Mixed calcium and zinc salts of dicarboxylic acids derived from rosin and dipentene: preparation and thermal stabilization for PVC
Mei Li†
ac,
Jinwen Zhang*c,
Kun Huangab,
Shouhai Liab,
Jianchun Jianga and
Jianling Xia*ab
aInstitute of Chemical Industry of Forestry Products, CAF, Key Lab of Biomass Energy and Material, National Engineering Lab for Biomass Chemical Utilization, Key Lab on Forest Chemical Engineering, SFA, Nanjing 210042, Jiangsu Province, P. R. China. E-mail: xiajianling@126.com; Fax: +86 25 85482454; Tel: +86 25 85482453
bInstitute of Forest New Technology, CAF, Beijing 100091, P. R. China
cSchool of Mechanical and Materials Engineering, Materials Science and Engineering Program, Composite Materials and Engineering Center, Washington State University, Pullman, WA 99164, USA. E-mail: jwzhang@wsu.edu; Fax: +1-509-335-5077; Tel: +1-509-335-8723
First published on 19th November 2014
Abstract
Maleated dipentene (DPMA) and acrylopimaric acid (APA) were prepared by the Diels–Alder addition of dipentene with maleic anhydride and gum rosin with acrylic acid, respectively, and subsequently converted to the corresponding zinc salts (DPMA-Zn, APA-Zn) and calcium salts (DPMA-Ca, APA-Ca). Their chemical structures were confirmed by FT-IR analysis. The effects of the mixed DPMA-Ca/DPMA-Zn and APA-Ca/APA-Zn stabilizers on the PVC thermal stability were studied. In comparison, two commercial products, calcium stearate (CaSt2) and zinc stearate (ZnSt2), and two homemade salts of a dimer fatty acid (C36DA), zinc salt (C36DA-Zn) and calcium salt (C36DA-Ca), were also employed as controls in the study of PVC thermal stabilization. The thermal stability of PVC samples was determined using thermogravimetric analysis (TGA), Congo red test and discoloration test. Dynamic mechanical properties of the PVC compounds were also studied. The results showed that PVC compounds stabilized by the mixed DPMA-Ca/DPMA-Zn and APA-Ca/APA-Zn stabilizers displayed comparable modulus and glass transition temperatures but exhibited overall superior thermal stability compared with the CaSt2/ZnSt2 stabilized PVC.
1. Introduction
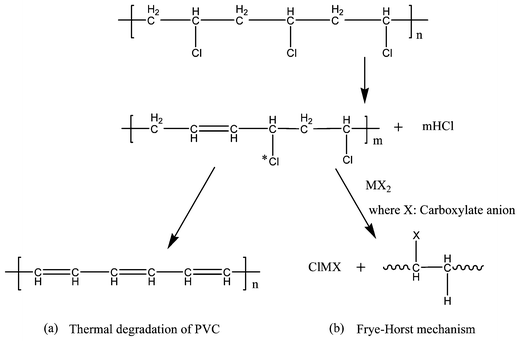
Poly(vinyl chloride) (PVC) is one of the most used thermoplastics and finds applications in both rigid and soft plastic products. It is well known that PVC is susceptible to degradation at elevated temperatures, giving off hydrochloric acid (HCl) that in turn accelerates the degradation process. Thermal degradation of PVC (Scheme 1(a)) occurs in a zipper elimination pattern which subsequently results in the formation of conjugated double bonds.1–3 With the elimination of one HCl molecule, an allylic chlorine structure is subsequently formed. This allylic chlorine (Scheme 1(a)) stimulates the next loss of an HCl molecule and this process repeats, leading to the chain or zip dehydrochlorination. The splitting-off of HCl from the polymer backbone affects the physical, chemical and mechanical properties of the PVC polymer.Until the discovery of thermal stabilizers, PVC was not a very useful industrial polymer because of the uncontrollable degradation at elevated temperatures. Technology advances in stabilizers, lubricants and processing have all contributed to today's huge production and wide application of PVC. Various thermal stabilizers have been designed to impart desired thermal stability to the final products. Now the main thermal stabilizers include lead salts, metal soaps and organotin compounds. Because the highly toxic lead salts pose serious concerns on environment and human health and organotin compounds are still expensive,4 industry gradually shifts its focus to the non-toxic and more economic calcium and zinc carboxylates.5,6
PVC processing stabilization can be gauged by short-term stability (or early color) and long-term stability (often called point-to-black).7 Another definition of short-term stability is “off-the-mill” color, and the long-term stability is the point at which the degradation either has made the compound no longer reclaimable or begins to endanger the processing equipment.7 Calcium soaps can impart long-term thermal stability to PVC products by absorbing HCl but they also tent to cause initial discoloration of PVC. In contrast, zinc soaps can effectively inhibit initial discoloration by substituting labile chlorine atoms in PVC chains but provide a poor long-term stability because of the phenomenon of “zinc burn”.8,9 By applying Ca and Zn thermal stabilizers together at an appropriate ratio, synergistic effect with both acceptable initial color and long-term stability for PVC products can be acheived.10–13 The use of metal mono-carboxylates as stabilizers for PVC has been extensively studied, including zinc, calcium, aluminium, barium, antimony, lithium, magnesium, sodium, potassium and cadmium stearates and laureates.9,11,12,14–16 Most of the mixed calcium and zinc thermal stabilizers derived from mono-carboxylates do not offer good long-term stability for PVC products. For example, the mixed calcium stearate (CaSt2)/zinc stearate (ZnSt2) thermal stabilizers could not provide excellent long-term thermal stability, whose static stability time was ∼23 min at 180 °C under the optimum Ca/Zn ratio.17 In order to have both excellent initial stabilization effect and long-term thermal stability, auxiliary thermal stabilizers such as pentaerythritol and organotin,18 epoxidized sunflower oil,19 β-diketone,20 polyols,21 hydrotalcites,22 zeolites23 and inorganic phosphates24 were combined with the mixed Ca/Zn stabilizer in attempt of improving the efficiency of stabilization. However, no satisfactory stabilization effects have been achieved by using the above auxiliary stabilizers.
According to the Frye–Horst mechanism (Scheme 1(b)), an esterification reaction takes place where the chlorine atom is bonded to the molecule in the presence of metal (M++) salt of fatty acid. Therefore, it can be inferred that the performance of thermal stabilizers is affected by the structure of carboxylate anion. Some studies introduced hydroxyl, aromatic and alicyclic groups into the structures of thermal stabilizers or used salts of polycarboxylic acids to offer better thermal stabilization and improved compatibility with PVC.14,25–28 It was noted that metal soaps of dicarboxylic acids were fairly heat stable and might be suitable as stabilizer of PVC.26
In this work, zinc and calcium thermal stabilizers were prepared from rosin and dipentene-derived dicarboxylic acids. Rosin has a fused-ring structure and dipentene a bridged ring structure, and both exhibit significant molecular rigidity comparable to that of aromatic compounds. Diels–Alder additions of dipentene with maleic anhydride and gum rosin with acrylic acid were performed, respectively, to give maleated dipentene (DPMA) and acrylopimaric acid (APA) which were subsequently converted to the corresponding zinc soaps (DPMA-Zn, APA-Zn) and calcium soaps (DPMA-Ca, APA-Ca) (Scheme 2). Thermal stabilizing effects of these salts for PVC were studied. For comparison, uses of a commercial mixed calcium stearate (CaSt2)/zinc stearate (ZnSt2) stabilizer and a homemade mixed zinc salt of dimer fatty acid (C36DA-Zn)/calcium salt of dimer fatty acid (C36DA-Ca) stabilizer (Scheme 2) as controls in PVC stabilization were also studied. The objective of this work is to demonstrate that the calcium and zinc salts of dicarboxylic acids derived from cyclic natural chemicals can offer better thermal stabilization for PVC than the fatty acid-derived counterparts.
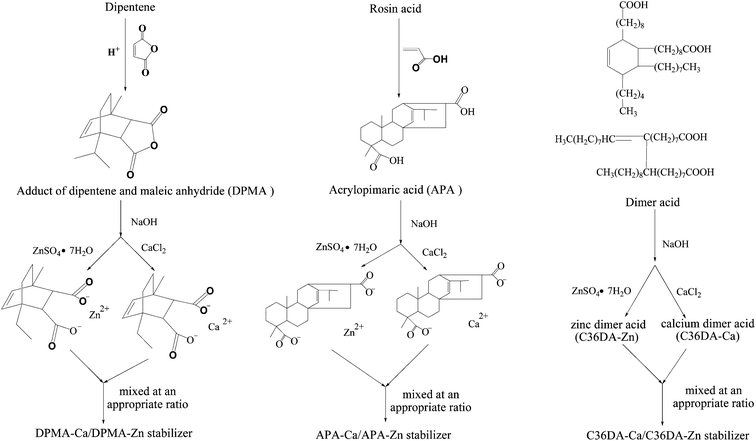 | ||
| Scheme 2 Synthesis routes of DPMA-Ca/DPMA-Zn stabilizer, APA-Ca/APA-Zn stabilizer and C36DA-Ca/C36DA-Zn stabilizer. | ||
2. Experimental
2.1 Materials
All materials were used as received. Gum rosin (acid value: 170 mg KOH/g) was obtained from Guangxi Wuzhou Richeng Chemical Company, China. Acrylic acid were obtained from Nanjing Chemical Reagent Co., Ltd, China. Maleic anhydride (95.0%) were purchased from Sigma-Aldrich. Technical grade dipentene (active ingredient contained limonene ≥90%) was purchased from Suzhou Synthetic Chemical Co., Ltd. Dimer fatty acid (hydrogenated, dimers ≥95%, monomers ≤1.5%, trimers ≤3.5%, acid value: 190 mg KOH per g) was obtained from Shanghai Guxiang Chemical Company. NaOH and anhydrous ethanol were purchased from Fisher Scientific. Zinc sulfate (ZnSO4·7H2O, 99.9%) and calcium chloride (purified) were purchased from J. T. Baker. PVC (DG-1000K) was purchased from Tianjin Dagu Chemical Co., Ltd., China. Bis (2-ethylhexyl) terephthalate (DOTP) were obtained from Acros Organics. Commercial calcium stearate (CaSt2, calcium content: 6.59%) and zinc stearate (ZnSt2, zinc content: 10.28%) were purchased from Xilong Chemical company, China.2.2 Synthesis
Calcium soaps were prepared by the following procedure. A sodium soap solution (0.1 mol salt in 100 mL water) was slowly added to 0.1 mol CaCl2 solution (in 100 mL ethanol and 100 mL deionized water). The reaction was continued at 70 °C for 3 h. The precipitated Ca soap was washed several times with deionized water, filtered and dried in an oven under 400 mm Hg vacuum at 70 °C for 24 h. Zinc soaps were prepared by reacting zinc sulphate (ZnSO4·7H2O) and sodium soaps with a similar procedure.
Characterizations. 1H-NMR spectra of the compouds in deuterated chloroform (CDCl3) were recorded using a Bruker 300 MHz Spectrometer at room temperature. Chemical shifts relative to that of chlorform (7.26) were reported.
X-ray diffraction (XRD) analysis was performed on a Y-4Q X-ray power diffractometer (Shimadzu 6000). The diffraction angle was swept from 10 to 80° using Cu-Paα ray source (λ = 0.154178 nm).
Fourier transform infrared (FTIR) spectra were recorded on a NEXUS 670 FTIR spectrometer in attenuated total reflectance mode. The sample was scanned from 4000 to 400 cm−1 with a resolution of 4 for 32 times.
The metal (Ca and Zn) content of the thermal stabilizer was measured using an Optima 7000 inductively coupled plasma-atomic emission spectrometer (ICP-AES) (PerkinElmer, America).
2.4 Preparation of PVC films
All PVC compounds were formulated on the same basis in terms of individual ingredients (Table 1). The PVC compounds were prepared as follows. First, PVC powder (100 g), DOTP (50 g) and thermal stabilizer (3.0 g, Ca soap/Zn soap = 4/1) were mixed using a mechanical mixer at room temperature for 5 min. Subsequently, the mixture was compounded into a homogeneous mixture at 165 °C for 3 min using a Haake torque rheometer (Thermo, Germany). The films with a thickness of ∼1.5 mm were made using a mini hot press (model 3912, Carver) at 170 °C.| Ingredients | Formulations | |||
|---|---|---|---|---|
| I | II | III | IV | |
| PVC | 100 | 100 | 100 | 100 |
| DOTP | 50 | 50 | 50 | 50 |
| CaSt2 + ZnSt2 | 2.4 g + 0.6 g | 0 | 0 | 0 |
| C36DA-Ca + C36DA-Zn | 0 | 2.4 g + 0.6 g | 0 | 0 |
| DPMA-Ca + DPMA-Zn | 0 | 0 | 2.4 g + 0.6 g | 0 |
| APA-Ca + APA-Zn | 0 | 0 | 0 | 2.4 g + 0.6 g |
2.5 Determinations of thermal and mechanical properties
The discoloration test of the stabilized PVC samples was performed according to the ISO 305:1990(E) standard. The PVC film with a thickness of ∼1 mm was cut into squares with a length of 15 mm, and then each square was wrapped individually using aluminium-foil paper. The empty test tubes were first placed in a temperature-controlled oil bath at 185 °C for 20 min, and then to each test tube was added one wrapped square strip. From this point, one test tube was removed from the oil bath every 10 min. The effect of stabilizer was evaluated by comparing color change of the heated PVC strips.
Thermal decomposition kinetics was studied using a Q600 TGA instrument (TA Instruments). Each sample was scanned from 30 to 400 °C at heating rates of 5, 10, 15, 20 and 25 °C min−1, respectively. The TGA experiment was performed under a nitrogen atmosphere (flow rate = 100 mL min−1). The kinetic parameters such as activation energy (E, KJ mol−1) and pre-exponential factor (A) were evaluated using Kissinger equation:
 | (1) |
| Ek = −aR | (2) |
 | (3) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ versus temperature curve.
δ versus temperature curve.3. Results and discussion
3.1 Zinc dicarboxylates and calcium dicarboxylates
Fig. 3 shows the FTIR spectra of zinc dicarboxylates (DPMA-Zn, APA-Zn, C36DA-Zn) and calcium dicarboxylates (DPMA-Ca, APA-Ca, C36DA-Ca). The absorption peaks at 1551, 1542, 1525, 1578, 1561, 1409 and 1445 cm−1 were due to the asymmetric stretching vibration of carboxylate. The absorption peaks at 1710 cm−1 in the FTIR spectrum of dimer fatty acid and 1693 cm−1 in the FTIR spectrum of APA, which were attributed to the carboxyls, almost disappeared in the spectra of APA-Zn, C36DA-Zn, APA-Ca and C36DA-Ca. Similarly, the absorption peaks at 1853 and 1773 cm−1 in the FTIR spectrum of DPMA, which were attributed to the anhydride, almost disappeared in the spetra of DPMA-Ca and DPMA-Zn. Ca and Zn contents in DPMA-Ca (12.7% Ca), DPMA-Zn (24.2% Zn), APA-Ca (10.0% Ca), APA-Zn (16.0% Zn), C36DA-Ca (6.29% Ca) and C36DA-Zn (9.35% Zn) were measured using ICP-AES. The FTIR and ICP-AES analyses suggested that APA, DPMA and dimer fatty acid were successfully converted to calcium and zinc soaps.Fig. 4 shows the XRD spectra of zinc dicarboxylates (DPMA-Zn, APA-Zn, C36DA-Zn) and calcium dicarboxylates (DPMA-Ca, APA-Ca, C36DA-Ca). In general, the XRD spectrum of a crystalline sample presents a series of sharp diffraction peak, while the XRD spectrum of an amorphous sample exhibits one or two broad diffuse X-ray peaks. Fig. 4 shows that C36DA-Ca and C36DA-Zn exhibited amorphous structures, while DPMA-Ca, DPMA-Zn, APA-Ca and APA-Zn demonstrated certain levels of crystalline structures. Further, the calcium salts of APA and DPMA exhibited slightly better crystalline structures.
3.2 Effects of various thermal stabilizers on the thermal stability of PVC
The films of four PVC compounds stabilized by different thermal stabilizers were heated at 185 °C for 30 min and then were examined for the change in microstructure using SEM. In Fig. 5, the micrographs of unheated samples indicates that the mixing was well done for each compound and the aggregation of the particles was not observed. The plasticizer DOTP seemly solvated the PVC and additive particles thoroughly. After heated, only the PVC sample with CaSt2/ZnSt2 (Fig. 5a1) shows some dark spots in the SEM image which was probably caused by thermal degradation, the rest samples still presented homogeneous micrographs. This result indicates the thermal stabilizers derived from diacids offered better thermal stability for PVC. | ||
| Fig. 5 SEM microphotographs of PVC samples with different stabilizers before and after heating test. | ||
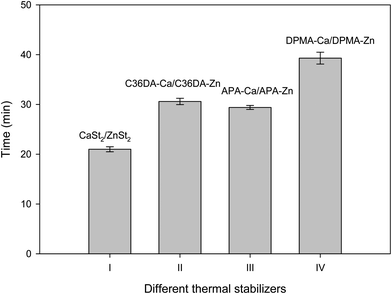
The static thermal stability time (Tss) were measured by the Congo red testing. Fig. 6 shows the effects of different stabilizers on Tss of the PVC compounds at 185 °C. The results indicate that DPMA-Ca/DPMA-Zn exhibited significantly higher long-term heat stability than other stabilizers. The Tss values of different formulations followed the order of DPMA-Ca/DPMA-Zn (48 min) > C36DA-Ca/C36DA-Zn (31 min) ≥ APA-Ca/APA-Zn (29 min 25 s) > CaSt2/ZnSt2 (20 min 55 s).
Table 2 shows the results of discoloration tests on the PVC strips containing different thermal stabilizers at 185 °C. The PVC strips containing CaSt2/ZnSt2 stabilizer exhibited excellent early color retention within 10 min but turned completely black within 30 min. In contrat, the PVC samples stabilized by the APA-Ca/APA-Zn stabilizer did not exhibit excellent early color retention but resulted in longer stability time compared with those stabilized by C36DA-Ca/C36DA-Zn and CaSt2/ZnSt2. This may be because the reactivity of the carboxylate anion of rosin acid was lower than that of the fatty acid in substituting the active chlorine atoms (Scheme 1(b)). Therefore, the activity of the carboxylate anion of the fused ring in the APA-Ca/APA-Zn thermal stabilizer was lower than that of the thermal stabilizer derived from C36DA, stearic acid and DPMA. However, zinc and calcium salts of rosin-derived dicarboxylic acid (APA) had higher metal ion contents than the C36DA and stearic acid-derived counterparts, which enabled APA-Ca/APA-Zn to be more effective in reducing HCl release within a specific degradation time when compared to theC36DA-Ca/C36DA-Zn ratio and CaSt2/ZnSt2. As a result, though APA-Ca/APA-Zn was not an excellent HCl scavenger in the early stage of PVC processing, it provided a long-term good thermal stability.
The calcium and zinc content of DPMA salts were higher than that of APA-Ca/APA-Zn thermal stabilizer and both controls. The carboxylate anion of the DPMA-Ca/DPMA-Zn thermal stabilizer probably had a comparable activity to that of the carboxylate anion of fatty acids. As a result, the PVC films stabilized by DPMA-Ca/DPMA-Zn exhibited the highest thermal stability than APA-Ca/APA-Zn thermal stabilizer and both controls. It can be seen that the PVC strip of DPMA-Ca/DPMA-Zn had the lightest color after 30 min and required the longest time (60 min) to be completely black.
The results of SEM, Congo test and discoloration test of PVC samples at 185 °C all indicate that the introduction of fused-ring and bridge-ring molecular into the structures of thermal stabilizers and use of polyacids offered better thermal stabilization than fatty acid in PVC application. At the same time the stabilizers containing a bridge-ring molecular structure had improved the initial color.
3.3 Thermal degradation kinetics
Fig. 7 shows the TGA and DTG curves of PVC compositions stabilized by different thermal stabilizers, while Fig. 8 displays the kinetic parameters. Compared with the two PVC compositions stabilized with the two controls (CaSt2/ZnSt2 and C36DA-Ca/C36DA-Zn), the PVC compound stabilized with APA-Ca/APA-Zn and DPMA-Ca/DPMA-Zn showed very similar thermal degradation temperatures. According to the Kissinger equation, the Ea values of different formulations followed the order of DPMA-Ca/DPMA-Zn (136.9 KJ mol−1) > APA-Ca/APA-Zn (113.0 KJ mol−1) > C36DA-Ca/C36DA-Zn (109.7 KJ mol−1) > CaSt2/ZnSt2 (101.4 KJ mol−1). The PVC sample containing DPMA-Ca/DPMA-Zn was found to have the highest activation energy of thermal degradation (Ea). Generally, a higher E suggests a higher resistance to thermal degradation. Therefore, the TGA results indicate that DPMA-Ca/DPMA-Zn was more effective on stabilizing PVC than that both the commercial thermal stabilizer and the APA-Ca/APA-Zn thermal stabilizer. | ||
| Fig. 8 Plots of ln(βi/Tp2) versus 1000/Tp and kinetic parameters for the PVC samples stabilized with four thermal stabilizers. | ||
3.4 Dynamic mechanical properties
Fig. 9 shows the changes of storage modulus (E′), loss modulus (E′′) and damping (tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ) of different PVC films with temperature. All compositions displayed a similar trend of E′, E′′ and tan
δ) of different PVC films with temperature. All compositions displayed a similar trend of E′, E′′ and tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ with temperature. Table 3 shows the Tg measured from the peak temperature of tan
δ with temperature. Table 3 shows the Tg measured from the peak temperature of tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ and E′ of different PVC samples at several temperatures. The APA-Ca/APA-Zn and DPMA-Ca/DPMA-Zn stabilized PVC exhibited slightly higher E′ than both controls, which was probably due to the more rigid fused ring and bridge-ring structures in APA-Ca/APA-Zn and DPMA-Ca/DPMA-Zn, respectively. In general, the dynamic mechanical properties of the PVC samples exhibited little dependence on the thermal stabilizer used, except for the one with C36DA-Ca/C36DA-Zn which exhibited lower storage modulus.
δ and E′ of different PVC samples at several temperatures. The APA-Ca/APA-Zn and DPMA-Ca/DPMA-Zn stabilized PVC exhibited slightly higher E′ than both controls, which was probably due to the more rigid fused ring and bridge-ring structures in APA-Ca/APA-Zn and DPMA-Ca/DPMA-Zn, respectively. In general, the dynamic mechanical properties of the PVC samples exhibited little dependence on the thermal stabilizer used, except for the one with C36DA-Ca/C36DA-Zn which exhibited lower storage modulus.
 | ||
Fig. 9 Storage modulus (E′), loss modulus (E′′) and tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ of the PVC samples with different thermal stabilizers. δ of the PVC samples with different thermal stabilizers. | ||
| Formulations | Tg (°C) | E′ at −50 °C (MPa) | E′ at −30 °C (MPa) | E′ at 30 °C (MPa) | E′ at 50 °C (MPa) |
|---|---|---|---|---|---|
| CaSt2/ZnSt2 | 31.9 | 1819.0 | 1581.7 | 96.8 | 36.5 |
| C36DA-Ca/C36DA-Zn | 33.6 | 1286.2 | 1042.2 | 47.8 | 15.2 |
| APA-Ca/APA-Zn | 35.5 | 1984.8 | 1629.0 | 68.4 | 25.2 |
| DPMA-Ca/DPMA-Zn | 31.5 | 1904.0 | 1567.6 | 95.4 | 35.3 |
4. Conclusions
Zinc and calcium salts of dipentene-derived anhydride (DPMA) and rosin-derived dicarboxylic acid (APA) were successfully prepared and had higher metal ion contents than that of dimer fatty acid (C36DA) and stearic acid (St) counterparts. DPMA-Ca/DPMA-Zn exhibited the highest thermal stabilizing effect among all four thermal stabilizers tested, while APA-Ca/APA-Zn also exhibited higher stabilizing effect than both controls (C36DA-Ca/C36DA-Zn and CaSt2/ZnSt2). The static stability time (Congo red test), discoloration time (discoloration test) and activation energy (TGA anaylsis) of the stabilized PVC compositions indicate the stabilizing effects of the four mixed Zn/Ca stabilizers followed the order of DPMA-Ca/DPMA-Zn > APA-Ca/APA-Zn ≥ C36DA-Ca/C36DA-Zn > CaSt2/ZnSt2, suggesting that the salts of dicarboxylic acids had higher stabilizing effect than that of monocarboxylic acid (St). The higher thermal stability of the PVC sample achieved with use of DPMA-Ca/DPMA-Zn and APA-Ca/APA-Zn was due to the presences of fused-ring bridge-ring structures in the molecules of the thermal stabilizers and relatively higher metal ion contents. The results from this study demonstrate that rosin acid and dipentene can be potential feedstocks for PVC thermal stabilizers.Acknowledgements
The authors are grateful for the financial support from the Basic research funding earmarked for the national commonweal research institutes, CAF (grant number: CAFYBB2012047) and Special funds of technology development research for the scientific research institutes “Technology research and development of bio-based phenol-free heat stabilizers for PVC”. The authors are also grateful for the technical support and facilities provided by the Composite Materials and Engineering Center at Washington State University.References
- P. Šimon, Polym. Degrad. Stab., 1990, 29, 155–163 CrossRef.
- R. Bacaloglu and M. Fisch, Polym. Degrad. Stab., 1994, 45, 301–313 CrossRef CAS.
- I. C. McNeill, L. Memetea and W. J. Cole, Polym. Degrad. Stab., 1995, 49, 181–191 CrossRef CAS.
- Y. J. Lin, J. R. Wang, D. G. Evans and D. Q. Li, J. Phys. Chem. Solids, 2006, 67, 998–1001 CrossRef CAS PubMed.
- Y. Z. Bao, Z. M. Huang, S. X. Li and Z. X. Weng, Polym. Degrad. Stab., 2008, 93, 448–455 CrossRef CAS PubMed.
- M. K. Naqvi, P. A. Unnikrishnan, Y. N. Sharma and I. S. Bhardwaj, Eur. Polym. J., 1984, 20, 95–98 CrossRef CAS.
- J. Edenbaum, in Mixed-metal stabilizers for polyvinyl chloride, Van Nostrand Reinhold Compary, New York, 1992, pp. 272–296 Search PubMed.
- G. Wypych, in Principles of stabilization, ChemTec Publishing, Toronto, 2nd edn, 2008, pp. 299–305 Search PubMed.
- K. B. Abbås and E. M. Sörvik, J. Vinyl Addit. Technol., 1980, 2, 87–94 CrossRef.
- W. Manzoor, S. M. Yousaf and Z. Ahmad, Polym. Degrad. Stab., 1996, 51, 295–299 CrossRef CAS.
- D. Balköse, H. I. Gökçel and S. E. Göktepe, Eur. Polym. J., 2001, 37, 1191–1197 CrossRef.
- G. Lévai, G. Ocskay, Z. Nyitrai and G. Meszlényi, Polym. Degrad. Stab., 1989, 25, 61–72 CrossRef.
- M. Beltrán, J. C. García and A. Marcilla, Eur. Polym. J., 1997, 33, 453–462 CrossRef.
- H. I. Gökçel, D. Balköse and U. Köktürk, Eur. Polym. J., 1999, 35, 1501–1508 CrossRef.
- S. Hollande and J. L. Laurnt, Polym. Degrad. Stab., 1997, 55, 141–145 CrossRef CAS.
- E. V. Farone, B. P. Labovitz and N. A. Kruse, US Pat., 20 130 137 806 A1, 2013.
- L. Fang, Y. H. Song, X. N. Zhu and Q. Zheng, Polym. Degrad. Stab., 2009, 94, 845–850 CrossRef CAS PubMed.
- M. Wang, J. Y. Xu, H. Wu and S. Y. Guo, Polym. Degrad. Stab., 2006, 91, 2101–2109 CrossRef CAS PubMed.
- M. T. Benaniba, N. Belhaneche-Bensemra and G. Gelbard, Polym. Degrad. Stab., 2003, 82, 245–249 CrossRef CAS.
- Y. S. Xue and H. J. Guo, Plastics Aids, 2006, 59, 16–19 Search PubMed.
- H. Ikeda, H. Goto, Y. Higaki, M. Sunami, K. Nakano, Y. Nakamura and T. lida, J. Appl. Polym. Sci., 2001, 79, 2029–2037 CrossRef CAS.
- E. V. Farone, B. P. Labovitz and N. A. Kruse, EP Pat., 0 256 872 B2, 1995.
- S. Atakul, D. Balköse and S. Ülkü, J. Vinyl Addit. Technol., 2005, 11, 47–56 CrossRef CAS.
- C. Razvan, R. Beck, A. Kurzinger, A. W. Purzer and M. Rosenthal, DE Pat., 3 941 902 C1, 1991.
- Y. B. Liu, W. Q. Liu and M. H. Hou, Polym. Degrad. Stab., 2007, 92, 1565–1571 CrossRef CAS PubMed.
- E. U. Ikhuoria, F. E. Okieimen and A. I. Aigbodion, J. Appl. Sci. Environ. Manage., 2005, 9, 127–130 Search PubMed.
- J. S. Kim, H. Kim, J. Yoon, K. Heo and M. Ree, J. Polym. Sci., Part A: Polym. Chem., 2005, 43, 4079–4088 CrossRef CAS.
- E. Ureta and M. E. Cantú, J. Appl. Polym. Sci., 2000, 77, 2603–2605 CrossRef CAS.
- J. N. Xin, P. Zhang, K. Huang and J. W. Zhang, RSC Adv., 2014, 4, 8525–8532 RSC.
- N. J. Halbrook and R. V. Lawrence, Ind. Eng. Chem. Prod. Res. Dev., 1972, 11, 200–202 CAS.
- K. Huang, J. W. Zhang, M. Li, J. L. Xia and Y. H. Zhou, Ind. Crops Prod., 2013, 49, 497–506 CrossRef CAS PubMed.
Footnote |
| † Mei Li is a visiting student at Washington State University. |
| This journal is © The Royal Society of Chemistry 2014 |