A biodegradable thermosensitive hydrogel with tuneable properties for mimicking three-dimensional microenvironments of stem cells†
Abstract
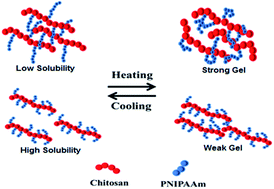
Employing stem cells in therapeutic applications strongly depends on the extracellular three-dimensional (3D) microenvironment and cell carrier properties. In this work, chitosan-g-poly(N-isopropylacrylamide) (CS-g-PNIPAAm) was synthesized as a stem cell mimicking microenvironment. The influence of various polymerization conditions, such as acid concentration, reaction temperature and monomer feed, on the grafting parameters of this thermo-responsive hydrogel, was systematically investigated. We found that the resulting copolymers with a small amount of long poly(N-isopropylacrylamide) (PNIPAAm) side chains are low-soluble at low temperatures, but can form stronger hydrogels (almost 5 folds) at high temperatures, whereas copolymers with a high amount of short PNIPAAm side chains are more soluble at low temperatures, however, they cannot form strong hydrogels at high temperatures. In a physiological pH, an optimized balance between the solubility (as the pre-requirement for cell dispersion and injectability) of copolymers at ambient temperature and enhanced gel mechanical strength (as the essential parameter of stem cell microenvironments) at body temperature can be achieved through controlled reaction conditions. Mesenchymal stem cells (MSCs) were cultured in the CS-g-PNIPAAm hydrogels. Further analysis of confocal images confirms MSCs can maintain their viability and increase the cellular biomass inside hydrogels. Sectional analysis demonstrates that cells are uniformly distributed within the hydrogels. Our results confirm that the CS-g-PNIPAAm with manipulated properties could provide a potential 3D microenvironment for stem cell culture, differentiation and in vivo injection.

 Please wait while we load your content...
Please wait while we load your content...