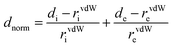Synthesis, crystal structures and phase transformation of the new solid-state forms of tetrandrine†
Zhengzheng Zhoua,
Henry H. Y. Tong*b,
Liang Lic,
Fanny L. Y. Shekd,
Yang Lve and
Ying Zheng*a
aState Key Laboratory of Quality Research in Chinese Medicine, Institute of Chinese Medical Sciences, University of Macau, Macao SAR, China. E-mail: yzheng@umac.mo; Fax: +86 853 28841358; Tel: +86 853 88224687
bSchool of Health Sciences, Macao Polytechnic Institute, Macao SAR, China. E-mail: henrytong@ipm.edu.mo; Fax: +86 853 28753159; Tel: +86 853 83998632
cDepartment of Forensic Medicine, Zhongshan School of Medicine, Sun Yat-sen University, Guangzhou, China
dMaterial Characterization and Preparation Facility, Hong Kong University of Science and Technology, Hong Kong SAR, China
eBeijing Key Laboratory of Polymorphic Drugs, Institute of Materia Medica, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China
First published on 14th November 2014
Abstract
In this study, the solid-state forms and phase transformation of tetrandrine (Tet) were investigated and characterized using X-ray diffraction and thermal analysis. Crystal structures of form I, acetonitrile and acetone solvates were elucidated using single-crystal X-ray diffraction. Form I and the acetonitrile solvate were both determined to crystallize in the orthorhombic space group P212121 with Z′ = 1. The unit cell parameters were a = 7.12371 (10) Å, b = 11.92092 (16) Å, and c = 38.3027 (2) Å for form I; a = 9.70383 (10) Å, b = 15.06419 (15) Å, and c = 26.1648 (3) Å for the acetonitrile solvate, which present obvious differences. The acetone solvate was found to crystallize in the monoclinic space group P21 with Z′ = 2. The unit cell parameters were a = 18.7432 (5) Å, b = 9.9123 (3) Å, and c = 19.3410 (6) Å for the acetone solvate. Significant differences are observed among the host molecular skeletons of these three crystal structures from the overlay diagrams. The crystal voids and Hirshfeld surface 2D fingerprint plots were calculated for the first time. The solvatomorphic forms have better dissolution than form I.
1. Introduction
Different polymorphs, hydrates and solvates of certain drug substance are believed to have diverse physiochemical, thermal and mechanical properties. For BCS II class compounds, these properties may further influence their oral bioavailability. Hence, it is important and valuable to understand the polymorphic properties of promising drugs to avoid problems caused by polymorphic transitions during processing.1–3This study focuses on a drug with poor-solubility and -bioavailability called tetrandrine (Tet), which is a bisbenzylisoquinoline alkaloid. It is the major bioactive component isolated from the root of Stephania tetrandra S. Moore, which has been used to treat hypertension and inflammation in China for many years.4,5 It was also reported to be a nonselective calcium channel blocker and multiple-drug-resistant protein antagonist.6–12 Tetrandrine in tablet form is a prescribed drug in China to treat rheumatism and analgesia.13 In general, a large dose (6–15 tablets per day) is required for current commercial tablets because of its poor solubility in water (0.46 μg mL−1), moderate lipophilicity (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P = 2.5 at pH = 7.0), low and variable oral bioavailability.14
P = 2.5 at pH = 7.0), low and variable oral bioavailability.14
Although several formulations have been developed to solve the poor solubility and bioavailability including phospholipid complex and solid lipid nanoparticles,14,15 there has been no detailed investigation on the solid-state properties of Tet, which may account for its poor aqueous solubility and oral absorption.16 To the best of our knowledge, only one single crystal of Tet has been reported.17 However, no detailed solid-state study has been performed to elucidate the polymorphism and solvate forms of Tet. In the present study, a polymorphic landscape study has been performed on Tet to reveal its potential solid-state forms. The crystal structures, crystal voids and phase transformation among different solid-state forms are discussed in detail.
2. Experimental section
2.1 Materials
Tetrandrine raw material (purity 98.0%) was purchased from Chengdu Preferred Biological Technology Co., LTD. (Chengdu, China). Sodium dodecyl sulfate (SDS) and solvents in this study were purchased from Merck (Darmstadt, Germany) with analytical reagent quality. Distilled water was obtained from a millipore Direct-Q ultra-pure water system (Millipore, Bedford, USA).2.2 Sample preparation and methods
Differential Scanning Calorimetry (DSC) thermograms were recorded with a Shimadzu DSC-60A system using the TA60 program. Approximately 2.0–5.0 mg of the sample (Mettler Toledo AG135 balance, Switzerland) was weighed into an aluminum pan. Dry nitrogen was used as the purge gas rate at 35 mL min−1 with the heating rate of 10 K min−1. The temperature calibration was performed with pure indium (purity 99.999%, mp 156.6 °C). Thermal Gravimetric Analysis (TGA) was performed on a Platinum-HT pan using a TA TGA Q5000 thermogravimetric analyzer. The heating rate of 10 K min−1 under dry nitrogen purge was used in the temperature range of 30–500 °C.
Fourier-Transform Infrared Spectroscopy (FT-IR) spectra were recorded from KBr disks using a Perkin Elmer 400 spectrophotometer (Buckinghamshire, UK). Ground KBr powder (drying at approximately 600 °C in the muffle furnace for 2 hours before using) was used as the background in the measurements. The number of scans was 16 times, and the resolution was 4 cm−1. The samples were scanned from 4000 to 400 cm−1. Raman spectra were collected on a micro-Raman system (Jobin Y von T64000), which was equipped with three lasers of 514, 633, 758 nm wavelength in the range of 4000 to 150 cm−1.
2.3 Theoretical calculation
The Hirshfeld surface calculation for crystal structures was performed using program Crystal Explorer 3.1. The solvent-free void, surface areas and volumes of the voids were simultaneously calculated using software packages PLATON and Crystal Explorer 3.1.24,25 The Hirshfeld surface was calculated based on the 0.5 isosurface of the weight function w(r).26,27where ρi(r) is a spherical atomic electron distribution located at the i th nucleus. The 2D fingerprint plot was produced from these two functions. To observe the close contacts among large atoms, a normalized contact distance (dnorm) was mapped, where rvdW is the van der Waals radius of the appropriate atom inside or outside the surface. More information was obtained from dnorm because it was positive for contacts greater than the van der Waals (vdW) radius separations.
2.4 Powder dissolution
The dissolution study was performed using the USP II dissolution apparatus dissolution-tester 700 (Erweka, Germany) at 37 ± 0.5 °C with a paddle speed of 100 rpm. Tet samples of 20.0 ± 0.2 mg were added into 500 mL SDS solution (0.5%, w/v). Five mL of the solution was withdrawn at 10, 20, 30, 45, 60, 90, 120 min and filtered through 0.45 μm filters. After each withdrawal, an equal volume of the dissolution medium was added to maintain a constant volume. The sample of dissolved Tet was measured using UV spectrum (Eppendorf biospectrometer, Germany) at 280 nm. All dissolution experiments were performed in triplicate.The similarity of the dissolution profiles was compared using the following equation:28
3. Results and discussion
3.1 X-ray diffraction analysis
The crystallographic data of form I, acetonitrile solvate, and acetone solvate are shown in Table 1. The absolute structure could be obtained from the Flack parameters,29 which are −0.01 (14), 0.06 (13) and 0.00 (13) for form I, acetonitrile solvate and acetone solvate, respectively. No inter/intra molecule hydrogen bonding was found in the unit cell of the solvates. The crystalline volumes of Tet significantly increased from 3252.7 Å3 (form I) to 3587.4 Å3 (acetone solvate) and 3824.8 Å3 (acetonitrile solvate) possibly because of the solvent adduction. The cavity voids of two solvates were calculated using the PLATON program. The acetonitrile solvate of Tet has a total potential solvent area volume of 860.1 Å3 per unit cell volume of 3824.8 Å3 (22.5%), whereas the total potential solvent area volume is 471.1 Å3 per unit cell volume of 3587.4 Å3 (13.1%) for the acetone solvate. The differences of solvent area volumes may be caused by the stoichiometry ratio discrepancy. The stoichiometry ratios of two solvatomorphs are significantly different: 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (acetonitrile solvate) and 1
2 (acetonitrile solvate) and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (acetone solvate). The results of stoichiometry ratios from crystal analysis are consistent with the TGA results and suggest that the solvatomorphs formed their own cavities to fit the solvents, which are observed in the packing diagrams (Fig. 1).
1 (acetone solvate). The results of stoichiometry ratios from crystal analysis are consistent with the TGA results and suggest that the solvatomorphs formed their own cavities to fit the solvents, which are observed in the packing diagrams (Fig. 1).
| Form I | Acetonitrile solvate | Acetone solvate | |
|---|---|---|---|
| Formula | C38H42N2O6 | C38H42N2O6·2(C2H3N) | 2(C38H42N2O6·C3H6O) |
| Mw | 622.74 | 704.87 | 1360.62 |
| Crystal system | Orthorhombic | Orthorhombic | Monoclinic |
| Space group | P212121 | P212121 | P21 |
| Temperature (K) | 100.00 (10) | 150.01 (10) | 100.00 (10) |
| a (Å) | 7.12371 (10) | 9.70383 (10) | 18.7432 (5) |
| b (Å) | 11.92092 (16) | 15.06419 (15) | 9.9123 (3) |
| c (Å) | 38.3027 (2) | 26.1648 (3) | 19.3410 (6) |
| α (°) | 90.00 | 90.00 | 90.00 |
| β (°) | 90.00 | 90.00 | 93.296 (3) |
| γ (°) | 90.00 | 90.00 | 90.00 |
| Cell volume (Å3) | 3252.71 (8) | 3824.78 (7) | 3587.38 (18) |
| Z/Z′ | 4/1 | 4/1 | 2/2 |
| Crystal size (mm) | 0.42 × 0.35 × 0.27 | 0.42 × 0.24 × 0.11 | 0.43 × 0.23 × 0.21 |
| Calcd density (g cm−3) | 1.272 | 1.224 | 1.260 |
| F (000) | 1328 | 1504 | 1454 |
| μ | 1.54184 | 1.54184 | 1.54184 |
| R1 [I > 2σ(I)] | 0.0323 | 0.0333 | 0.0433 |
| ωR2 (all) | 0.0797 | 0.0868 | 0.1105 |
| Goodnesss of fit on F2 | 1.081 | 1.048 | 1.048 |
| CCDC | 996340 | 996341 | 996342 |
 | ||
| Fig. 1 Packing profiles of form I (I), acetonitrile solvate (II), acetone solvate (III) viewed along a axis. The solvents are denoted with spheres for clarity. | ||
The calculated densities of form I, acetonitrile and acetone solvates are 1.272, 1.224 and 1.260 g cm−3, respectively. The density may be low because of the relatively loose molecular arrangements in the crystal lattices. Form I and the acetonitrile solvate belong to the same orthorhombic space group P212121, whereas the acetone solvate belongs to another space group P21. The asymmetric unit of the acetone solvate consists of dimeric Tet and acetone molecules (crystalline dimer, Z = Z′ = 2), which differs from form I and the acetonitrile solvate (Z = 4, Z′ = 1).
The unit cell parameters of form I and the reported anhydrous crystal were compared, which demonstrated slight differences.17 The axis direction and dimension of the reported crystal were a = 38.368 (6) Å, b = 7.230 (2) Å, c = 12.046 (2) Å, which are different from those of form I (a = 7.12371 (10) Å, b = 11.92092 (16) Å, c = 38.3027 (2)). Moreover, the cell volume and calculated density of form I vs. the reported anhydrous crystal are 3341.6 Å3 vs. 3252.71 Å3 and 1.24 g cm−3 vs. 1.272 g cm−3, respectively, which may be attributed to the slight conformational changes.
3.2 Conformational analysis
The dihedral angles of benzene ring planes were calculated to demonstrate the conformational changes (Fig. 2). The dihedral angles of form I, acetonitrile solvate and acetone solvate (dimeric molecules) were: A/B: 61.3°, 86.3°, 81.5°, 96.4°; A/D: 77.1°, 76.7°, 89.6°, 90.7°; A/E: 26.1°, 43.8°, 35.0°, 31.9°; B/D: 47.8°, 13.7°, 41.6°, 37.7°; B/E: 45.4°, 95.7°, 69.9°, 102.1° and D/E: 80.3°, 95.1°, 99.8°, 77.8°, respectively. The dihedral angles significantly changed among the polymorphs and solvates of Tet, which was also observed in the overlap diagrams of these three main molecular skeletons (Fig. 2II). As shown in Fig. 2III, the dimeric molecules of the acetone solvate could not be completely overlapped. The dihedral angles of the six-member ring (C and F) with an adjoin benzene ring (B and A) were calculated as A/F: 5.1, 4.8 and B/C: 18.6, 11.3 for the dimer, respectively. The dihedral angles of B/C have significant discrepancies because the atom C8 on one molecule of the dimeric acetone solvate was split into C8A and C8B (position disorder). As shown in Fig. 2, the conformations of acetonitrile and acetone solvates are distinctly different from that of form I, which indicates that the solvates are conformational pseudo polymorphism, where the adduct solvent in the channel void space in the molecule results in the chain arrangement and lattice transformation. The detailed crystal structures discrepancy of atomic and hydrogen coordinates, bond lengths, angles and torsion angles among form I, acetonitrile solvate and acetone solvate are tabulated in ESI (Table S1–S12†).3.3 Crystal voids calculation
Crystal voids are calculated using the program Crystal Explorer, which is based on the sum of spherical atomic electron densities at the appropriate nuclear positions (procrystal electron density). Table 2 shows that the acetonitrile solvate has the largest void volume (575.72 Å3) and surface area (1361.30 Å2), whereas the acetone solvate has the smallest crystal void volume (446.49 Å3) and surface area (1313.18 Å2), which suggests that the acetonitrile solvate has the largest porosity, and the acetone solvate had the smallest porosity. Consistently with the porosity, the calculated void volume of the acetonitrile solvate is 15.05%, which is larger than that of the acetone solvate (12.45%). The results indicate that the molecule provides an appropriate volume accessible to different solvents; hence, a possible space is accessible to acetonitrile or acetone in the porous crystal. Although many attempts and experiments have been performed to form new solvates with other solvents including methanol, ethanol, propanol, isopropanol, acetic ether, n-butyl alcohol, dioxane and tetramethylene oxide in the polymorphism screening, no new solvate could be obtained. It is inferred that only the suitable solvent can fit into the cavity or channel in the molecule packing and minimize the molecular energy to facilitate the crystallization process.| Volume (Å3) | Surface area (Å2) | Cell volume (Å3) | Per cell volume (%) | |
|---|---|---|---|---|
| a 1 au of electron density = 6.748e Å−3. | ||||
| Form I | 499.97 | 1320.15 | 3252.71 | 15.37 |
| Acetonitrile solvate | 575.72 | 1361.30 | 3824.78 | 15.05 |
| Acetone solvate | 446.49 | 1313.18 | 3587.38 | 12.45 |
3.4 Hirshfeld 2D fingerprint plots
The 2D fingerprint plots of Hirshfeld surfaces were calculated using the program Crystal Explorer (Fig. 3).30–32 The plots clearly show the intermolecular interactions (plot of di versus de, where di and de are the distances from the surface to the nearest atom inside and outside the surface, respectively). The fingerprint plots showed obvious differences between form I and the two solvates. The small wings (1a, di = 1.0 Å, de = 1.9 Å and 1b, di = 1.9 Å, de = 1.0 Å) of form I occurred because of the weak C–H⋯π interactions. The acetonitrile solvate shows additional and unique small spikes (2a, di = 1.0 Å, de = 1.4 Å and 2b, di = 1.4 Å, de = 1.0 Å) that arose from the weak interactions of the N–H⋯C and O–H⋯C types. The acetone solvate shows the characteristic weak C–H⋯π interactions at 3a, di = 1.0 Å, de = 1.6 Å and 3b, di = 1.6 Å, de = 1.0 Å (acetone methyl group hydrogen as the donor and the host molecule benzene ring as the acceptor). The hydrogen bond donor corresponds to the upper spike (de > di), whereas the hydrogen bond acceptor corresponds to the lower spike (de < di). The weak interactions of H–H type in form I (1c and d), acetonitrile (2e and f) and acetone solvate (3c and d) show obvious differences, which were also confirmed using the packing analysis (different molecular arrangements).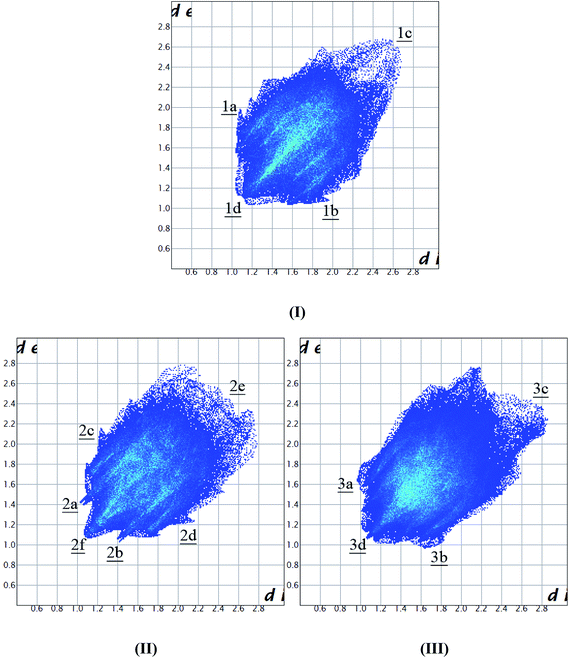 | ||
| Fig. 3 2D fingerprint plots for form I (I), acetonitrile solvate (II) and acetone solvate (III). de and di are the distances to the nearest atom exterior and interior to the surface. | ||
3.5 Powder X-ray diffraction and vibrational spectrum analysis
The polymorphic samples of Tet prepared with multiple methods were characterized using PXRD (Fig. 4.). Form I has the characteristic peaks at 8.8, 11.9, 13.9, 14.0, 15.8 and 20.0° (2θ). The acetonitrile solvate shows the characteristic peaks at 2θ 6.7, 9.6, 9.7, 13.5, 17.9, 21.9 and 22.3°. The acetone solvate presents obvious characteristic peaks at 2θ 6.3, 10.8, 13.3 and 19.1°. The calculated PXRD patterns of form I, acetonitrile solvate and acetone solvate were compared with the experimental patterns obtained from the bulk samples (Fig. 4). The results show that the main characteristic peak with high intensity was consistent with one another, which indicates a high phase purity of the bulk samples.FT-IR and Raman spectra were obtained as shown in Fig. 5. The CH3 and CH2 stretching vibration absorption between form I and the two solvates show differences in the peak range of 2800–3000 cm−1, which indicates that the hydrogen from the solvates or the chemical environments changes affects the IR absorption (Fig. 5I). The acetonitrile and acetone solvates have ν (C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N) = 2327 cm−1 and ν (C
N) = 2327 cm−1 and ν (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) = 1710 cm−1, respectively. However, the stretching band of C
O) = 1710 cm−1, respectively. However, the stretching band of C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N (2327 cm−1) shows a small peak because it was seriously affected by C
N (2327 cm−1) shows a small peak because it was seriously affected by C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O from CO2 in the atmosphere. The carbonyl group of the acetone solvate has a sharp peak in the IR diagram. The number and area of peaks in the fingerprint region of 1200–400 cm−1 exhibit differences because of the molecular arrangement and solvent adduction. The Raman spectrum (785 nm) of form I is distinguishable from those of the two solvates in the range of 1300–150 cm−1, such as the peaks at 1274, 1223, 1166, 1100, 1062, 1025, 996, 511, 311, 287 and 181 cm−1. Compared with form I in the Raman spectrum, the number and intensity of the two solvates significantly decrease. The acetone solvate has a characteristic peak at 1361 cm−1 (Fig. 5II). The combination of IR and Raman spectra could clearly distinguish the polymorphs and solvates.
O from CO2 in the atmosphere. The carbonyl group of the acetone solvate has a sharp peak in the IR diagram. The number and area of peaks in the fingerprint region of 1200–400 cm−1 exhibit differences because of the molecular arrangement and solvent adduction. The Raman spectrum (785 nm) of form I is distinguishable from those of the two solvates in the range of 1300–150 cm−1, such as the peaks at 1274, 1223, 1166, 1100, 1062, 1025, 996, 511, 311, 287 and 181 cm−1. Compared with form I in the Raman spectrum, the number and intensity of the two solvates significantly decrease. The acetone solvate has a characteristic peak at 1361 cm−1 (Fig. 5II). The combination of IR and Raman spectra could clearly distinguish the polymorphs and solvates.
 | ||
| Fig. 5 FT-IR diagrams (I) and Raman patterns (II) of form I (a), acetonitrile solvate (b) and acetone solvate (c). | ||
3.6 Thermal behaviors and phase transformation analysis
The DSC profiles of form I, acetonitrile solvate and acetone solvate are shown in Fig. 6I. The acetonitrile solvate shows endothermic peaks at temperature T = 92.4 °C (356.5 mJ, 72.9 J g−1), whereas the acetone solvate exhibits endothermic peaks at temperature T = 98.6 °C (73.0 mJ, 27.0 J g−1) prior to the melting point. The TG results (Fig. 6II and III) show that both solvates lost weight near the temperature of the endothermic peaks in the DSC profiles. The calculated and experimental weight losses of the acetonitrile and acetone solvates are 11.6%, 11.4% and 8.5%, 6.2%, respectively. The acetone solvent could slip from the crystal lattice, which possibly caused the variance between the calculated and experimental weight losses on TG for the acetone solvate.To elucidate the thermodynamic stability and phase conversion between form I and the two solvates, VT-PXRD was used in the experiments. The VT-PXRD patterns of the acetonitrile solvate converted into a meta-stable phase at 60–75 °C, which was accompanied with characteristic peaks (2θ: 9.6°, 9.7° and 14.7°) disappearance and significant peaks intensity reduction (15° < 2θ < 40°, Fig. 6IV). At 90 °C, an amorphous state was formed, and all characteristic peaks disappeared. The results indicated that the endothermic peak of DSC at 92.4 °C was a phase transformation point. When the temperature raised to 130 °C, some characteristic peaks of form I (2θ: 8.8°, 11.9, 13.9°, 14.0° and 15.8°) appeared, which were more obvious with heating to 145 °C. The phase transformation in the VT-PXRD plots occurred in a broader temperature range than the endothermic peak of the DSC data because the VT-PXRD experiment was conducted in vacuum. Compared with acetonitrile solvate, the characteristic peak at 2θ (6.3°) of acetone solvate disappeared and new peaks were generated above the temperature of 100 °C (Fig. 6V). The characteristic peaks of form I was consistent with phase transformation of acetonitrile solvate occurred at 120 °C. Two solvates showed similar VT-PXRD patterns with minor discrepancy.
3.7 Powder dissolution test
Using form I as a reference, the powder dissolution of three crystalline Tet were performed in 0.5% sodium lauryl sulfate to maintain the sink condition. The calculated f2 factor of acetonitrile and acetone solvates was 19.2 and 31.3, respectively, which suggests that they are not similar to form I. The two solvates present much higher dissolution than form I (Fig. 7). | ||
| Fig. 7 Powder dissolution test of tetrandrine form I, acetonitrile solvate and acetone solvate in 0.5% SDS dissolution media. | ||
4. Conclusions
This study demonstrates that Tet can convert into solvatomorphism in acetonitrile and acetone solutions using the solvent slow-evaporation method. The solvates are meta-stable solid forms and can lose the solvent at the phase transformation temperature upon thermal analysis. The solvatomorphs were formed because of the solvent adduction and the conformational change of the host molecule, which also affects their three-dimensional crystal packing. The adduct solvents may belong to channel solvents in the crystal structures by occupying the void space because only crystal voids and no hydrogen bonding were observed in the crystal lattice. The dissolution test demonstrates that the two solvates have better dissolution profiles than form I.Acknowledgements
The authors are grateful to Mr Xiaolong Feng from the Instrumental Analysis & Research Center of Sun Yat-Sen University for the single-crystal X-ray diffraction data collection. Financial support from the Macao Science and Technology Development Fund (112/2013/A3) is gratefully acknowledged.References
- J. P. Brog, C. L. Chanez, A. Crochet and K. M. Fromm, RSC Adv., 2013, 3, 16905–16931 RSC
.
- S. R. Byrn, R. R. Pfeiffer, G. Stephenson, D. J. W. Grant and W. B. Gleason, Chem. Mater., 1994, 6, 1148–1158 CrossRef CAS
.
- H. G. Brittain, Polymorphism in pharmaceutical solids, 2nd edn, Informa Healthcare, New York, 2009, vol. 192, p. 640 Search PubMed
.
- W. Xu, B. G. Debeb, L. Lacerda, J. Li and W. A. Woodward, Cancers, 2011, 3, 2274–2285 CrossRef CAS PubMed
.
- X. H. Cai, S. Wang and B. A. Chen, Chin. J. Nat. Med., 2011, 9, 0473–0480 CAS
.
- J. P. Felix, V. F. King, J. L. Shevell, M. L. Garcia, G. J. Kaczorowski, I. R. Bick and R. S Slaughter, Biochemistry, 1992, 31, 11793–11800 CrossRef CAS
.
- C. Y. Kwan, Y. M. Leung, T. K. Kwan and E. E Daniel, Life Sci., 2001, 68, 841–847 CrossRef CAS
.
- G. Wang and J. R. Lemos, Life Sci., 1995, 56, 295–306 CrossRef CAS
.
- Z. L. Liu, T. Hirano, S. Tanaka, K. Onda and K. Oka, J. Pharm. Pharmacol., 2003, 55, 1531–1537 CrossRef CAS PubMed
.
- T. H. Wang, J. Y. Wan, X. Gong, H. Z. Li and Y. Cheng, Oncol. Rep., 2012, 28, 1681–1686 CAS
.
- X. Zhu, M. Sui and W. Fan, Anticancer Res., 2005, 25, 1953–1962 CAS
.
- J. M. Wu, Y. Chen, J. C. Chen, T. Y. Lin and S. H. Tseng, Cancer Lett., 2010, 287, 187–195 CrossRef CAS PubMed
.
- J. Cao, Z. W. Yang, F. Li, H. Y. Zhao and Y. C Zhang, World Clin. Drugs, 2013, 34, 75–79 CAS
.
- Y. Q. Zhao, L. P. Wang, C. Ma, K. Zhao, Y. Liu and N. P. Feng, Int. J. Nanomed., 2013, 8, 4169–4181 Search PubMed
.
- S. Li, Z. Ji, M. Zou, X. Nie, Y. Shi and G. Cheng, AAPS PharmSciTech, 2011, 12, 1011–1018 CrossRef CAS PubMed
.
- R. K. Harris, P. Y. Ghi, H. Puschmann, D. C. Apperley and U. J. Griesser, Org. Process. Red. Dev., 2005, 9, 902–910 CrossRef CAS
.
- C. J. Gilmore, R. F. Bryan and S. M. Kupchan, J. Am. Chem. Soc., 1976, 98, 1947–1952 CrossRef CAS
.
- CrysAlisPro, Version C. 1.171. 35.20, Agilent Technologies Inc., Santa Clara, CA, USA, 2011 Search PubMed
.
- L. J. Farrugia, J. Appl. Crystallogr., 2012, 45, 849–854 CrossRef CAS
.
- O. V. Dolomanov, L. J. Bourhis, R. J. Gildea, J. A. K. Howard and H. Puschmann, J. Appl. Crystallogr., 2009, 42, 339–341 CrossRef CAS
.
- G. M. Sheldrick, Acta Crystallogr., Sect. A: Found. Crystallogr., 2008, 64, 112–122 CrossRef CAS PubMed
.
- G. M. Sheldrick, SHELXT\L-97, program for crystal structure solution and refinement, University of Gottingen, Gottingen, Germany, 1997 Search PubMed
.
- P. V. D. Sluis and A. L. Spek, Acta Crystallogr., Sect. A: Found. Crystallogr., 1990, 46, 194–201 CrossRef
.
- A. L. Spek, Acta Crystallogr., 2009, D65, 148–155 CrossRef PubMed
.
- M. J. Turner, J.
J. McKinnon, D. Jayatilaka and M. A. Spackman, CrystEngComm, 2011, 13, 1804–1813 RSC
.
- J. J. McKinnon, D. Jayatilaka and M. A. Spackman, Chem. Commun., 2007, 7, 3814–3816 RSC
.
- J. J. Lee, A. N. Sobolev, M. J. Turner, R. O. Fuller, B. B. Iversen, G. A. Koutsantonis and M. A. Spackman, Cryst. Growth Des., 2014, 14, 1296–1306 CAS
.
- P. Costa and J. M. S. Lobo, Eur. J. Pharm. Sci., 2001, 13, 123–133 CrossRef CAS
.
- H. D. Flack, Acta Crystallogr., 1983, A 39, 876–881 CrossRef
.
- M. A. Spackman and J. J. McKinnon, CrystEngComm, 2002, 4, 378–392 RSC
.
- J. J. McKinnon, M. A. Spackman and A. S. Mitchell, Acta Crystallogr., 2004, B60, 627–668 CrossRef CAS PubMed
.
- D. E. Braun, T. Gelbrich, V. Kahlenberg, R. Tessadri, J. Wieser and U. J. Griesser, Cryst. Growth Des., 2009, 9, 1054–1065 CAS
.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 996340–996342. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c4ra10457a |
| This journal is © The Royal Society of Chemistry 2014 |