Effect of sulphidation temperature on the performance of NiO–MoO3/γ-Al2O3 catalysts for sulphur-resistant methanation
Baowei Wang*,
Zongyuan Hu,
Sihan Liu,
Minhong Jiang,
Yuqin Yao,
Zhenhua Li and
Xinbin Ma*
Key Laboratory for Green Chemical Technology of the Ministry of Education, School of Chemical Engineering and Technology, Tianjin University, Tianjin 300072, China. E-mail: wangbw@tju.edu.cn
First published on 17th October 2014
Abstract
The effect of the sulphidation temperature on the activity and selectivity of a NiO–MoO3/γ-Al2O3 catalyst for sulphur-resistant methanation was systematically investigated. The prepared catalysts were subsequently characterised by N2-physisorption, temperature-programmed sulphidation, X-ray diffraction, Raman spectroscopy, X-ray photoelectron spectroscopy, and transmission electron microscopy. The results obtained from characterisation demonstrated that the NiMoO4 species in the NiO–MoO3/γ-Al2O3 catalyst was sulphided when the sulphidation temperature was at or above 300 °C. Evaluation of the catalysts in sulphur-resistant methanation from syngas indicated that the sample sulphided at 400 °C has the highest likelihood of possessing a greater NiMoS type I structure. The catalytic activity decreased when the sulphidation temperature was above 400 °C. This decrease was primarily caused by the formation of MoS2 crystals and the progressive transformation of the NiMoS phase with increasing sulphidation temperature. The NiMoS type II structure did not display good performance for sulphur-resistant methanation because it resulted in the over-sulphidation of the NiMoS structure to form crystalline MoS2, which exhibited lower methanation activity.
Introduction
Recently, the study and development of synthetic natural gas (SNG) production has attracted increasingly more attention and entered an era of rapid development due to environmental contamination and energy shortage crises.1 Notably, the production of SNG is a relatively reasonable and clean way to adequately utilise coal. Methanation is a key reaction for producing SNG from syngas. Transition metals, such as nickel, are commonly used as methanation catalysts because they possess high methanation activity. Not only does this type of catalyst show a high degree of CO conversion, but it also possesses good CH4 selectivity.2,3 The methanation reaction over Ni-based catalysts occurs as follows:| CO + 3H2 → CH4 + H2O | (1) |
As is evident in eqn (1), methanation reactions over Ni-based catalysts require a H2/CO molar ratio of no less than 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. However, for most coal gasification processes, it is almost impossible to generate syngas with such a high H2/CO ratio. Thus, the adjustment of the H2/CO ratio is inevitably performed via a water–gas shift (WGS) reaction in syngas:
1. However, for most coal gasification processes, it is almost impossible to generate syngas with such a high H2/CO ratio. Thus, the adjustment of the H2/CO ratio is inevitably performed via a water–gas shift (WGS) reaction in syngas:
| CO + H2O → CO2 + H2 | (2) |
| 2CO + 2H2 → CH4 + CO2 | (3) |
MoS2-based catalysts can significantly reduce the cost of methanation reactions from syngas and react at low H2/CO ratios (H2/CO = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Moreover, it is widely recognised that MoS2-based catalysts exhibit sulphur tolerance during methanation, whereas Ni-based catalysts are very sensitive to sulphur poisoning.4 Hence, this sensitivity leads to certain operating limitations of Ni-based catalysts, which also increases the need for MoS2-based catalysts in industry.
1). Moreover, it is widely recognised that MoS2-based catalysts exhibit sulphur tolerance during methanation, whereas Ni-based catalysts are very sensitive to sulphur poisoning.4 Hence, this sensitivity leads to certain operating limitations of Ni-based catalysts, which also increases the need for MoS2-based catalysts in industry.
To date, due to the specific properties of MoS2-based catalysts, such as high catalytic activity and sulphur-resistant ability, these catalysts have been broadly applied to various hydrotreatment reactions, such as hydrodesulphurisation, the Fischer–Tropsch synthesis, and alcohol synthesis.5–8 The active phase of the MoS2-based catalyst has been extensively recognised as MoS2, which is formed by sulphiding an oxidic precursor. Although the sulphidation of the oxidic precursor can occur under reaction conditions, its catalytic performance completely relies on the raw materials used and optimal operational conditions. For catalysts prepared from precursors containing oxygen, sulphidation plays a key role in determining the catalytic performances of such catalysts.9–11 It is therefore essential to adjust well-controlled sulphided conditions for the MoS2-based catalyst in order to obtain better catalytic performance.12 Following sulphidation, it must be determined whether molybdenum oxysulphide or MoS2 exist on the catalyst, as the species present is correlated with the activity. Hence, it is believed that Mo-based sulphides might be promising catalysts for methanation.
Unlike monometallic MoS2-based catalysts, bimetallic MoS2-based catalysts, such as CoO–MoO3/γ-Al2O3 and NiO–MoO3/γ-Al2O3, show higher catalytic activity in hydrotreating reactions.13–15 Sulphided Ni–Mo and Co–Mo catalysts supported on Al2O3 have been used extensively in several hydrotreating processes, such as hydrodesulphurisation (HDS), hydro-denitrogenation (HDN), hydrodeoxygenation (HDO), and hydrogenation (HYD). It is well accepted that the active component of hydrotreatment catalysts comprises nanosized MoS2 particles with cobalt or nickel atoms decorating their edges and corners;16 these particles form the Co(Ni)–Mo–S phase. Two types of the Co(Ni)–Mo–S phase have been described in the literature.17–19 Type I, characterised by incomplete sulphidation, is formed after low-temperature sulphidation; it has a lower degree of hydrodesulphurisation (HDS) activity than type II, which is formed after high-temperature sulphidation and is fully sulphided.
In our previous study,20 it was found that the CoO–MoO3/γ-Al2O3 catalyst sulphided at 400 °C exhibited the highest catalytic activity because it possessed the most CoMoS type I structures. A CoMoS type I structure, which is formed at low temperature, can transform into CoMoS type II when the sulphidation temperature increases. In addition, the CoMoS type II structure did not favor sulphur-resistant methanation reaction.
Although there are ample studies in the literature on the application of sulphided Ni–Mo catalysts in hydrotreating HDN reactions, no studies have focused on their ability to participate in sulphur-resistant methanation. In this work, we attempted to investigate the effects of the sulphidation temperature on the catalytic performance of NiO–MoO3/Al2O3 for sulphur-resistant methanation from syngas. The catalysts were sulphided at a range of temperatures; characterized by BET, TPS, XRD, RS, XPS and TEM; and evaluated in a fixed-bed reactor. In addition, the properties of as-prepared catalysts were further investigated, in order to understand the correlations between structure and activity.
Experimental
Catalyst preparation
A commercial γ-Al2O3 powder (Qianye Non-metallic Material Co. Ltd, China) was used as the catalyst support. The monometallic NiO/γ-Al2O3 and MoO3/γ-Al2O3 catalysts were prepared by the incipient wetness impregnation method. The bimetallic NiO–MoO3/γ-Al2O3 catalysts were prepared by the co-impregnation method. The γ-Al2O3 support was impregnated by aqueous solution of nickel nitrate hexahydrate (Ni(NO3)2·6H2O) (Kemiou Chemical Reagent Co. Ltd, Tianjin), aqueous solution of heptamolybdate tetrahydrate (NH4)6Mo7O24·4H2O (Kemiou Chemical Reagent Co. Ltd, Tianjin), mixed aqueous solution of heptamolybdate tetrahydrate and nickel nitrate hexahydrate, respectively. The samples were dried at room temperature for 48 h, subsequently dried at 120 °C for 4 h and calcined at 600 °C for 5 h with a heating rate of 5 °C min−1. According to our previous work,21,22 the amount of Ni oxides and Mo oxides in the catalyst were approximately 5.0 wt% and 25.0 wt% for three catalysts, respectively. It was found that the exact loadings of Ni and Mo measured by ICP (Varian-MPX) in the final catalyst were consistent with the amount estimated.Catalyst characterisation
N2 adsorption and desorption isotherms of the catalysts were obtained at −196 °C using a Tristar-3000 apparatus (Micro-meritics, United States) to determine the textural properties of the catalysts (i.e., specific surface areas and pore volumes). Each sample was degassed at 300 °C for 4 h before the adsorption measurement. The X-ray diffraction (XRD) pattern was obtained using a Rigaku D/max-2500 X-ray diffractometer (40 kV, 200 mA) with Ni-filtered Cu-Kα radiation (λ = 1.54056 Å). The scan speed was 5° min−1 with a scanning angle ranging from 5° to 90°. The phases were identified by comparison to powder diffraction data from the Joint Committee on Powder Diffraction Standards (JCPDS). The laser Raman spectra were obtained using an InVia-Reflex (Renishaw, England) laser Raman spectrometer, integrated with highly sensitive research-grade microscopes. The 532 nm emission line from the Ar+ ion laser (Spectra Physics) was employed for the incident light, and this 6 mW beam was focused on the samples using the system microscope. The laser beam intensity and the spectrum slit width were 8 mW and 25 μm, respectively. The samples were pressed into pellets for the measurements. X-ray photoelectron spectroscopy (XPS) analysis of all catalyst samples was performed using a PHI-1600 ESCA XPS system with monochromatic Mg–K radiation and a chamber pressure of 2 × 10−10 Torr. The binding energies were calibrated to the C 1s line at 284.6 eV. The peak areas were measured using a plani-metric technique that assumed a linear baseline. The morphology and structure of the catalysts were characterised via a Tecnai G2F20 (200 kV) high-resolution transmission electron microscope (TEM) (FEI, Holland), which has a maximum resolution of 0.15 nm/200 kV. For analysis, each sample was ultrasonically dispersed in ethanol and then deposited onto a copper grid.Sample sulphidation for characterisation
Sulphidation experiments were performed in a quartz tube reactor (inner diameter 10 mm and length 500 mm), similar to previous studies.20,23 In each experiment, the NiO–MoO3/γ-Al2O3 catalyst (3 mL) with a particle diameter between 0.43 mm to 0.85 mm was sandwiched between quartz fibres in the middle of the reactor and subjected to 3 vol% H2S in H2 (Dalian Date Gas Co. Ltd, China) at a flow rate of 100 mL min−1. For each sulphidation process, the catalysts were heated to the desired temperature at a rate of 3 °C min−1 and maintained at that temperature for 4 h. Afterward, catalysts were cooled down to room temperature under nitrogen atmosphere and stored in a vacuum dryer.Evaluation of catalytic activity
Catalytic performance was evaluated using a continuous-flow, fixed-bed reactor. The tubular, stainless steel reactor had an inner diameter of 12 mm and a length of 700 mm. The experimental apparatus has been described in detail in our previous study.24 Prior to the reaction, the catalyst (3 mL) was sulphided in situ at 400 °C for about 4 h in a 3 vol% H2S/H2 flow at atmospheric pressure. The following conditions were applied to evaluate the catalytic activity: syngas (H2/CO = 1.0) containing 0.12 vol% H2S and 20 vol% N2, a gas hourly space velocity of 5000 h−1, a temperature of 550 °C and a pressure of 3.0 MPa. The outlet gases were online analysed using an Agilent 7890A GC system equipped with two sets of TCD and one FID. The external standard method was used to calibrate the GC results and obtain the composition of each component in the outlet gases. The catalytic activity was represented by the conversion of CO and yield of CH4, which were obtained after 20 h reaction. The CO conversion, the CH4 selectivity, the CO2 selectivity, and CH4 yield were calculated using the following equations as previously shown:25
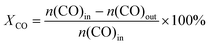 | (4) |
 | (5) |
 | (6) |
 | (7) |
| YCH4 = XCO × SCH4 × 100% | (8) |
Results and discussion
Effect of the sulphidation temperature on the textural and structural properties of NiO–MoO3/γ-Al2O3 catalysts
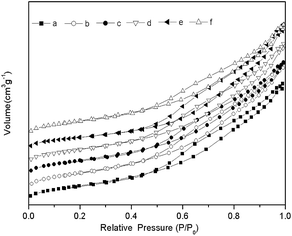
The BET surface area, pore volumes and the average pore diameter of the NiO–MoO3/γ-Al2O3 catalyst and its sulphided samples are given in Table 1. The BET surface area and pore volume of the sulphided samples were clearly smaller than those of non-sulphided sample. The average pore sizes of the sulphided samples were mainly larger than those of the non-sulphided sample, except for the sample sulphided at 400 °C. It was found that the BET specific surface area slightly decreased, while the particle size increased upon the sulphidation temperature. The reason for this could be attributed to blockage of the pores and the formation of bulk and crystalline MoS2 with the increase of sulphidation temperature. It could thus be deduced that the BET surface area generally decreased with increasing particle size. Hysteresis loops, presented in Fig. 1, were clearly observed for the NiO–MoO3/γ-Al2O3 catalyst and its sulphided samples. These hysteresis loops are typical type IV with H3 shape according to the IUPAC classification, which reveals a slit-shaped mesoporous structure. Furthermore, the hysteresis loops for all sulphided samples were quite similar to one another, which indicated that the structure of the samples was not destroyed during the sulphidation process.To understand the sulphidation conditions, the sulphidation behaviours of the NiO/γ-Al2O3, MoO3/γ-Al2O3 and NiO–MoO3/γ-Al2O3 catalysts were investigated via TPS techniques. Fig. 2 presents the TPS profiles of these catalysts. As shown in Fig. 2(a), there were two H2S consumption regions in the hydrogen sulphide consumption profile of the NiO/γ-Al2O3 sample. This result indicates that the Ni species would be sulphide until the sulphidation temperature was above approximately 320 °C. Bastow et al.21 reported that the Ni3S2 phase initially appeared, and then it gradually changed to the NiS phase with increasing sulphidation temperature. This was consistent with the second weak H2S consumption region, i.e., the Ni3S2 phase was gradually sulphided to NiS as the sulphidation temperature rose from 550 °C to 700 °C. As shown in Fig. 2(b), there were three H2S consumption regions for the MoO3/γ-Al2O3 catalyst. The first region, below 200 °C, was associated with a simple O–S exchange to form MoOxSy species.26,27 The second H2S consumption region (250–500 °C) was attributed to further sulphidation of MoOxSy to form a MoS2 structure. The last region (>550 °C) arose from the sulphidation of a Mo–O–Al structure, which indicated that the MoO3/γ-Al2O3 catalyst was completely sulphide.28 The TPS profile of the NiO–MoO3/γ-Al2O3 catalyst is displayed in Fig. 2(c). Three H2S consumption regions are clearly observed in the TPS profile of the NiO–MoO3/γ-Al2O3 catalyst, including H2S consumption regions of the NiO/γ-Al2O3 and MoO3/γ-Al2O3 catalysts during sulphidation. The sulphided regions are broader than those of the NiO/γ-Al2O3 and MoO3/γ-Al2O3 catalyst. This result could be attributed to the sulphidation of the Ni and Mo species in the catalyst. As reported in the literature,29 catalyst sulphidation occurs in three main stages: (1) initial exchange of surface oxygen for sulphur; (2) reduction of molybdenum accompanied by the release of hydrogen sulphide; and (3) slow sulphiding of the remaining oxygen. The results of the TPS profiles in this study were in good agreement with the sulphiding stages described above.
 | ||
| Fig. 2 TPS profiles of (a) NiO/γ-Al2O3 catalyst, (b) MoO3/γ-Al2O3, catalyst, and (c) NiO–MoO3/γ-Al2O3 catalyst. | ||
Fig. 3 presents the X-ray diffraction patterns of the NiO–MoO3/γ-Al2O3 samples sulphided at 200, 300, 400, 500, and 600 °C. For comparison, the XRD pattern of the non-sulphided sample is also included. Three primary γ-Al2O3 diffraction peaks were detected at 2θ = 37.5°, 45.7°, and 66.6°, which were also found in the XRD spectra of the sulphided samples. There was an additional feature observed at 2θ = 26.7°, which might be due to β-NiMoO4 (tetrahedral Mo coordination).30 The formation of these crystallites, which was caused by the interaction between the Ni and Mo species, was characterized by being highly dispersed on the support. In addition, no diffraction peaks corresponding to crystallographic MoO3 or nickel oxides were detected in the non-sulphided sample, which meant that molybdenum oxide and nickel oxide completely dispersed on the γ-Al2O3 support.24,31 When the catalyst was sulphided at 200 °C, there were no distinct diffraction peaks for the MoS2 phase, indicating no MoS2 crystal formation, i.e., the catalyst was not sulphided at 200 °C. Instead, weak diffraction peaks with 2θ at approximately 33° and 59° characteristic for the (100) and (110) planes of the hexagonal phase of MoS2 [PDF 37-1492] occurred after sulphidation at 300 °C. This result indicates that the oxidic precursor was initially sulphided at 300 °C, in accordance with the TPS results. Furthermore, an enhanced intensity of these diffraction lines occurred with the increase in sulphidation temperature, demonstrating that large MoS2 crystallites were formed as the sulphidation temperature increased. Moreover, a NiMoS4 phase was observed at 2θ = 38° for samples sulphided at temperature of 400 °C or above.32 It could be deduced that the NiMoS structure formed when the sulphidation temperature was 400 °C or above.
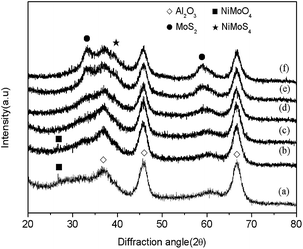 | ||
| Fig. 3 X-ray diffraction patterns of NiO–MoO3/γ-Al2O3 sulphided at different temperatures: (a) non-sulphided, (b) 200 °C, (c) 300 °C, (d) 400 °C, (e) 500 °C, and (f) 600 °C. | ||
In accordance with the XRD spectra, the laser Raman spectra of the NiO–MoO3/γ-Al2O3 catalyst and samples sulphided at different temperatures are illustrated in Fig. 4. The Raman spectrum of the NiO–MoO3/γ-Al2O3 catalyst (non-sulphided) revealed a dominant asymmetric feature at 961 cm−1, which was attributed to the β-NiMoO4 phases33 (Fig. 4(a)), and was in agreement with the XRD results in Fig. 3. In addition, the Raman bands at approximately 925 cm−1, 359 cm−1, 579 cm −1, and 227 cm−1 were associated with the symmetric stretching and bending modes of the terminal Mo![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond, and the Mo–O–Mo symmetric stretch and deformation modes of the amorphous octahedrally coordinated Mo6+(O) species, respectively.34–37 The intensity of these bands decreased with the increase of sulphidation temperature. With respect to the sulphided samples, a dominant peak at 961 cm−1, which was attributed to the NiMoO4 phases, was not found. This result was likely caused by the sulphidation of the NiMoO4 phases during the sulphidation process.
O bond, and the Mo–O–Mo symmetric stretch and deformation modes of the amorphous octahedrally coordinated Mo6+(O) species, respectively.34–37 The intensity of these bands decreased with the increase of sulphidation temperature. With respect to the sulphided samples, a dominant peak at 961 cm−1, which was attributed to the NiMoO4 phases, was not found. This result was likely caused by the sulphidation of the NiMoO4 phases during the sulphidation process.
 | ||
| Fig. 4 Laser Raman spectra of NiO–MoO3/γ-Al2O3 sulphided at different temperatures: (a) non-sulphided, (b) 200 °C, (c) 300 °C, (d) 400 °C, (e) 500 °C, and (f) 600 °C. | ||
The Raman bands at 383 cm−1, 408 cm−1, and 281 cm−1 are ascribable to the MoS2 phases.38,39 Additionally, the intensity of these bands gradually strengthened with the increase of sulphidation temperature. As the sulphidation temperature rose, there were increasingly more MoS2 phases formed. All of these phenomena were in agreement with XRD analysis. Notably, the Raman bands at 848 cm−1 and 1002 cm−1, corresponding to MoO3,40 were also found in these sulphided samples and not found in the non-sulphided samples. It was observed that the Raman bands at 848 cm−1 and 1002 cm−1 were overlapped by the dominant asymmetric feature at 961 cm−1. It was probable that exposure of the samples to air following sulphidation caused partial sulphided sample re-oxidation and thereby made it easy for MoO3 to form.
In addition, XPS experiments allowed evaluation of the part of Mo and Ni involved in the different phases encountered on the catalyst surface. For simplicity, the deconvolution of Mo 3d and Ni 2p3/2 spectra for only catalysts sulphided at three representative temperatures (200 °C, 400 °C, and 600 °C) were reported (Fig. 5 and 6). The deconvolution of Mo 3d spectra has been well documented.41–44 Molybdenum could exist as disulphide MoS2 (MoIV), where the doublet was located at 229.0 ± 0.1 eV (MoIV 3d5/2) and 232.1 ± 0.1 eV (MoIV 3d3/2). The existence of a Mo oxide phase (MoOx or NiMoO4, MoVI) was elucidated by two contributions located at 232.2 ± 0.1 eV (MoVI 3d5/2) and 235.3 ± 0.1 eV (MoVI 3d3/2). According to the literature, the last phase was an intermediate state Mo oxysulphide species (MoOxSy, MoV). For this species, the doublet appeared at 230.2 ± 0.1 eV (MoV 3d5/2) and 233.4 ± 0.1 eV (MoV 3d3/2). In addition, in the same part of the spectra, two bands appeared corresponding to the S 2s contributions that were decomposed in two different sulphur species (226.0 ± 0.1 eV and 227.6 ± 0.2 eV), which were attributed to the S2− (MoS2) and S22− (MoOxSy) species, respectively.45 These sulphur species must be subtracted from the total spectrum of Mo 3d. Proper deconvolution of the registered peaks provided the data that is summarized in Table 2. There was a significant influence of the sulphidation temperature on the distribution of Mo species. The highest atomic percentage of the (MoIV) sulphide phase and the lowest atomic percentages of the (MoV, MoVI) phases were obtained for the NiO–MoO3/γ-Al2O3 catalyst. This confirmed that the Mo contained in the catalyst was well sulphided at 600 °C, i.e., the sulphidation degree of NiO–MoO3/γ-Al2O3 was correlated with the sulphidation temperature.
 | ||
| Fig. 5 Deconvolution of XPS Mo 3d spectra of the catalysts sulphided at different temperatures: 200 °C (a), 400 °C (b), and 600 °C (c). | ||
 | ||
| Fig. 6 Deconvolution of XPS Ni 2p3/2 spectrum of the catalysts sulphided at different temperatures: 200 °C (a), 400 °C (b), and 600 °C (c). | ||
| ST (°C) | MoIV | MoV | MoVI | |||
|---|---|---|---|---|---|---|
| BE (eV) | % atom | BE (eV) | % atom | BE (eV) | % atom | |
| 200 | 229.2 | 22.3 | 230.2 | 33.1 | 232.4 | 44.6 |
| 400 | 229.1 | 56.5 | 230.3 | 24.3 | 232.5 | 19.2 |
| 600 | 229.3 | 84.1 | 230.2 | 10.3 | 232.4 | 5.6 |
For the analysis of surface Ni species, there were three major contributions that could have been responsible for the decomposition of the Ni 2p envelope: (i) the NiSx sulphided phase (which could arise from Ni2S3, Ni9S8, or NiS, with binding energy between 852.9 and 853.8 eV), (ii) oxidic nickel on the Al2O3 carrier (binding energy of 856.9 eV), and (iii) the NiMoS phase (with binding energy between 853.5 and 854 eV).46,47 The relative quantities of Ni species (NiII oxide, NiSx, and NiMoS) according to the nature of the support are reported in Table 3. A higher proportion of NiMoS phase was obtained on NiO–MoO3/γ-Al2O3 sulphided at 600 °C, in which 80.8% of the nickel atoms were engaged. This value was higher than those obtained for catalysts sulphided at the low temperature, demonstrating that NiMoO4 phases were transformed into NiMoS4 upon sulphidation.
| ST (°C) | NiMoS phase | NiSx phase | NiII phase | |||
|---|---|---|---|---|---|---|
| BE (eV) | % atom | BE (eV) | % atom | BE (eV) | % atom | |
| 200 | 853.5 | 46.6 | 852.9 | 15.9 | 855.8 | 37.5 |
| 400 | 853.4 | 67.4 | 852.5 | 14.5 | 855.9 | 18.1 |
| 600 | 853.5 | 80.8 | 852.8 | 11.4 | 855.4 | 7.8 |
The surface XPS atomic ratios of the sulphided samples are also compiled in Table 4. For sulphided catalysts, the Mo/Al ratios markedly decreased with the increase in sulphidation temperature, and a similar trend was observed for the Ni/Al ratios. The drastic decrease in both Mo/Al and Ni/Al ratios upon sulphidation suggested the formation of large sulphide particles at the surface of the aluminium oxide. The S/Al and S/(Ni + Mo) ratios substantially increased, however, as the sulphidation temperature rose, thus indicating that the degree of sulphidation depends, to a large extent, on the temperature. Furthermore, catalysts sulphided at 600 °C had higher S/(Ni + Mo) and S/Al ratios, which indicated a larger degree of sulphidation of these catalysts.
| ST (°C) | S/(Ni + Mo) | Mo/Al | Ni/Al | S/Al |
|---|---|---|---|---|
| 200 | 0.800 | 0.127 | 0.0403 | 0.134 |
| 400 | 1.80 | 0.101 | 0.0380 | 0.227 |
| 600 | 2.05 | 0.0974 | 0.0252 | 0.279 |
The HRTEM images of catalysts sulphided at different temperatures are shown in Fig. 7. Information is provided regarding the morphology of MoS2, the distribution of slab length (L), and the number of fringes (N). Fig. 7 presents images of typical crystallites of the NiO–MoO3/γ-Al2O3 phase sulphided at different temperatures. Obviously, no distinct stacked MoS2 crystals were found in the catalysts sulphided at or below 300 °C, which was in good agreement with the results of XRD and Raman spectroscopy. Nanosized spot-like entities were found to exist in these catalysts, and these entities were assigned to non-crystalline Mo oxysulfide particles.48 These entities were scarce in the catalysts sulphided above 300 °C, which was attributed to the high-temperature sulphidation of these entities. With the increase in sulphidation temperature, the micrographs consistently displayed increasingly more stripes that represented MoS2 phase. There were abundant black thread-like fringes corresponding to the MoS2 slabs with different stack height and length evenly distributed on the surface of Al2O3. In addition, the stacking number and length of stacked MoS2 crystals are presented in Table 5 by statistical analysis of the HRTEM images. For the catalyst sulphided at 300 °C, the average stack length was 2.14 nm, and the average stacking number was 1.73 layers. With a further increase in the sulphidation temperature, larger MoS2 particles were predominantly formed as seen in the case of catalysts sulphided at higher temperatures. The stacking number and length of stacked MoS2 crystallites increased with increasing sulphidation temperature. In addition, HRTEM showed that the MoS2 slabs for those catalysts sulphided at high temperature were larger and more stacked than those at low temperature, which suggested that less MoedgeIV active sites were available on the catalyst surface.43 The results of TEM demonstrated that the degree of sulphidation for MoO3 strengthened and large MoS2 phase crystallites formed, which were in good accordance with XRD and Raman results.
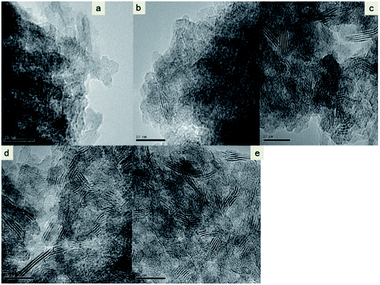 | ||
| Fig. 7 TEM images of the NiO–MoO3/γ-Al2O3 catalyst sulphided at (a) 200 °C, (b) 300 °C, (c) 400 °C, (d) 500 °C, and (e) 600 °C. | ||
| ST (°C) | Average length L (nm) | Average stacking number |
|---|---|---|
| 200 | — | — |
| 300 | 2.14 | 1.73 |
| 400 | 2.66 | 1.96 |
| 500 | 3.58 | 3.23 |
| 600 | 4.17 | 3.65 |
The sulphidation temperature was found to have a pronounced influence on both textural and structural properties of the NiO–MoO3/γ-Al2O3 catalysts. Furthermore, based on the results of TPS, XRD, Raman spectroscopy, and TEM, we divided the temperature range into two zones and will separately discuss the variations observed in the properties of the resulting sulphides.
(I) Sulphidation temperature below 400 °C: in this region, no significant influence on the texture of the NiO–MoO3/γ-Al2O3 sulphides is observed. Based on the results of the N2 adsorption–desorption (Table 1 and Fig. 1), the BET surface, pore volumes, and average pore sizes were essentially the same. As previously reported in the literature,49,50 β-NiMoO4, which was more easily converted into NiMoS active sites during the sulphidation process, was the precursor of the NiMoS structure. The XRD and RS results elucidated that NiMoO4 species existed in the NiO–MoO3/γ-Al2O3 catalyst. Therefore, the Ni species and Mo species could form a NiMoS structure during the sulphidation process. The NiMoS structure was formed by the Ni atom located on the edge of the MoS2 crystal.16 In other words, the formation of the MoS2 crystal was a prerequisite for the formation of the NiMoS structure. At low sulphide temperature, no NiMoS phase formed until the MoS2 particles were formed. The XRD and RS results demonstrated that the formation of MoS2 occurred when the sulphidation temperature was 300 °C. The TEM results indicated, however, that MoS2 crystals were visible in the catalyst until the sulphidation temperature reached 400 °C. Thus, it could be concluded that the NiO–MoO3/γ-Al2O3 catalyst underwents only partial sulphidation at 300 °C.
(II) Sulphidation temperature in the range of 400–600 °C: a drastic decrease in the specific surface area accompanied by a significant increase in the size of the MoS2 particles was observed in this temperature range (Table 1). XRD, Raman, and XPS analyses demonstrated that the intensity of sulphidation was progressively enhanced with the increase of sulphidation temperature. Previous reports in the literature stated that the type of NiMoS structures could be divided into NiMoS type I and NiMoS type II, which formed incomplete sulphidation and full sulphide, respectively.28 This demonstrated that the NiMoS type formation depended on the sulphidation temperature. According to the results of XPS, NiMoS phases were formed during the sulphidation process. A higher proportion of the NiMoS phase was obtained at higher sulphidation temperature. In addition, TEM revealed MoS2 slabs with significantly higher stacking and an average higher length as the sulphidation temperature rose. It was observed from a combination of XPS quantification and HRTEM micrographs that there were more and more stripes representing MoS2 as the temperature increased from 400 to 600 °C. This strongly suggested that NiMoS type II was formed at high sulphidation temperatures. It can be deduced that the NiMoS phase can be changed with the sulphidation temperature.
Effect of the sulphidation temperature on catalytic activity
The activities of the NiO–MoO3/γ-Al2O3 catalysts sulphided at different temperatures were tested, and the results are shown in Fig. 8. For comparison, the activity of the non-sulphided NiO–MoO3/γ-Al2O3 catalyst was also given. Even though the non-sulphided catalyst showed superior stability as compared to the other catalysts, it exhibited minimum CO conversion among these catalysts. This result indicates that the NiO–MoO3/γ-Al2O3 oxidic precursor did not possess better methanation activity and that sulphidation played a vital role in determining the catalytic performance of such a catalyst, as reported in the literature.10 When the sulphidation temperature was at or lower than 400 °C, the catalytic activities were greatly improved with increasing sulphidation temperature. The XRD and RS results showed that the NiMoO4 species was sulphided until the sulphidation temperature was above 300 °C. This illustrated that appropriately sulphided NiMoO4 species exhibited much better catalytic activity, which can be due to the formation of the NiMoS structure during the catalyst sulphidation. When the sulphidation temperature was above 400 °C, the catalytic activity of NiO–MoO3/γ-Al2O3 decreased with increasing sulphidation temperature. In addition, the CH4 yield exhibited the same trend as the CO conversion and reached the maximum value at the sulphidation temperature of 400 °C. However, the CO conversion gradually decreased as the reaction progressed, which might have been due to the aggregation of MoS2 nanoparticles and the formation of MoS2 crystallites during the methanation reaction in high temperature. As shown in Fig. 8, the selectivity of CH4, CO2, and C2H6 by several catalysts was more or less the same (the value changed less than 3%), i.e., the calcination temperature had no significant effect on the selectivity. In general, the NiO–MoO3/γ-Al2O3 catalyst sulphided at 400 °C exhibited the best catalytic activities for sulphur-resistant methanation among others, involving in the highest CO conversion and CH4 yield. | ||
| Fig. 8 CO conversion and selectivity of NiO–MoO3/γ-Al2O3 catalysts sulphided at different temperatures: (a) non-sulphided, (b) 200 °C, (c) 300 °C, (d) 400 °C, (e) 500 °C, and (f) 600 °C. | ||
As discussed above, the BET specific surface areas of the catalysts decreased with increasing sulphidation temperature. The decrease in BET specific surface is one of the factors leading to the decrease in catalytic activity, but not the key factor. In the NiMoS catalyst system, Ni atoms are generally incorporated into Mo vacancies at the edge locations of the MoS2 crystallites. Only the NiMoS structure, which was formed by interactions between the edge atoms of MoS2 and Ni atoms, has been reported to show catalytic activity, while Mo sites on the basal plane area were almost inactive.51 Moreover, the TEM results illuminated that the stacking number and the length of stacked MoS2 distinctly increased when the catalyst was sulphided at temperatures higher than 400 °C. The formation of MoS2 crystals decreased the edge atoms of MoS2 where the Ni atoms were located. The quantification of the active MoIV sites was achieved by XPS, and the morphology of MoS2 slabs was examined by TEM. XPS quantification revealed that the MoS2 slabs of catalysts sulphided at high temperature are longer, the stacking is higher, and apparent MoedgeIV active sites are relegated. This strongly suggested that NiMoS type II was formed at high sulphidation temperatures. Presumably, the active structure changed from NiMoS type I to NiMoS type II with increasing sulphidation temperature. Therefore, it could be speculated that the deterioration in the catalytic activity of NiO–MoO3/γ-Al2O3 sulphidation at higher temperatures occurred for the following reasons: (1) a decrease in the BET specific surface area; (2) an increase in the amount of stacked MoS2 crystals accompanied by a decrease in the MoedgeIV active sites; and (3) an increasingly progressive transformation of the NiMoS phase with the increase of sulphidation temperature.
In HDS reactions, the NiMoS type II structure that was formed at a high sulphidation temperature of 600 °C exhibited better catalytic activity than the NiMoS type I structure that formed at a sulphidation temperature of 400 °C. Nevertheless, in the sulphur-resistant methanation system, the catalyst sulphided at 400 °C had a higher catalytic activity than the catalyst sulphided at 600 °C. This result suggested that the active reaction centres for HDS and methanation were different. In addition, the combined results of XRD, RS, and TEM revealed that NiMoS type II formed at 600 °C with numerous crystalline MoS2 species. The crystalline MoS2 structure showed lower methanation activity. As a result, these phenomena indicated that the formation of a NiMoS type II structure did not favour a sulphur-resistant methanation reaction, similar to the CoMoS type II structure.
In agreement with a previous study,18 regardless of which promoter (cobalt or nickel) was used in the MoS2-based catalysts for methanation, the Co(Ni)–Mo–S type I structure exhibited higher catalytic activity, and formation of a Co(Ni)–Mo–S type II structure did not favour the sulphur-resistant methanation reaction. However, this result was completely different from the HDS reactions in which the Co(Ni)–Mo–S type II structure formed at high sulphidation temperatures displayed catalytic activity approximately twice as high as the Co(Ni)–Mo–S type I structure.
Conclusions
The effect of sulphidation temperature on the performance of the NiO–MoO3/γ-Al2O3 catalyst toward sulphur-resistant methanation was investigated in this study. The present work led to the following main conclusions: (1) the NiO–MoO3/γ-Al2O3 catalyst possessed higher catalytic activity after the sulphidation of NiMoO4, and the best sulphidation temperature for the NiO–MoO3/γ-Al2O3 catalyst was approximately 400 °C; (2) when the sulphidation temperature was higher than 400 °C, the activity of the NiO–MoO3/γ-Al2O3 catalyst decreased with increasing sulphidation temperature, which was attributed to the transformation of the NiMoS phase that progressively increases with sulphidation temperature and the increase of stacked MoS2 crystals that accompanied less MoedgeIV active sites; (3) the NiMoS type II structure did not display good performance for sulphur-resistant methanation because the formation of this species accompanied crystalline MoS2 in catalysts, which showed lower methanation activity.Notes and references
- A. L. Kustov, A. M. Frey, K. E. Larsen, T. Johannessen, J. K. Nørskov and C. H. Christensen, Appl. Catal., A, 2007, 320, 98–104 CrossRef CAS PubMed.
- I. Chen and D. W. Shiue, Ind. Eng. Chem. Res., 1988, 27, 1391–1396 CrossRef CAS.
- A. V. Pashigreva, G. A. Bukhtiyarova, O. V. Klimov, Y. A. Chesalov, G. S. Litvak and A. S. Noskov, Catal. Today, 2010, 149, 19–27 CrossRef CAS PubMed.
- J. Happel, M. Yoshikiyo, F. S. Yin, M. Otarod, H. Y. Cheh, M. A. Hnatow, L. Bajars, H. S. Meyer and I. Chen, Ind. Eng. Chem. Prod. Res. Dev., 1986, 25, 214–219 CrossRef CAS.
- Y. Okamoto, S. y. Ishihara, M. Kawano, M. Satoh and T. Kubota, J. Catal., 2003, 217, 12–22 CAS.
- P. Afanasiev, J. Catal., 2010, 269, 269–280 CrossRef CAS PubMed.
- M. Nagai and K. Matsuda, J. Catal., 2006, 238, 489–496 CrossRef CAS PubMed.
- G. Z. Bian, F. Li, Y. L. Fu and K. Fujimoto, Appl. Catal., A, 1998, 170, 255–268 CrossRef CAS.
- P. Arnoldy, J. A. M. van den Heijkant, G. D. de Bok and J. A. Moulijn, J. Catal., 1985, 92, 35–55 CrossRef CAS.
- X. R. Shi, J. G. Wang and K. Hermann, J. Phys. Chem. C, 2010, 114, 6791–6801 CAS.
- M. de Boer, A. J. van Dillen, D. C. Koningsberger and J. W. Geus, J. Phys. Chem., 1994, 98, 7862–7870 CrossRef CAS.
- P. J. Mangnus, E. K. Poels and J. A. Moulijn, Ind. Eng. Chem. Res., 1993, 32, 1818–1821 CrossRef CAS.
- Y. E. Licea, S. L. Amaya, A. Echavarría, J. Bettini, J. G. Eon, L. A. Palacio and A. C. Faro, Catal. Sci. Technol., 2014, 4, 1227 CAS.
- I. V. Deliy, E. N. Vlasova, A. L. Nuzhdin, E. Y. Gerasimov and G. A. Bukhtiyarova, RSC Adv., 2014, 4, 2242 RSC.
- C. Bouvier, Y. Romero, F. Richard and S. Brunet, Green Chem., 2011, 13, 2441–2451 RSC.
- N. Y. Topsøe and H. Topsøe, J. Catal., 1983, 84, 386–401 CrossRef.
- H. Topsøe and B. S. Clausen, Catal. Rev.: Sci. Eng., 1984, 26, 395–420 Search PubMed.
- H. Topsøe and B. S. Clausen, Appl. Catal., 1986, 25, 273–293 CrossRef.
- H. Topsøe, B. S. Clausen, R. Candia, C. Wivel and S. Mørup, J. Catal., 1981, 68, 433–452 CrossRef.
- M. H. Jiang, B. W. Wang, Y. Q. Yao, Z. H. Li, X. B. Ma, S. D. Qin and Q. Sun, Catal. Sci. Technol., 2013, 3, 2793–2800 CAS.
- B. Wang, G. Ding, Y. Shang, J. Lv, H. Wang, E. Wang, Z. Li, X. Ma, S. Qin and Q. Sun, Appl. Catal., A, 2012, 431–432, 144–150 CrossRef CAS PubMed.
- M. Jiang, B. Wang, Y. Yao, Z. Li, X. Ma, S. Qin and Q. Sun, Catal. Sci. Technol., 2013, 3, 2793 CAS.
- M. H. Jiang, B. W. Wang, J. Lv, H. Y. Wang, Z. H. Li, X. B. Ma, S. D. Qin and Q. Sun, Appl. Catal., A, 2013, 466, 224–232 CrossRef CAS PubMed.
- B. W. Wang, G. Z. Ding, Y. G. Shang, J. Lv, H. Y. Wang, E. D. Wang, Z. H. Li, X. B. Ma, S. D. Qin and Q. Sun, Appl. Catal., A, 2012, 431–432, 144–150 CrossRef CAS PubMed.
- M. Jiang, B. Wang, Y. Yao, H. Wang, Z. Li, X. Ma, S. Qin and Q. Sun, Appl. Catal., A, 2014, 469, 89–97 CrossRef CAS PubMed.
- T. F. Hayden and J. A. Dumesic, J. Catal., 1987, 103, 366–384 CrossRef CAS.
- J. C. Muijsers, T. Weber, R. M. Vanhardeveld, H. W. Zandbergen and J. W. Niemantsverdriet, J. Catal., 1995, 157, 698–705 CrossRef CAS.
- J. A. R. van Veen, H. A. Colijn, P. A. J. M. Hendriks and A. J. van Welsenes, Fuel Process. Technol., 1993, 35, 137–157 CrossRef CAS.
- P. Zeuthen, P. Blom, B. Muegge and F. E. Massoth, Appl. Catal., 1991, 68, 117–130 CrossRef CAS.
- F. J. Maldonado-Hódar, L. M. P. Madeira and M. F. Portela, J. Catal., 1996, 164, 399–410 CrossRef.
- F. Y. A. El Kady, S. A. Shaban and A. O. Abo El Naga, Transition Met. Chem., 2011, 36, 237–244 CrossRef CAS.
- X. Q. Wang and U. S. Ozkan, J. Mol. Catal. A: Chem., 2005, 232, 101–112 CrossRef CAS PubMed.
- P. Dufresne, E. Payen, J. Grimblot and J. P. Bonnelle, J. Phys. Chem., 1981, 85, 2344–2351 CrossRef CAS.
- G. Mestl, J. Mol. Catal. A: Chem., 2000, 158, 45–65 CrossRef CAS.
- H. C. Hu and I. E. Wachs, J. Phys. Chem., 1995, 99, 10911–10922 CrossRef CAS.
- D. S. Kim, K. Segawa, T. Soeya and I. E. Wachs, J. Catal., 1992, 136, 539–553 CrossRef CAS.
- G. Mestl and T. K. K. Srinivasan, Catal. Rev.: Sci. Eng., 1998, 40, 451–570 CAS.
- S. L. González-Cortés, T. C. Xiao, P. M. F. J. Costa, B. Fontal and M. L. H. Green, Appl. Catal., A, 2004, 270, 209–222 CrossRef PubMed.
- C. Lamonier, C. Martin, J. Mazurelle, V. Harlé, D. Guillaume and E. Payen, Appl. Catal., B, 2007, 70, 548–556 CrossRef CAS PubMed.
- A. N. Desikan, L. Huang and S. T. Oyama, J. Phys. Chem., 1991, 95, 10050–10056 CrossRef CAS.
- L. Fang, S. P. Xu, L. Cao, Y. Chi, T. Zhang and D. F. Xue, J. Phys. Chem. C, 2007, 111, 7396–7402 Search PubMed.
- J. C. M. Th. Weber, J. H. M. C. van Wolput, C. P. J. Verhagen and J. W. Niemantsverdriet, J. Phys. Chem., 1996, 100, 14144–14150 CrossRef.
- T. K. T. Ninh, L. Massin, D. Laurenti and M. Vrinat, Appl. Catal., A, 2011, 407, 29–39 CrossRef CAS PubMed.
- R. Hernández-Huesca, J. Mérida-Robles, P. Maireles-Torres, E. Rodríguez-Castellón and A. Jiménez-López, J. Catal., 2001, 203, 122–132 CrossRef.
- S. W. A. Galtayries and J. Grimblot, J. Electron Spectrosc. Relat. Phenom., 1997, 87, 31–44 CrossRef.
- B. Guichard, M. Roy-Auberger, E. Devers, C. Legens and P. Raybaud, Catal. Today, 2008, 130, 97–108 CrossRef CAS PubMed.
- J. Escobar, M. C. Barrera, J. A. Toledo, M. A. Cortés-Jácome, C. Angeles-Chávez, S. Núñez, V. Santes, E. Gómez, L. Díaz, E. Romero and J. G. Pacheco, Appl. Catal., B, 2009, 88, 564–575 CrossRef CAS PubMed.
- H. R. Reinhoudt, A. D. van Langeveld, P. J. Kooyman, R. M. Stockmann, R. Prins, H. W. Zandbergen and J. A. Moulijn, J. Catal., 1998, 179, 443–450 CrossRef CAS.
- J. L. Brito and J. Laine, Appl. Catal., 1991, 72, L13–L15 CrossRef CAS.
- S. Chaturvedi, J. A. Rodriguez and J. L. Brito, Catal. Lett., 1998, 51, 85–93 CrossRef CAS.
- J. G. Kushmerick and P. S. Weiss, J. Phys. Chem. B, 1998, 102, 10094–10097 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2014 |