References of other approaches: (a) F. Dolhem, F. A. Tahli, C. Lièvre and G. Demailly, Eur. J. Org. Chem., 2005, 23, 5019 CrossRef; (b) V. S. Sudhir, N. Y. P. Kumar, R. B. N. Baig and S. Chandrasekaran, J. Org. Chem., 2009, 74, 7588 CrossRef PubMed; (c) Y. S. Reddy, A. P. J. Pal, P. Gupta, A. A. Ansari and Y. D. Vankar, J. Org. Chem., 2011, 76, 5972 CrossRef CAS PubMed.
- R. Panchadhayee and A. K. Misra, J. Carbohydr. Chem., 2008, 27, 148 CrossRef CAS.
- M. A. Fernández-Herrera, H. López-Muñoz, J. M. V. Hernández-Vázquez, M. López-Dávila, S. Mohan, M. L. Escobar-Sánchez, L. Sánchez-Sánchez, B. M. Pinto and J. Sandoval-Ramírez, Eur. J. Med. Chem., 2011, 46, 3877 CrossRef PubMed.
- (a) B. Elchert, J. Li, J. Wang, Y. Hui, R. Rai, R. Ptak, P. Ward, J. Y. Takemoto, M. Bensaci and C.-W. T. Chang, J. Org. Chem., 2004, 69, 1513 CrossRef CAS PubMed; (b) Y. Hui and C.-W. T. Chang, Org. Lett., 2002, 4, 2245 CrossRef CAS PubMed.
- (a) R. Chinchilla and C. Nájera, Chem. Soc. Rev., 2011, 40, 5084 RSC; (b) N. W. Polaske, D. V. McGrath and J. R. McElhanon, Macromolecules, 2010, 43, 1270 CrossRef CAS.
- R. Srinivasan, M. Uttamchandani and S. Q. Yao, Org. Lett., 2006, 8, 713 CrossRef CAS PubMed.
- K. D. Bodine, D. Y. Gin and M. S. Gin, J. Am. Chem. Soc., 2004, 126, 1638 CrossRef CAS PubMed.
- (a) P. Chattopadhyay and N. D. Adhikary, J. Org. Chem., 2012, 77, 5399 CrossRef PubMed; (b) L. Marmuse, S. A. Nepogodiev and R. A. Field, Org. Biomol. Chem., 2005, 3, 2225 RSC; (c) S. G. Agalave, S. R. Maujan and V. S. Pore, Chem.–Asian J., 2011, 6, 2696 CrossRef CAS PubMed; (d) W. Shi, R. S. Coleman and T. L. Lowary, Org. Biomol. Chem., 2009, 7, 3709 RSC; (e) I. Kumar, N. A. Mir, C. V. Rode and B. P. Wakhloo, Tetrahedron: Asymmetry, 2012, 23, 225 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra11035h |
| This journal is © The Royal Society of Chemistry 2014 |