Xylariterpenoids A–D, four new sesquiterpenoids from the Xylariaceae fungus†
Abstract
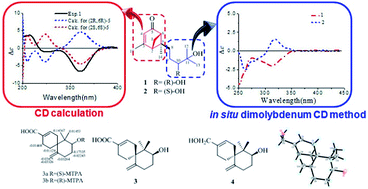
Four new sesquiterpenoids, sesquiterpenoids A–D (1–4), were isolated from solid cultures of the Xylariaceae fungus (no. 63-19-7-3). Their structures were determined through NMR analyses, CD calculation, the in situ dimolybdenum CD method, the modified Mosher's method, and X-ray data analysis. The cytotoxicities of all compounds against HL-60, SMMC-7721, A-549, MCF-7 and SW480 human cancer cell lines were assayed.

 Please wait while we load your content...
Please wait while we load your content...