A ratiometric fluorescent probe for highly selective and sensitive detection of hypochlorite based on the oxidation of N-alkylpyridinium†
Lin Wang,
Lingliang Long*,
Liping Zhou,
Yanjun Wu,
Chi Zhang,
Zhixiang Han,
Junli Wang* and
Zulin Da
Scientific Research Academy & School of Chemistry and Chemical Engineering, Jiangsu University, Zhenjiang, Jiangsu 212013, P. R. China. E-mail: Longlingliang@163.com; Junleewang@yahoo.com; Fax: +86-511-88797815
First published on 31st October 2014
Abstract
A simple and efficient ratiometric fluorescent probe 1 for hypochlorite has been rationally constructed based on a novel hypochlorite promoted oxidation of N-alkylpyridinium reaction. Notably, this oxidation reaction was first employed for developing fluorescent hypochlorite probe. Upon addition of hypochlorite, probe 1 presented a ratiometric response, with the emission wavelength displaying a remarkable blue shift (up to 143 nm). Probe 1 also provided highly selective and sensitive response to hypochlorite. The detection limit was measured to be 0.093 μM. The sensing reaction product, compound 2, was isolated and confirmed by NMR spectra and mass spectrometry. TD-DFT calculation demonstrated that the intramolecular charge transfer process in compound 2 was significantly inhibited, which result in the large blue shift of emission. Probe 1 has been successfully applied for hypochlorite detection in natural water samples. Living cell imaging experiments established that probe 1 could detect not only artificially loaded but also endogenous hypochlorite in living cells.
Introduction
Reactive oxygen species (ROS) and reactive nitrogen species (RNS) play crucial roles in many physiological processes.1 Hypochlorite (OCl−), one of the most important ROS, is synthesized from peroxidation of chloride ions catalyzed by the enzyme myeloperoxidase (MPO).2 It acts as an effective antimicrobial agent in living organisms and plays a dominant role in immune systems.3 Nevertheless, excessive or misplaced production of OCl− in living systems may lead to extensive oxidative stress and oxidative damage, which can induce serious diseases such as arthritis,4 cardiovascular diseases,5 atherosclerosis,6 neuron degeneration,7 and cancer.8 On the other hand, OCl− is widely used in our daily lives, such as in household bleach, disinfection of drinking water, cooling-water treatment, and cyanide treatment. Typically, it is used in the concentration range of 10–104 μM.9 However, high concentrations of OCl− in solution are a potential health hazard to humans and animals.10 Thereby, the development of sensitive and selective methods for OCl− detection is urgently required.Among various detection techniques, the fluorescent probe, which features high sensitivity, simplicity and potential for in vivo imaging, has been regarded as the most promising technique for detection of OCl− in both biological and environmental system.11 In the past few years, a number of small-molecule fluorescent probes for OCl− have been constructed.12 And the corresponding design strategies are based on the oxidation reaction mediated by OCl−, including oxidation of p-methoxyphenol to benzoquinone,12a,b thioether to sulfonate,12c–e thioether to sulfoxide,12f–g dibenzoylhydrazine to dibenzoyldiimide,12h,i hydroxamic acid to acyl nitroso,12j selenide to selenoxide,12k–m cuprous ion to cupric ion,12n oxime to aldehyde,12o,p and others.13 Even though these oxidation-reaction based probes showed excellent selectivity for OCl− over other ROS/RNS, however, most of them responded to OCl− only relying on an increase or decrease in fluorescence intensity. As the change in fluorescence intensity is the only detection signal, factors such as environmental conditions, instrumental efficiency, and probe distribution can interfere with the signal output.14 A ratiometric fluorescent method provides an alternative approach, which can overcome the above factors by built-in correction of two emission bands.15 But unfortunately, up to now, very few examples of ratiometric fluorescent probe for OCl− have been constructed.16 These probes still suffered from severe limitations of working in high pH condition,16a,b long response time,16c complicated synthetic procedures,16d,e excitation at two wavelengths,16f or emission in the Ultraviolet region.16g These limitations seriously retarded the application of the probe in real biological and environmental samples. Thus, there is a great need to develop new ratiometric fluorescent probes for detection of OCl− with favorable characteristics using a novel design approach. In this work, we reported an efficient ratiometric fluorescent probe for OCl− based on a novel OCl− promoted oxidation of N-alkylpyridinium reaction. The probe can well address the existing issues, and has been successfully applied to detect OCl− in natural water samples. Moreover, bio-imaging studies demonstrated that the probe could detect not only artificially loaded but also endogenous OCl− in living cells.
Results and discussion
Design and synthesis
To rationally develop an efficient ratiometric probe for OCl−, a suitable oxidation reaction promoted by OCl− is in highly sought. It is known that the N-alkylpyridinium can be transformed into N-alkylpyridone by an oxidant (Fig. 1a).17 Although the mechanism has not been described in the literature until now, we anticipated that this oxidation reaction could be exploited as an interesting platform for ratiometric fluorescent OCl− probes. Therefore, we designed compound 1 as a candidate of ratiometric fluorescent probe for OCl− (Fig. 1b). In compound 1, the N-ethylpyridinium moiety was used as the recognition group, which is essentially conjugated with a 7-diethylamino-coumarin dye via a vinyl spacer. The selection of the 7-diethylamino-coumarin dye as fluorophore is due to that this dye possesses several favourable fluorescence properties such as high fluorescence quantum yield, absorption and emission spectra in the visible region. More importantly, it can be used as electron donor in an intramolecular charge transfer (ICT) based system.18 As the N-ethylpyridinium is a typical electron deficient group, thus upon excitation, strong ICT process in compound 1 will be preceded from the 7-diethylamino-coumarin dye to N-ethylpyridinium moiety. We envisioned that the N-ethylpyridinium group in compound 1 could be converted into N-ethylpyridone group by treating with OCl−, thereby compound 1 is transformed to 2. Due to the electron withdrawing ability of N-ethylpyridone group is much weaker than that of N-ethylpyridinium group, the ICT process in compound 2 will be dramatically inhibited, which results in a blue shift emission. Therefore, upon treatment with OCl−, compound 1 will show a substantial ratiometric response.Compound 1 can be conveniently synthesized by condensation of 7-diethylamino-coumarin aldehyde with N-ethyl-4-methyl-pyridium bromide under the catalysis of piperidine (see ESI†). The reference compound 3 was prepared by reaction of 7-diethylamino-coumarin aldehyde with 4-methylpyridine in the presence of p-toluenesulfonic acid. Structural identification of the compounds was confirmed by 1H NMR, 13C NMR, and ESI-MS spectroscopy.
Optical properties
The fluorescence emission spectra and absorption spectra of probe 1 were examined in 20 mM potassium phosphate buffer/THF (v/v 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, pH 7.4) at room temperature. Probe 1 displayed fluorescence emission (Φf = 0.38) centered at 631 nm, which is obvious red shift relative to the reference compound 3 (494 nm, Φf = 0.69) (Fig. S1†). As the electron withdrawing ability of N-ethylpyridinium moiety in probe 1 is stronger than that of pyridine moiety in compound 3, the drastic red shift emission for probe 1 is apparently attributed to the stronger ICT process from 7-diethylamino-coumarin dye to N-ethylpyridinium moiety. Additionally, the large red shift emission further implied that the variation of electron withdrawing ability of the electron acceptor in such probe system would afford obvious shift in emission wavelength. Thus, probe 1 could be used as promising ratiometric probe for OCl− providing that the strong electron withdrawing N-ethylpyridinium moiety in probe 1 can be converted to weak electron withdrawing N-ethylpyridone moiety by OCl−. The absorption spectra of probe 1 also exhibited red shift compared with compound 3 (Fig. S2†). The maximal absorption bands of probe 1 and compound 3 are centered at 496 and 430 nm respectively. The red shift in absorption spectra further demonstrated that the strong ICT process occurred from 7-diethylamino-coumarin dye to N-ethylpyridinium moiety.
3, pH 7.4) at room temperature. Probe 1 displayed fluorescence emission (Φf = 0.38) centered at 631 nm, which is obvious red shift relative to the reference compound 3 (494 nm, Φf = 0.69) (Fig. S1†). As the electron withdrawing ability of N-ethylpyridinium moiety in probe 1 is stronger than that of pyridine moiety in compound 3, the drastic red shift emission for probe 1 is apparently attributed to the stronger ICT process from 7-diethylamino-coumarin dye to N-ethylpyridinium moiety. Additionally, the large red shift emission further implied that the variation of electron withdrawing ability of the electron acceptor in such probe system would afford obvious shift in emission wavelength. Thus, probe 1 could be used as promising ratiometric probe for OCl− providing that the strong electron withdrawing N-ethylpyridinium moiety in probe 1 can be converted to weak electron withdrawing N-ethylpyridone moiety by OCl−. The absorption spectra of probe 1 also exhibited red shift compared with compound 3 (Fig. S2†). The maximal absorption bands of probe 1 and compound 3 are centered at 496 and 430 nm respectively. The red shift in absorption spectra further demonstrated that the strong ICT process occurred from 7-diethylamino-coumarin dye to N-ethylpyridinium moiety.
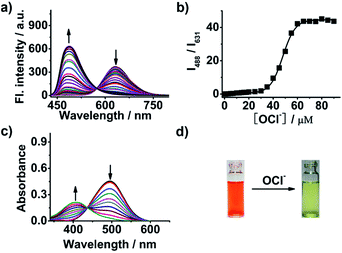
Optical response of probe 1 to OCl−
The fluorescence spectra changes of probe 1 in the presence of different concentrations of OCl− were investigated. Upon addition of OCl−, the fluorescence emission of probe 1 at 631 nm gradually decreased; concurrently, a new emission at 488 nm appeared and increased (Fig. 2a). A well-defined isoemission point was clearly found at 473 nm. These phenomena suggested that the N-ethylpyridinium moiety in probe 1 indeed can be converted into N-ethylpyridones by the oxidation of OCl−, which is in good agreement with the afore-mentioned design concept. Importantly, the emission wavelengths of probe 1 before and after addition of OCl− exhibited a very large blue shift (up to 143 nm). Such a large emission shift has been rarely achieved for the ratiometric OCl− probes reported to date. And the large emission shift makes the two emission peaks resolved well, which provides a good opportunity to conduct the ratiometric detection. Notably, the emission ratio (I488/I631) displayed a dramatic increase from 0.012 to 43.674 (3639-fold enhancement) after 90 μM of OCl− added (Fig. 2b). In addition, the emission ratios (I488/I631) also showed a good linearity with OCl− concentration in the range of 0–30.0 μM (Fig. S3†). The detection limit was measured to be 0.093 μM (Fig. S4†). The ratiometric response of probe 1 to OCl− also elicited the variation of fluorescence emission colors. After addition of OCl−, the visual fluorescence color of probe 1 varied from red to green (Fig. 1b). Moreover, probe 1 responded to OCl− rapidly. When 150 μM OCl− were introduced, the fluorescence intensity at 488 nm instantly increased and reached a plateau after 20 second reaction (Fig. S5†), and the pseudo-first-order rate constant (k′) was determined to be 15.50 min−1 (Fig. S6†). The ratiometric responses of probe 1 to OCl− at different pH values were investigated. When the OCl− was added, large enhancement of the emission ratio (I488/I631) were observed in the pH range of 5.36–10.5 (Fig. S7†). Therefore, probe 1 can function well in the physiological pH condition. Moreover, the larger ratiometric response of probe 1 to OCl− under basic conditions denoted that probe 1 detects OCl− rather than HOCl, as the pKa of HOCl is around 7.46.12o Probe 1 detection of OCl− in the solvents with different THF volume fraction was explored. After treating probe 1 with OCl−, little fluorescence enhancement at 488 nm was observed in the pure aqueous solution (Fig. S8†), similar to the reported results.19 However, with the increase of THF volume fraction in the testing solvent, larger fluorescence enhancement was noted. Thus, the response of probe 1 to OCl− is more sensitive in the solvent with higher THF volume fraction.The UV-Vis absorption spectra of probe 1 with various OCl− concentrations (0–90 μM) were investigated and shown in Fig. 2c. The free probe exhibits a broad absorption centered at 496 nm, which is ascribed to the ICT transition. However, when increasing concentration of OCl− was added, the absorption at 496 nm was gradually decreased with a new absorption peak appearing at 410 nm. Meanwhile, the visible solution color of probe 1 exhibited an obvious change from orange to yellow (Fig. 2d). The blue shift in UV-Vis absorption spectra confirmed that the ICT process in probe 1 was inhibited after reaction with OCl−.
Selectivity studies
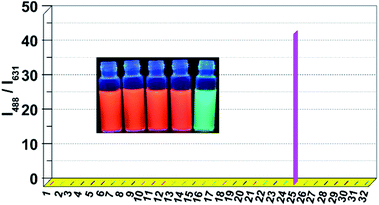
To study the selectivity of probe 1 to OCl−, the ratiometric response of probe 1 to various relevant species were studied. Fortunately, under the experiment condition, only OCl− caused significant enhancement of the emission ratio (I488/I631), while other ROS/RNS (H2O2, ˙OH, 1O2, and NO˙), anions (F−, Cl−, Br−, I−, ClO3−, ClO4−, CO32−, SO42−, NO3−, NO2−, SCN−, BF4−, CH3COO−, HSO3−), cations (Cu2+, Fe3+, Zn2+, Pb2+, Ni2+) and biologically relevant species (Gly, Val, Phe, Ala, Leu, Lys, glucose) induced no visible effect on the emission ratio of probe 1 (Fig. 3). Thus, probe 1 displayed selective response to OCl−. In addition, the visual fluorescence responses of probe 1 to various species demonstrated that the probe can be used conveniently for OCl− detection by naked eye under a normal UV lamp (Fig. 3 inset). The competition experiments were also carried out. Upon the addition of OCl− (90 μM) to a solution of probe 1 (10 μM) in the presence of other potentially competing species (90 μM) respectively, remarkable enhancements of the emission ratio (I488/I631) were observed (Fig. S9†). These findings suggested that probe 1 could be employed to detect OCl− selectively even in milieus that contain potential interferents.Reaction products of probe 1 with OCl−
As designed, the ratiometric response of probe 1 to OCl− is due to the N-ethylpyridinium moiety in probe 1 was oxidized to N-ethylpyridone by OCl−, and therefore probe 1 was transformed to compound 2. To confirm this, probe 1 was reacted with OCl−, and the reaction product was isolated for standard characterization. The 1H NMR spectra of probe 1 and the isolated product are shown in Fig. 4. The signals of the protons on carbon a and carbon b in probe 1 appear at 8.92 (Ha) and 8.09 (Hb) ppm, respectively. However, in the 1H NMR spectra of the isolated product 2, the signals at 8.92 and 8.09 ppm disappeared, and three new signals at 7.43, 7.43 and 6.98 ppm appeared, which were assigned to the protons on carbon a′, carbon i′ and carbon b′ in compound 2, respectively. Thus, the N-alkylpyridinium moiety in probe 1 was indeed converted to N-alkylpyridone by the oxidation of OCl−. The conversion of probe 1 to compound 2 by OCl− was further confirmed by ESI mass spectrometry, where a major peak at m/z 365.35 is assigned to [2 + H]+ (Fig. S11†). Another evidence for the N-ethylpyridinium moiety in probe 1 reaction with OCl− was provided from the optical response of reference compound 3 to OCl−. Compared with the molecule structure of probe 1, the pyridine moiety was instead of the N-ethylpyridinium moiety in compound 3. Upon addition of OCl−, the fluorescence emission and absorption spectra of compound 3 gave almost no changes (Fig. S12†), which is in sharp contrast with the response of probe 1 to OCl−. Together with these evidences, we can conclude that the N-ethylpyridinium moiety being oxidized to N-ethylpyridone was indeed responsible for the ratiometric response of probe 1 to OCl−. | ||
| Fig. 4 Partial 1H NMR (400 MHz) spectra of (1) probe 1, (2) the isolated product of probe 1 + OCl−. The solvent is CDCl3. | ||
Theoretical calculation
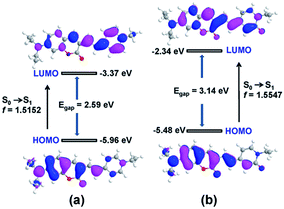
To get insight into the optical response of probe 1 to OCl−, probe 1 and compound 2 were examined by time-dependent density functional theory (TD-DFT) calculations at the B3LYP/6-31+G** level using Gaussian 09 program. The TD-DFT calculation indicated that the S0 → S1 (HOMO → LUMO) electronic transitions with oscillator strength f = 1.5152 and 1.5547 are identified as the allowable transitions of probe 1 and compound 2, respectively (Table S1†). Therefore, the HOMO–LUMO transitions contribute to the fluorescence for probe 1 and compound 2. The electron distributions in HOMO and LUMO of 1 and 2 are shown in Fig. 5. For probe 1, the HOMO is distributed primarily on 7-diethylamino-coumarin and vinyl moieties, whereas the LUMO is delocalized over the vinyl and N-ethylpyridinium moieties. Thus, it is clear that, upon excitation, the ICT process will take place from 7-diethylamino-coumarin to the N-ethylpyridinium moiety. However, for the compound 2, both the HOMO and LUMO are localized on the entire molecule (including the 7-diethylamino-coumarin moiety, vinyl moiety, and N-ethylpyridones moiety). Therefore, upon excitation, the ICT process between 7-diethylamino-coumarin and N-ethylpyridones moieties will be inhibited. Moreover, the energy gap between the HOMO and LUMO of 2 was larger than that of 1, in line with the blue shift of absorption and emission spectra of compound 2. Thus, based on the TD-DFT calculations, the optical properties of probe 1 and compound 2 have been theoretically revealed.Detection of OCl− in natural water samples
It is important to monitor the level of OCl− in natural water samples as the OCl− was often used in our daily lives. The water samples were obtained from Yangtzi River, pond water (from the campus of Jiangsu University) and tap water. After the probe treated with the water samples, ratiometic values were determined. The OCl− in Yangtzi River and pond water was not detected, while the concentration of OCl− in tap water was quantified to be 8.65 ± 0.08 μM. And then, the water samples were spiked with standard OCl− solution. The probe 1 was able to measure the concentrations of spiked OCl− with good recovery (Table S2†). Therefore, probe 1 can potentially be used for OCl− detection in natural water samples.Fluorescence imaging of OCl− in living cells
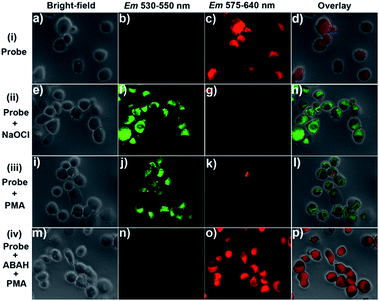
Next, we studied bio-imaging applications of probe 1 for OCl− detection in living cells. The probe was firstly used to track artificially loaded OCl− in RAW264.7 macrophage cells. The RAW264.7 cells were stained with probe 1 (1 μM) for 30 min, and then washed three times with phosphate-buffered saline (PBS). As illustrated in Fig. 6c, apparent fluorescence in the red channel could be observed. This indicated that probe 1 can easily penetrate the cells membrane and distribute throughout the cells. In addition, almost no fluorescence in the green channel could be found (Fig. 6b), denoting the probe was highly stable, and did not react with other species in living cells. However, when the probe 1-stained cells was further treated with NaOCl (20 μM) for 30 min, strong fluorescence was observed in the green channel (Fig. 6f), but only faint fluorescence in the red channel (Fig. 6g). These results suggested that probe 1 could selectively respond to artificially loaded OCl− inside cells.Inspired by the results of artificially loaded OCl− imaging, we further evaluated the feasibility of probe 1 to image the phorbol myristate acetate (PMA) induced endogenous OCl− in RAW264.7 cells. The PMA is known to activate the generation of ROS and RNS in macrophage cells, including OCl−.20 After stained with probe 1 for 30 min, the RAW264.7 cells were further stimulated with PMA (2.5 μg mL−1) for 2 h. Then the fluorescence images of the cells were recorded. As shown in Fig. 6j, strong fluorescence was observed in the green channel, and concurrently, the fluorescence in the red channel was dramatically decreased (Fig. 6k), implying the response of probe 1 to the endogenous OCl−. In a control experiment, the living RAW264.7 cells were stained with probe 1 for 30 min, and then the cells were incubated with 100 μM MPO inhibitor 4-aminobenzoic acid hydrazide (ABAH)21 for 30 min, the cells were further stimulated with PMA (2.5 μg mL−1) for 2 h. Almost no fluorescence can be found in the green channel (Fig. 6n), but strong fluorescence still existed in the red channel (Fig. 6o). Thus, the OCl− cannot be produced by the cells in the presence of the MPO inhibitor and the MPO is vital for the generation of endogenous OCl−. The experiments demonstrated that probe 1 is capable of selective imaging endogenous OCl− in living RAW264.7 cells.
The cytotoxicities of probe 1 to RAW264.7 macrophage cells were measured with the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay. As shown in Fig. S14,† the cell viabilities remained above 90% after incubation with 5 μM probe 1 for 24 h. Thus, the toxicity of probe 1 is low to the cultured cells.
Conclusions
In summary, we have developed a novel ratiometric fluorescent probe, compound 1, for OCl−. The probe was designed based on a novel N-alkylpyridinium oxidation chemistry where the N-alkylpyridinium is selectively oxidized to N-alkylpyridone by OCl−. It is worth noting that this oxidation reaction was firstly used for fluorescent OCl− probe design. Compared with the currently available OCl− ratiometric probes, the desirable features of probe 1 includes high selectivity and sensitivity, function well in physiological pH condition, simple synthetic procedure, fast response to OCl−, excitation and emission in the visible region, as well as large emission shift. The probe has been successfully applied to detect OCl− in natural water samples. Moreover, the fluorescence imaging studies demonstrated that the probe could detect not only artificially loaded but also endogenous OCl− in living cells. We expect that the probe will be a useful tool for assessing OCl− level in real environmental and biological samples. Moreover, the OCl− promoted oxidation of N-alkylpyridinium reaction will be widely applicable for developing other OCl− fluorescent probes.Acknowledgements
This research was supported by the National Natural Science Foundation of China (21202063, 21105038), the Ministry of Science and Technology of China for the International Science Linkages Program (2011DFG52970), the Ministry of Education of China for Changjiang Innovation Research Team (IRT1064), the Natural Science Foundation of Jiangsu Province (BK2012281), Jiangsu Innovation Research Team, and the Research Foundation of Jiangsu University (11JDG078).Notes and references
- (a) R. S. Balaban, S. Nemoto and T. Finkel, Cell, 2005, 120, 483–495 CrossRef CAS PubMed; (b) M. T. Lin and M. F. Beal, Nature, 2006, 443, 787–795 CrossRef CAS PubMed.
- J. E. Harrison and J. Schultz, J. Biol. Chem., 1976, 251, 1371–1374 CAS.
- Z. M. Prokopowicz, F. Arce, R. Biedron, C. L. Chiang, M. Ciszek, D. R. Katz, M. Nowakowska, S. Zapotoczny, J. Marcinkiewicz and B. M. Chain, J. Immunol., 2010, 184, 824–835 CrossRef CAS PubMed.
- M. J. Steinbeck, L. J. Nesti, P. F. Sharkey and J. Parvizi, J. Orthop. Res., 2007, 25, 1128–1135 CrossRef CAS PubMed.
- S. Sugiyama, K. Kugiyama, M. Aikawa, S. Nakamura, H. Ogawa and P. Libby, Arterioscler., Thromb., Vasc. Biol., 2004, 24, 1309–1314 CrossRef CAS PubMed.
- A. Daugherty, J. L. Dunn, D. L. Rateri and J. W. Heinecke, J. Clin. Invest., 1994, 94, 437–444 CrossRef CAS PubMed.
- D. I. Pattison and M. J. Davies, Chem. Res. Toxicol., 2001, 14, 1453–1464 CrossRef CAS PubMed.
- M. Benhar, D. Engelberg and A. Levitzki, EMBO Rep., 2002, 3, 420–425 CrossRef CAS PubMed.
- (a) T. Aokl and M. Munemorl, Anal. Chem., 1983, 55, 209–212 CrossRef; (b) J. G. March and B. M. Simonet, Talanta, 2007, 73, 232–236 CrossRef CAS PubMed.
- (a) R. B. R. Mesquita and A. O. S. S. Rangel, Talanta, 2005, 68, 268–273 CrossRef CAS PubMed; (b) L. C. Adam and G. Gordon, Anal. Chem., 1995, 67, 535–540 CrossRef CAS.
- (a) J. Du, M. Hu, J. Fan and X. Peng, Chem. Soc. Rev., 2012, 41, 4511–4535 RSC; (b) Y. Yang, Q. Zhao, W. Feng and F. Li, Chem. Rev., 2013, 113, 192–270 CrossRef CAS PubMed; (c) X. Chen, T. Pradhan, F. Wang, J. S. Kim and J. Yoon, Chem. Rev., 2012, 112, 1910–1956 CrossRef CAS PubMed.
- (a) Z. Sun, F. Liu, Y. Chen, P. K. H. Tam and D. Yang, Org. Lett., 2008, 10, 2171–2174 CrossRef CAS PubMed; (b) W. Zhang, C. Guo, L. Liu, J. Qin and C. Yang, Org. Biomol. Chem., 2011, 9, 5560–5563 RSC; (c) S. Kenmoku, Y. Urano, H. Kojima and T. Nagano, J. Am. Chem. Soc., 2007, 129, 7313–7318 CrossRef CAS PubMed; (d) Y. Koide, Y. Urano, K. Hanaoka, T. Terai and T. Nagano, J. Am. Chem. Soc., 2011, 133, 5680–5682 CrossRef CAS PubMed; (e) Q. A. Best, N. Sattenapally, D. J. Dyer, C. N. Scott and M. E. McCarroll, J. Am. Chem. Soc., 2013, 135, 13365–13370 CrossRef CAS PubMed; (f) T. Kim, S. Park, Y. Choi and Y. Kim, Chem.–Asian J., 2011, 6, 1358–1361 CrossRef CAS PubMed; (g) S. Liu, M. Vedamalai and S. Wu, Anal. Chim. Acta, 2013, 800, 71–76 CrossRef CAS PubMed; (h) X. Chen, X. Wang, S. Wang, W. Shi, K. Wang and H. Ma, Chem.–Eur. J., 2008, 14, 4719–4724 CrossRef CAS PubMed; (i) Y. Liu, Y. Sun, J. Du, X. Lv, Y. Zhao, M. Chen, P. Wang and W. Guo, Org. Biomol. Chem., 2011, 9, 432–437 RSC; (j) Y. Yang, H. J. Cho, J. Lee, I. Shin and J. Tae, Org. Lett., 2009, 11, 859–861 CrossRef CAS PubMed; (k) Z. Lou, P. Li, Q. Pan and K. Han, Chem. Commun., 2013, 49, 2445–2447 RSC; (l) S. Liu and S. Wu, Org. Lett., 2013, 15, 878–881 CrossRef CAS PubMed; (m) S. T. Manjare, J. Kim, Y. Lee and D. G. Churchill, Org. Lett., 2014, 16, 520–523 CrossRef CAS PubMed; (n) X. Lou, Y. Zhang, Q. Li, J. Qin and Z. Li, Chem. Commun., 2011, 47, 3189–3191 RSC; (o) G. Wu, F. Zeng and S. Wu, Anal. Methods, 2013, 5, 5589–5596 RSC; (p) X. Cheng, H. Jia, T. Long, J. Feng, J. Qin and Z. Li, Chem. Commun., 2011, 47, 11978–11980 RSC.
- (a) G. Li, D. Zhu, Q. Liu, L. Xue and H. Jiang, Org. Lett., 2013, 15, 2002–2005 CrossRef CAS PubMed; (b) Y. Xiao, R. Zhang, Z. Ye, Z. Dai, H. An and J. Yuan, Anal. Chem., 2012, 84, 10785–10792 CrossRef CAS PubMed; (c) L. Yuan, W. Lin, Y. Yang and H. Chen, J. Am. Chem. Soc., 2012, 134, 1200–1211 CrossRef CAS PubMed; (d) X. Chen, K. Lee, E. Ha, K. M. Lee, Y. Y. Seo, H. K. Choi, H. N. Kim, M. J. Kim, C. Cho, S. Y. Lee, W. Lee and J. Yoon, Chem. Commun., 2011, 47, 4373–4375 RSC; (e) J. Hwang, M. G. Choi, J. Bae and S. Chang, Org. Biomol. Chem., 2011, 9, 7011–7015 RSC; (f) T. Guo, L. Cui, J. Shen, R. Wang, W. Zhu, Y. Xu and X. Qian, Chem. Commun., 2013, 49, 1862–1864 RSC; (g) J. Shepherd, S. A. Hilderbrand, P. Waterman, J. W. Heinecke, R. Weissleder and P. Libby, Chem. Biol., 2007, 14, 1221–1231 CrossRef CAS PubMed; (h) F. Wei, Y. Lu, S. He, L. Zhao and X. Zeng, Anal. Methods, 2012, 4, 616–618 RSC; (i) Y. Zhou, J. Li, K. Chu, K. Liu, C. Yao and J. Li, Chem. Commun., 2012, 48, 4677–4679 RSC; (j) R. Zhang, B. Song, Z. Dai, Z. Ye, Y. Xiao, Y. Liu and J. Yuan, Biosens. Bioelectron., 2013, 50, 1–7 CrossRef PubMed.
- (a) K. Komatsu, Y. Urano, H. Kojima and T. Nagano, J. Am. Chem. Soc., 2007, 129, 13447–13454 CrossRef CAS PubMed; (b) D. Srikun, E. W. Miller, D. W. Domaille and C. J. Chang, J. Am. Chem. Soc., 2008, 130, 4596–4597 CrossRef CAS PubMed.
- (a) K. Kikuchi, H. Takakusa and T. Nagano, TrAC, Trends Anal. Chem., 2004, 23, 407–415 CrossRef CAS; (b) J. V. Mello and N. S. Finney, Angew. Chem., Int. Ed., 2001, 40, 1536–1538 CrossRef CAS.
- (a) W. Lin, L. Long, B. Chen and W. Tan, Chem.–Eur. J., 2009, 15, 2305–2309 CrossRef CAS PubMed; (b) L. Long, D. Zhang, X. Li, J. Zhang, C. Zhang and L. Zhou, Anal. Chim. Acta, 2013, 775, 100–105 CrossRef CAS PubMed; (c) J. Park, H. Kim, Y. Choi and Y. Kim, Analyst, 2013, 138, 3368–3371 RSC; (d) L. Yuan, W. Lin, Y. Xie, B. Chen and J. Song, Chem.–Eur. J., 2012, 18, 2700–2706 CrossRef CAS PubMed; (e) G. Chen, F. Song, J. Wang, Z. Yang, S. Sun, J. Fan, X. Qiang, X. Wang, B. Dou and X. Peng, Chem. Commun., 2012, 48, 2949–2951 RSC; (f) L. Yuan, W. Lin, J. Song and Y. Yang, Chem. Commun., 2011, 47, 12691–12693 RSC; (g) S. Goswami, S. Paul and A. Manna, Dalton Trans., 2013, 10097–10101 RSC.
- T. Fujii, S. Yoshifuji, K. Yoshida, M. Ohba, S. Ikegami and M. Kirisawa, Chem. Pharm. Bull., 1975, 23, 993–1002 CrossRef CAS.
- (a) X. Lv, J. Liu, Y. Liu, Y. Zhao, Y. Sun, P. Wang and W. Guo, Chem. Commun., 2011, 47, 12843–12845 RSC; (b) L. Long, X. Li, D. Zhang, S. Meng, J. Zhang, X. Sun, C. Zhang, L. Zhou and L. Wang, RSC Adv., 2013, 3, 12204–12209 RSC.
- J. Hou, J. Yang, K. Li, Y. Liao, K. Yu, Y. Xie and X. Yu, Chem. Commun., 2014, 50, 9947–9950 RSC.
- M. Wolfson, L. C. McPhail, V. N. Nasrallah and R. Snyderman, J. Immunol., 1985, 135, 2057–2062 CAS.
- S. Kumar, S. Patel, A. Jyoti, R. Keshari, A. Verma, M. Barthwal and M. Dikshit, Cytometry, Part A, 2010, 77, 1038–1048 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details; synthesis and characterization of compounds; spectroscopic data. See DOI: 10.1039/c4ra10633d |
| This journal is © The Royal Society of Chemistry 2014 |