Efficient pyrrolidine catalyzed cycloaddition of aziridines with isothiocyanates, isoselenocyanates and carbon disulfide “on water”†
Mani Sengoden,
Murugan Vijay,
Emayavaramban Balakumar and
Tharmalingam Punniyamurthy*
Department of Chemistry, Indian Institute of Technology Guwahati, Guwahati-781039, India. E-mail: tpunni@iitg.ernet.in; Fax: +91 0361 2690762
First published on 3rd October 2014
Abstract
The cycloaddition of aziridines with isothiocyanates, isoselenocyanates and carbon disulfide has been described using pyrrolidine as catalyst on water at moderate temperature. This protocol features the use of commercial amine as catalyst and water as solvent affording a potential route for the construction of five membered heterocycles in high yields.
Introduction
Water is the most abundant, cheap, safe and environmentally benign solvent in nature. Development of effective methods for the use of water as a reaction medium for organic synthesis has thus received much attention in recent years.1–3 The cycloaddition of aziridines with isothiocyanates affords efficient approach for the construction of functionalized five membered heterocycles that are important in biological and medicinal sciences.4 For examples, Pd,5 Ni,6 Fe,3s Zn,7 PBu3,8 NaI,9 HBF4 (ref. 10) and Ph4SbBr11 based systems have been studied for the reaction of aziridines with carbodiimides, carbon disulfide, isocyanates, isothiocyantes or isoselenocycantes as catalyst or stoichiometric reagents. Herein, we wish to report an efficient pyrrolidine catalyzed cycloaddition reaction of aziridines with isothiocyanates, isoselenocyanates and carbon disulfide on water at moderate temperature under air.12 The reaction occurs in aqueous suspension via a urea type intermediate affording a potential route for the construction of five membered heterocycles under mild reaction conditions (Scheme 1). | ||
| Scheme 1 Amine catalyzed cycloaddition of aziridine with heterocumulenes via urea type intermediate. | ||
Results and discussion
First, the reaction conditions were optimized employing phenyl isothiocyanate (1a) and 1-isopropyl-2-phenylaziridine (2a) as model substrates in the presence of various amine catalysts on water at varied temperature (Table 1). Gratifyingly, the reaction occurred to give the desired thiazolidin-2-ylidene (3a) in 46% yield when the substrates 1a and 2a were stirred with 25 mol% pyrrolidine for 12 h on water at ambient conditions (entry 1). Increasing the reaction temperature to 50 °C led to completion of the process in 6 h with 97% yield (entry 2). In a set of screened amine catalysts, pyrrolidine, morpholine, piperidine, L-proline, N-methylaniline, diisopropylamine, N-Boc pyrrolidine, triethylamine, DBU, NaHCO3 and K2CO3, the former afforded the superior results (entries 2–13). Control experiments confirmed that without the amine catalyst the reaction produced 3a after 12 h in <9% yield (entry 14 and 15).| Entry | Catalyst | T (°C) | t (h) | Yield 3ab (%) |
|---|---|---|---|---|
| a Reaction conditions. 1a (0.5 mmol), 2a (0.5 mmol), catalyst (25 mol%), H2O (1.0 mL), 50 °C, air.b Determined by 400 MHz 1H NMR.c 20 mol% of catalyst used. DBU = 1,8-diazabicyclo[5.4.0]-undec-7-ene. | ||||
| 1 | Pyrrolidine | 28 | 12 | 46 |
| 2 | Pyrrolidine | 50 | 6 | 97 |
| 3 | Morpholine | 50 | 6 | 36 |
| 4 | Piperidine | 50 | 6 | 38 |
| 5 | L-Proline | 50 | 6 | 75 |
| 6 | N-Methylaniline | 50 | 6 | 25 |
| 7 | Diisopropylamine | 50 | 6 | 20 |
| 8 | N-Boc-pyrrolidine | 50 | 6 | 9 |
| 9 | Triethylamine | 50 | 6 | 9 |
| 10 | DBU | 50 | 6 | 15 |
| 11 | NaHCO3 | 50 | 6 | 12 |
| 12 | K2CO3 | 50 | 6 | 13 |
| 13 | Pyrrolidine | 50 | 10 | 86c |
| 14 | — | 50 | 12 | 9 |
| 15 | — | 28 | 12 | 4 |
With the optimal conditions in hand, the generality of the protocol was studied for the reactions of a series of substituted isothiocyanates with aziridine 2a (Table 2). The reactions were efficient to afford the target products in high yields. For examples, isothiocyanates 1b–h having 2-methoxy, 2-methyl, 3-fluoro, 4-ethyl, 4-methoxy, 4-methyl and 4-nitro substituents in the phenyl ring underwent reaction to give the heterocycles 3b–h in 69–88% yields. Likewise, the substrates 1i–k bearing 2,4-, 3,4- and 3,5-dimethyl substituents readily underwent reaction to provide the target products 3i–k in 79–85% yields. Furthermore, aliphatic isothiocyanate 1l was compatible affording the target heterocycle 3l in 70% yield. Similarly, 1-naphthyl isothiocyanate 1m underwent reaction to give the desired 3m in 82% yield.
| Entry | Isothio-cyanate | R | t (h) | Productb (yield, %) |
|---|---|---|---|---|
| a Reaction conditions. 1b–m (0.5 mmol), 2a (0.5 mmol), H2O (1.0 mL), 50 °C, air.b Yield of isolated product. | ||||
| 1 | 1b | 2-MeOC6H4 | 7.0 | 3b (73) |
| 2 | 1c | 2-MeC6H4 | 8.5 | 3c (71) |
| 3 | 1d | 3-FC6H4 | 5.5 | 3d (69) |
| 4 | 1e | 4-EtC6H4 | 9.5 | 3e (80) |
| 5 | 1f | 4-MeOC6H4 | 10.5 | 3f (88) |
| 6 | 1g | 4-MeC6H4 | 9.5 | 3g (82) |
| 7 | 1h | 4-NO2C6H4 | 10.0 | 3h (75) |
| 8 | 1i | 2,4-Me2C6H3 | 10.0 | 3i (79) |
| 9 | 1j | 3,4-Me2C6H3 | 10.5 | 3j (81) |
| 10 | 1k | 3,5-Me2C6H3 | 9.0 | 3k (85) |
| 11 | 1l | α-Methylbenzyl | 9.5 | 3l (70) |
| 12 | 1m | 1-Naphthyl | 9.0 | 3m (82) |
Next, the reactions of substituted aziridines 2b–k with isothiocyanate 1b were examined and the results are summarized in Table 3. For examples, aryl aziridines 2b–e having allyl, benzyl, n-butyl and cyclohexyl substituents on the nitrogen atom underwent reaction to give the corresponding cycloaddition products 3n–q in 69–83% yields, whereas 1-tosyl-2-phenyl-aziridine 2f showed no reaction and the starting materials were recovered intact. Furthermore, the substrates 2g–k having electrons donating and electron withdrawing substituents such as 4-bromo, 4-fluoro, 4-methoxy, 4-methyl and 2,4-dimethyl groups readily underwent reaction to furnish the thiazolidin-2-ylidenes 3s–w in 73–89% yields.
| Entry | Aziridine | R | R′ | t (h) | Productb (yield, %) |
|---|---|---|---|---|---|
| a Reaction conditions. 1b (0.5 mmol), 2b–k (0.5 mmol), H2O (1.0 mL), 50 °C, air.b Yield of isolated product. n.d. = not detected. | |||||
| 1 | 2b | Allyl | Ph | 9.5 | 3n (70) |
| 2 | 2c | Bn | Ph | 10.5 | 3o (78) |
| 3 | 2d | n-Bu | Ph | 6.5 | 3p (83) |
| 4 | 2e | Cyclohexyl | Ph | 8.5 | 3q (69) |
| 5 | 2f | Ts | Ph | 12.0 | 3r n.d. |
| 6 | 2g | iPr | 4-BrC6H4 | 9.0 | 3s (85) |
| 7 | 2h | iPr | 4-FC6H4 | 10.5 | 3t (74) |
| 8 | 2i | iPr | 4-MeOC6H4 | 5.0 | 3u (89) |
| 9 | 2j | iPr | 4-MeC6H4 | 6.0 | 3v (77) |
| 10 | 2k | iPr | 2,4-Me2C6H3 | 8.5 | 3w (73) |
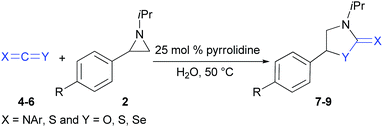
Finally, the reactions of isocyanate 4, isoselenocyanates 5a–e and carbon disulfide 6 with aziridines, were investigated (Table 4). However, isocyanate 4 showed no reaction and the starting materials were recovered intact (entry 1). In contrast, isoselenocyanates 5a–e readily underwent reaction with aziridine 2a to afford the target products 8a–e in 80–91% yield. Likewise, carbon disulfide 6 underwent reaction with aziridines 2a, 2g, 2i and 2j to furnish the heterocycles 9a–d in 73–85% yields.
| Entry | Substrate | X | Y | R | t (h) | Productb (yield, %) |
|---|---|---|---|---|---|---|
| a Reaction conditions. 4–5 (0.5 mmol) or 6 (2.5 mmol), 2 (0.5 mmol), H2O (1.0 mL), 50 °C, air.b Yield of isolated product. n.d. = not detected. | ||||||
| 1 | 4 | N(4-ClC6H4) | O | H | 3.5 | 7 n.d. |
| 2 | 5a | N(Ph) | Se | H | 4.0 | 8a (91) |
| 3 | 5b | N(2-MeOC6H4) | Se | H | 2.5 | 8b (80) |
| 4 | 5c | N(3-MeC6H4) | Se | H | 4.5 | 8c (83) |
| 5 | 5d | N(4-ClC6H4) | Se | H | 3.5 | 8d (89) |
| 6 | 5e | N(4-MeOC6H4) | Se | H | 6.0 | 8e (85) |
| 7 | 6 | S | S | H | 8.0 | 9a (73) |
| 8 | 6 | S | S | Br | 9.5 | 9b (85) |
| 9 | 6 | S | S | MeO | 5.5 | 9c (81) |
| 10 | 6 | S | S | Me | 7.0 | 9d (77) |
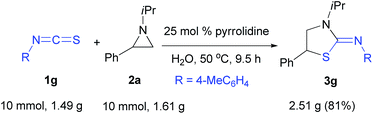
To reveal the scale up of the process, the reaction of 1g with 2a was studied (Scheme 2). As above, the reaction occurred to afford 3g in 81% yield. This result clearly suggests that the protocol may be employed on the gram scale synthesis of the target heterocycles.
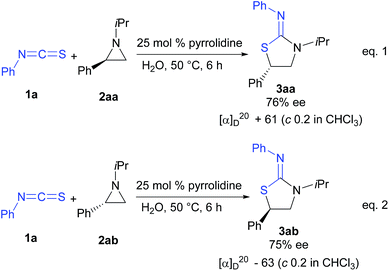
To gain insight into mechanism, optically active aziridines 2aa and 2ab having opposite configurations were reacted with isothiocyanate 1a under the optimized reaction conditions (eqn (1) and (2)). The reactions proceeded readily to yield the target heterocycles with inverted configurations (Scheme 3). These results suggest that the reaction involves SN2 pathway. The absolute configuration of 3ab was confirmed by single crystal X-ray analysis (Fig. 1). Furthermore, we were able to isolate and characterize the reactive urea type intermediate A when a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
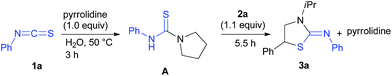
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of isothiocyanate 1a and pyrrolidine were reacted (Scheme 4). The isolated intermediate A readily underwent reaction with aziridine 2a to give the target product 3a. These results suggest that the reaction of pyrrolidine with heterocumulene can yield the reactive urea type intermediate a that may proceed reaction with aziridine via intermediate b (SN2) to afford c. The intramolecular cyclization of c may then give the target heterocycles and the catalyst to complete the catalytic cycle (Scheme 5).
1 mixture of isothiocyanate 1a and pyrrolidine were reacted (Scheme 4). The isolated intermediate A readily underwent reaction with aziridine 2a to give the target product 3a. These results suggest that the reaction of pyrrolidine with heterocumulene can yield the reactive urea type intermediate a that may proceed reaction with aziridine via intermediate b (SN2) to afford c. The intramolecular cyclization of c may then give the target heterocycles and the catalyst to complete the catalytic cycle (Scheme 5).
 | ||
| Fig. 1 ORTEP diagram of (Z)-N-(3-isopropyl-5-phenylthiazolidin-2-ylidene)aniline (3ab). Thermal ellipsoids are drawn at a 50% probability level.† | ||
Conclusions
In summary, we have developed an efficient and simple organocatalytic protocol for the (3 + 2)-cycloaddition reaction of isothiocyanates, isoselenocyanates and carbon disulfide with aziridines under mild conditions. The features of this process include the use of commercial pyrrolidine as the catalyst and involvement of urea type intermediate. These studies can open a new further development of the cycloaddition of isothiocyanates, isoselenocyanates and carbon disulfide with a variety of substrates.Experimental
General information
All reactions were performed in pure water (>5 MΩ cm@25 °C, total organic content < 30 ppb) obtained from Elix water purification system. Amines and alkenes were purchased from Aldrich and used as received. Selenium (99.9%) and amino acids were purchased from SRL. Aziridines13 and heterocumulenes3s were prepared according to the reported procedure. The reactions were monitored by analytical TLC on Merck silica gel G/GF 254 plates. The column chromatography was performed with Rankem silica gel (60–120 mesh). NMR (1H and 13C) spectra were recorded on DRX-400 Varian and Bruker Avance III 600 spectrometers. The data have been accounted as follows: chemical shifts (δ ppm) (multiplicity, coupling constant (Hz), integration). The abbreviations for multiplicity are as follows: s = singlet, d = doublet, t = triplet, q = quartet, m = multiplet, dd = doublet of doublets. Chemical shifts (δ) are reported relative to residual solvent signals (CHCl3, 7.24 ppm for 1H NMR and 77.23 ppm for 13C NMR). Melting points were determined with a Büchi B-545 apparatus and are uncorrected. Elemental analyses were recorded using Perkin Elmer CHNS analyzer. Optical rotation was determined by using Perkin Elmer-343 Polarimeter. FT-IR spectra were recorded using Perkin Elmer IR spectrometer. HPLC analysis was carried out using Waters-2489 with Daicel Chiralcel OJ, OJ-H columns using isopropanol and hexane as eluent.General procedure for the cycloaddition of aziridines with isothiocyanates and isoselenocyanates
Isothiocyanate or isoselenocyanate (0.5 mmol), aziridine (0.5 mmol) and pyrrolidine (0.125 mmol) were stirred in H2O (1 mL) at 50 °C under air (Tables 1–4). The progress of the reaction was monitored by TLC using ethyl acetate and hexane as eluent. The reaction mixture was then cooled to room temperature and extracted with diethyl ether (3 × 10 mL). The organic layer was washed with water (5 mL). Drying (Na2SO4) and evaporation of the solvent on a rotary evaporator gave a residue that was purified on a silica gel column chromatography using hexane and ethyl acetate as eluent.General procedure for the cycloaddition of aziridines with carbon disulfide
Carbon disulfide (2.5 mmol) was added portion wise for 0.5 h to a stirred solution of aziridine (0.5 mmol) and pyrrolidine (0.125 mmol) were stirred in H2O (1 mL) at 50 °C under air and the stirring continued for the appropriate time (Table 4). The progress of the reaction was monitored by TLC using ethyl acetate and hexane as eluent. The reaction mixture was then cooled to room temperature and extracted with diethyl ether (3 × 10 mL). The organic layer was washed with water (5 mL). Drying (Na2SO4) and evaporated on a rotary evaporator to give a residue that was purified on a silica gel column chromatography using hexane and ethyl acetate as eluent.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 ethyl acetate–hexane Rf = 0.60; colorless solid; yield 86% (127 mg); mp 108–109 °C; 1H NMR (400 MHz, CDCl3) δ 7.40–7.34 (m, 2H), 7.32–7.22 (m, 5H), 7.02–6.96 (m, 3H), 4.69–4.64 (m, 2H), 3.86 (dd, J = 10.0, 6.8 Hz, 1H), 3.54 (dd, J = 10.0, 7.6 Hz, 1H), 1.27 (d, J = 6.8 Hz, 3H), 1.23 (d, J = 6.8 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 158.1, 152.3, 139.3, 128.8, 128.2, 127.5, 123.0, 122.2, 52.9, 46.7, 46.3, 20.0, 19.1; FT-IR (KBr) 3075, 3034, 2964, 2934, 2856, 1613, 1584, 1178, 1061, 855, 766 cm−1; anal. calcd for C18H20N2S: C, 72.93; H, 6.80; N, 9.45; S, 10.82. Found: C, 72.85; H, 6.82; N, 9.48; S, 10.85%. Compound 3aa: [α]20D = +61.0 (c 0.2 in CHCl3); HPLC analysis: 76% ee [Daicel CHIRALCEL OJ column, hexane–iPrOH = 85
9 ethyl acetate–hexane Rf = 0.60; colorless solid; yield 86% (127 mg); mp 108–109 °C; 1H NMR (400 MHz, CDCl3) δ 7.40–7.34 (m, 2H), 7.32–7.22 (m, 5H), 7.02–6.96 (m, 3H), 4.69–4.64 (m, 2H), 3.86 (dd, J = 10.0, 6.8 Hz, 1H), 3.54 (dd, J = 10.0, 7.6 Hz, 1H), 1.27 (d, J = 6.8 Hz, 3H), 1.23 (d, J = 6.8 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 158.1, 152.3, 139.3, 128.8, 128.2, 127.5, 123.0, 122.2, 52.9, 46.7, 46.3, 20.0, 19.1; FT-IR (KBr) 3075, 3034, 2964, 2934, 2856, 1613, 1584, 1178, 1061, 855, 766 cm−1; anal. calcd for C18H20N2S: C, 72.93; H, 6.80; N, 9.45; S, 10.82. Found: C, 72.85; H, 6.82; N, 9.48; S, 10.85%. Compound 3aa: [α]20D = +61.0 (c 0.2 in CHCl3); HPLC analysis: 76% ee [Daicel CHIRALCEL OJ column, hexane–iPrOH = 85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15, flow rate: 1 mL min−1, λ = 215 nm, tR = 12.72 min (minor), 18.24 min (major)]. Compound 3ab: [α]20D = −63.0 (c 0.2 in CHCl3); HPLC analysis: 75% ee [Daicel CHIRALCEL OJ column, hexane–iPrOH = 85
15, flow rate: 1 mL min−1, λ = 215 nm, tR = 12.72 min (minor), 18.24 min (major)]. Compound 3ab: [α]20D = −63.0 (c 0.2 in CHCl3); HPLC analysis: 75% ee [Daicel CHIRALCEL OJ column, hexane–iPrOH = 85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15, flow rate: 1 mL min−1, λ = 215 nm, tR = 12.66 min (major), 17.21 min (minor)].
15, flow rate: 1 mL min−1, λ = 215 nm, tR = 12.66 min (major), 17.21 min (minor)].![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 ethyl acetate–hexane Rf = 0.54; colorless solid; yield 73% (118 mg); mp 107–108 °C; 1H NMR (400 MHz, CDCl3) δ 7.41 (d, J = 8.4 Hz, 2H), 7.32–7.23 (m, 3H), 7.00–6.96 (m, 1H), 6.92–6.90 (m, 1H), 6.86–6.82 (m, 2H), 4.75–4.68 (m, 1H), 4.64 (t, J = 7.2 Hz, 1H), 3.87 (dd, J = 9.6, 7.2 Hz, 1H), 3.80 (s, 3H), 3.54 (dd, J = 9.6, 7.6 Hz, 1H), 1.27 (d, J = 6.8 Hz, 3H), 1.23 (d, J = 6.8 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 159.1, 151.8, 141.7, 139.6, 128.7, 128.0, 127.5, 123.9, 123.0, 120.7, 111.6, 55.8, 53.2, 46.5, 46.4, 19.9, 19.1; FT-IR (KBr) 3056, 3032, 2973, 2947, 2926, 2867, 1623, 1585, 1493, 1187, 1064, 1024, 747 cm−1; anal. calcd for C19H22N2OS: C, 69.90; H, 6.79; N, 8.58; S, 9.82. Found: C, 69.98; H, 6.77; N, 8.55; S, 9.84%.
9 ethyl acetate–hexane Rf = 0.54; colorless solid; yield 73% (118 mg); mp 107–108 °C; 1H NMR (400 MHz, CDCl3) δ 7.41 (d, J = 8.4 Hz, 2H), 7.32–7.23 (m, 3H), 7.00–6.96 (m, 1H), 6.92–6.90 (m, 1H), 6.86–6.82 (m, 2H), 4.75–4.68 (m, 1H), 4.64 (t, J = 7.2 Hz, 1H), 3.87 (dd, J = 9.6, 7.2 Hz, 1H), 3.80 (s, 3H), 3.54 (dd, J = 9.6, 7.6 Hz, 1H), 1.27 (d, J = 6.8 Hz, 3H), 1.23 (d, J = 6.8 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 159.1, 151.8, 141.7, 139.6, 128.7, 128.0, 127.5, 123.9, 123.0, 120.7, 111.6, 55.8, 53.2, 46.5, 46.4, 19.9, 19.1; FT-IR (KBr) 3056, 3032, 2973, 2947, 2926, 2867, 1623, 1585, 1493, 1187, 1064, 1024, 747 cm−1; anal. calcd for C19H22N2OS: C, 69.90; H, 6.79; N, 8.58; S, 9.82. Found: C, 69.98; H, 6.77; N, 8.55; S, 9.84%.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 ethyl acetate–hexane Rf = 0.58; colorless solid; yield 71% (110 mg); mp 74–75 °C; 1H NMR (400 MHz, CDCl3) δ 7.38 (d, J = 7.2 Hz, 2H), 7.32–7.25 (m, 3H), 7.13 (d, J = 7.2 Hz, 1H), 7.07 (t, J = 7.2 Hz, 1H), 6.92 (t, J = 7.6 Hz, 1H), 6.86 (d, J = 7.6 Hz, 1H), 4.68–4.62 (m, 2H), 3.86 (dd, J = 9.6, 6.8 Hz, 1H), 3.54 (dd, J = 9.6, 8.0 Hz, 1H), 2.20 (s, 3H), 1.28 (d, J = 6.8 Hz, 3H), 1.25 (d, J = 6.8 Hz, 3H); 13C NMR (150 MHz, CDCl3) δ 157.7, 151.1, 139.6, 130.6, 130.2, 128.9, 128.2, 127.5, 126.4, 123.2, 121.4, 53.3, 46.7, 46.5, 20.0, 19.1, 18.2; FT-IR (KBr) 3058, 3023, 2971, 2929, 2870, 1626, 1591, 1488, 1456, 1401, 1362, 1243, 1212, 1179, 1110, 1066, 939 cm−1; anal. calcd for C19H22N2S: C, 73.51; H, 7.14; N, 9.02; S, 10.33. Found: C, 73.59; H, 7.13; N, 8.99; S, 10.29%.
9 ethyl acetate–hexane Rf = 0.58; colorless solid; yield 71% (110 mg); mp 74–75 °C; 1H NMR (400 MHz, CDCl3) δ 7.38 (d, J = 7.2 Hz, 2H), 7.32–7.25 (m, 3H), 7.13 (d, J = 7.2 Hz, 1H), 7.07 (t, J = 7.2 Hz, 1H), 6.92 (t, J = 7.6 Hz, 1H), 6.86 (d, J = 7.6 Hz, 1H), 4.68–4.62 (m, 2H), 3.86 (dd, J = 9.6, 6.8 Hz, 1H), 3.54 (dd, J = 9.6, 8.0 Hz, 1H), 2.20 (s, 3H), 1.28 (d, J = 6.8 Hz, 3H), 1.25 (d, J = 6.8 Hz, 3H); 13C NMR (150 MHz, CDCl3) δ 157.7, 151.1, 139.6, 130.6, 130.2, 128.9, 128.2, 127.5, 126.4, 123.2, 121.4, 53.3, 46.7, 46.5, 20.0, 19.1, 18.2; FT-IR (KBr) 3058, 3023, 2971, 2929, 2870, 1626, 1591, 1488, 1456, 1401, 1362, 1243, 1212, 1179, 1110, 1066, 939 cm−1; anal. calcd for C19H22N2S: C, 73.51; H, 7.14; N, 9.02; S, 10.33. Found: C, 73.59; H, 7.13; N, 8.99; S, 10.29%.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 ethyl acetate–hexane Rf = 0.54; colorless solid; yield 69% (108 mg); mp 91–92 °C; 1H NMR (400 MHz, CDCl3) δ 7.38–7.36 (m, 2H), 7.32–7.23 (m, 3H), 7.17–7.13 (m, 1H), 6.77–6.74 (m, 1H), 6.72–6.65 (m, 2H), 4.68–4.61 (m, 2H), 3.84 (dd, J = 10.0, 7.2 Hz, 1H), 3.53 (dd, J = 9.6, 7.6 Hz, 1H), 1.25 (d, J = 6.8 Hz, 3H), 1.21 (d, J = 6.8 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 164.4 (d, 1JCF = 243.4 Hz), 158.5, 154.1 (d, 3JCF = 9.9 Hz), 139.0, 129.9 (d, 3JCF = 9.9 Hz), 128.9, 128.3, 127.5, 118.0, 109.7 (d, 2JCF = 16.8 Hz), 109.5 (d, 2JCF = 17.5 Hz), 52.9, 46.8, 46.4, 20.0, 19.1; FT-IR (KBr) 3061, 3028, 2975, 2925, 2856, 1618, 1588, 1482, 1402, 1254, 1228, 1182, 1126, 1057, 963 cm−1; anal. calcd for C18H19FN2S: C, 68.76; H, 6.09; N, 8.91; S, 10.20. Found: C, 68.86; H, 6.07; N, 8.89; S, 10.23%.
9 ethyl acetate–hexane Rf = 0.54; colorless solid; yield 69% (108 mg); mp 91–92 °C; 1H NMR (400 MHz, CDCl3) δ 7.38–7.36 (m, 2H), 7.32–7.23 (m, 3H), 7.17–7.13 (m, 1H), 6.77–6.74 (m, 1H), 6.72–6.65 (m, 2H), 4.68–4.61 (m, 2H), 3.84 (dd, J = 10.0, 7.2 Hz, 1H), 3.53 (dd, J = 9.6, 7.6 Hz, 1H), 1.25 (d, J = 6.8 Hz, 3H), 1.21 (d, J = 6.8 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 164.4 (d, 1JCF = 243.4 Hz), 158.5, 154.1 (d, 3JCF = 9.9 Hz), 139.0, 129.9 (d, 3JCF = 9.9 Hz), 128.9, 128.3, 127.5, 118.0, 109.7 (d, 2JCF = 16.8 Hz), 109.5 (d, 2JCF = 17.5 Hz), 52.9, 46.8, 46.4, 20.0, 19.1; FT-IR (KBr) 3061, 3028, 2975, 2925, 2856, 1618, 1588, 1482, 1402, 1254, 1228, 1182, 1126, 1057, 963 cm−1; anal. calcd for C18H19FN2S: C, 68.76; H, 6.09; N, 8.91; S, 10.20. Found: C, 68.86; H, 6.07; N, 8.89; S, 10.23%.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 ethyl acetate–hexane Rf = 0.56; colorless solid; yield 80% (130 mg); mp 63–64 °C; 1H NMR (400 MHz, CDCl3) δ 7.40 (d, J = 7.6 Hz, 2H), 7.33–7.26 (m, 3H), 7.09 (d, J = 8.0 Hz, 2H), 6.91 (d, J = 8.0 Hz, 2H), 4.70–4.63 (m, 2H), 3.85 (dd, J = 10.0, 7.2 Hz, 1H), 3.54 (dd, J = 10.0, 7.6 Hz, 1H), 2.60 (q, J = 7.6 Hz, 2H), 1.27 (d, J = 6.8 Hz, 3H), 1.23–1.18 (m, 6H); 13C NMR (100 MHz, CDCl3) δ 157.9, 149.9, 139.3, 138.6, 128.8, 128.2, 128.1, 127.5, 121.9, 52.9, 46.6, 46.2, 28.3, 20.0, 19.0, 15.7; FT-IR (KBr) 3067, 3020, 2964, 2923, 2861, 1618, 1594, 1504, 1474, 1453, 1404, 1244, 1208, 1194, 1066, 848 cm−1; anal. calcd for C20H24N2S: C, 74.03; H, 7.46; N, 8.63; S, 9.88. Found: C, 73.93; H, 7.49; N, 8.67; S, 9.91%.
9 ethyl acetate–hexane Rf = 0.56; colorless solid; yield 80% (130 mg); mp 63–64 °C; 1H NMR (400 MHz, CDCl3) δ 7.40 (d, J = 7.6 Hz, 2H), 7.33–7.26 (m, 3H), 7.09 (d, J = 8.0 Hz, 2H), 6.91 (d, J = 8.0 Hz, 2H), 4.70–4.63 (m, 2H), 3.85 (dd, J = 10.0, 7.2 Hz, 1H), 3.54 (dd, J = 10.0, 7.6 Hz, 1H), 2.60 (q, J = 7.6 Hz, 2H), 1.27 (d, J = 6.8 Hz, 3H), 1.23–1.18 (m, 6H); 13C NMR (100 MHz, CDCl3) δ 157.9, 149.9, 139.3, 138.6, 128.8, 128.2, 128.1, 127.5, 121.9, 52.9, 46.6, 46.2, 28.3, 20.0, 19.0, 15.7; FT-IR (KBr) 3067, 3020, 2964, 2923, 2861, 1618, 1594, 1504, 1474, 1453, 1404, 1244, 1208, 1194, 1066, 848 cm−1; anal. calcd for C20H24N2S: C, 74.03; H, 7.46; N, 8.63; S, 9.88. Found: C, 73.93; H, 7.49; N, 8.67; S, 9.91%.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 ethyl acetate–hexane Rf = 0.53; colorless liquid; yield 88% (144 mg); 1H NMR (400 MHz, CDCl3) δ 7.37–7.35 (m, 2H), 7.31–7.22 (m, 3H), 6.91 (d, J = 9.2 Hz, 2H), 6.79 (d, J = 9.2 Hz, 2H), 4.68–4.60 (m, 2H), 3.83 (dd, J = 9.6, 6.8 Hz, 1H), 3.71 (s, 3H), 3.52 (dd, J = 9.6, 7.6 Hz, 1H), 1.24 (d, J = 6.8 Hz, 3H), 1.21 (d, J = 6.8 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 158.9, 155.7, 145.4, 139.2, 128.8, 128.1, 127.5, 123.1, 114.1, 55.4, 53.0, 46.7, 46.4, 20.0, 19.1; FT-IR (neat) 3032, 2970, 2934, 2871, 2059, 1870, 1614, 1504, 1455, 1402, 1364, 1290, 1237, 1179, 1126, 1103, 1066, 1035, 937 cm−1; anal. calcd for C19H22N2OS: C, 69.90; H, 6.79; N, 8.58; S, 9.82. Found: C, 69.98; H, 6.77; N, 8.55; S, 9.84%.
9 ethyl acetate–hexane Rf = 0.53; colorless liquid; yield 88% (144 mg); 1H NMR (400 MHz, CDCl3) δ 7.37–7.35 (m, 2H), 7.31–7.22 (m, 3H), 6.91 (d, J = 9.2 Hz, 2H), 6.79 (d, J = 9.2 Hz, 2H), 4.68–4.60 (m, 2H), 3.83 (dd, J = 9.6, 6.8 Hz, 1H), 3.71 (s, 3H), 3.52 (dd, J = 9.6, 7.6 Hz, 1H), 1.24 (d, J = 6.8 Hz, 3H), 1.21 (d, J = 6.8 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 158.9, 155.7, 145.4, 139.2, 128.8, 128.1, 127.5, 123.1, 114.1, 55.4, 53.0, 46.7, 46.4, 20.0, 19.1; FT-IR (neat) 3032, 2970, 2934, 2871, 2059, 1870, 1614, 1504, 1455, 1402, 1364, 1290, 1237, 1179, 1126, 1103, 1066, 1035, 937 cm−1; anal. calcd for C19H22N2OS: C, 69.90; H, 6.79; N, 8.58; S, 9.82. Found: C, 69.98; H, 6.77; N, 8.55; S, 9.84%.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 ethyl acetate–hexane Rf = 0.55; colorless solid; yield 82% (127 mg); mp 89–90 °C; 1H NMR (400 MHz, CDCl3) δ 7.40 (d, J = 8.4 Hz, 2H), 7.33–7.26 (m, 3H), 7.05 (d, J = 8.0 Hz, 2H), 6.86 (d, J = 8.0 Hz, 2H), 4.69–4.62 (m, 2H), 3.84 (dd, J = 9.6, 6.8 Hz, 1H), 3.53 (dd, J = 9.6, 8.0 Hz, 1H), 2.27 (s, 3H), 1.26 (d, J = 6.8 Hz, 3H), 1.22 (d, J = 6.8 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 158.0, 149.8, 139.3, 132.2, 129.4, 128.8, 128.1, 127.5, 121.9, 52.9, 46.7, 46.3, 21.0, 20.0, 19.1; FT-IR (KBr) 3025, 2971, 2928, 2870, 1624, 1602, 1505, 1470, 1454, 1402, 1363, 1270, 1242, 1212, 1191, 1126, 1106, 1066, 1030, 938 cm−1 anal. calcd for C19H22N2S: C, 73.51; H, 7.14; N, 9.02; S, 10.33. Found: C, 73.62; H, 7.11; N, 8.98; S, 10.29%.
9 ethyl acetate–hexane Rf = 0.55; colorless solid; yield 82% (127 mg); mp 89–90 °C; 1H NMR (400 MHz, CDCl3) δ 7.40 (d, J = 8.4 Hz, 2H), 7.33–7.26 (m, 3H), 7.05 (d, J = 8.0 Hz, 2H), 6.86 (d, J = 8.0 Hz, 2H), 4.69–4.62 (m, 2H), 3.84 (dd, J = 9.6, 6.8 Hz, 1H), 3.53 (dd, J = 9.6, 8.0 Hz, 1H), 2.27 (s, 3H), 1.26 (d, J = 6.8 Hz, 3H), 1.22 (d, J = 6.8 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 158.0, 149.8, 139.3, 132.2, 129.4, 128.8, 128.1, 127.5, 121.9, 52.9, 46.7, 46.3, 21.0, 20.0, 19.1; FT-IR (KBr) 3025, 2971, 2928, 2870, 1624, 1602, 1505, 1470, 1454, 1402, 1363, 1270, 1242, 1212, 1191, 1126, 1106, 1066, 1030, 938 cm−1 anal. calcd for C19H22N2S: C, 73.51; H, 7.14; N, 9.02; S, 10.33. Found: C, 73.62; H, 7.11; N, 8.98; S, 10.29%.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 ethyl acetate–hexane Rf = 0.45; yellow solid; yield 75% (128 mg); mp 68–69 °C; 1H NMR (400 MHz, CDCl3) δ 8.12 (d, J = 8.8 Hz, 2H), 7.39–7.28 (m, 5H), 7.04 (d, J = 8.8 Hz, 2H), 4.72 (t, J = 7.6 Hz, 1H), 4.70–4.63 (m, 1H), 3.91 (dd, J = 10.0, 7.2 Hz, 1H), 3.60 (dd, J = 10.0, 8.0 Hz, 1H), 1.27 (d, J = 6.8 Hz, 3H), 1.23 (d, J = 6.8 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 158.4, 158.3, 142.6, 138.5, 128.8, 128.3, 127.2, 124.8, 122.4, 52.8, 46.8, 46.6, 19.7, 19.1; FT-IR (KBr) 3063, 3038, 2973, 2926, 2869, 2852, 1614, 1571, 1498, 1328, 1218, 1185, 1162, 1108, 1066, 850 cm−1; anal. calcd for C18H19N3O2S: C, 63.32; H, 5.61; N, 12.31; S, 9.39. Found: C, 63.40; H, 5.60; N, 12.34; S, 9.36%.
9 ethyl acetate–hexane Rf = 0.45; yellow solid; yield 75% (128 mg); mp 68–69 °C; 1H NMR (400 MHz, CDCl3) δ 8.12 (d, J = 8.8 Hz, 2H), 7.39–7.28 (m, 5H), 7.04 (d, J = 8.8 Hz, 2H), 4.72 (t, J = 7.6 Hz, 1H), 4.70–4.63 (m, 1H), 3.91 (dd, J = 10.0, 7.2 Hz, 1H), 3.60 (dd, J = 10.0, 8.0 Hz, 1H), 1.27 (d, J = 6.8 Hz, 3H), 1.23 (d, J = 6.8 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 158.4, 158.3, 142.6, 138.5, 128.8, 128.3, 127.2, 124.8, 122.4, 52.8, 46.8, 46.6, 19.7, 19.1; FT-IR (KBr) 3063, 3038, 2973, 2926, 2869, 2852, 1614, 1571, 1498, 1328, 1218, 1185, 1162, 1108, 1066, 850 cm−1; anal. calcd for C18H19N3O2S: C, 63.32; H, 5.61; N, 12.31; S, 9.39. Found: C, 63.40; H, 5.60; N, 12.34; S, 9.36%.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 ethyl acetate–hexane Rf = 0.58; colorless solid; yield 79% (128 mg); mp 69–70 °C; 1H NMR (400 MHz, CDCl3) δ 7.38 (d, J = 7.2 Hz, 2H), 7.32–7.25 (m, 3H), 6.94 (s, 1H), 6.89 (d, J = 7.6 Hz, 1H), 6.76 (d, J = 7.6 Hz, 1H), 4.68–4.61 (m, 2H), 3.85 (dd, J = 9.2, 7.6 Hz, 1H), 3.52 (t, J = 8.4 Hz, 1H), 2.24 (s, 3H), 2.17 (s, 3H), 1.28 (d, J = 6.8 Hz, 3H), 1.24 (d, J = 6.8 Hz, 3H); 13C NMR (150 MHz, CDCl3) δ 157.8, 148.5, 139.7, 132.4, 131.0, 130.2, 128.8, 128.1, 127.5, 126.9, 121.2, 53.2, 46.6, 46.5, 21.0, 19.9, 19.1, 18.1; FT-IR (KBr) 3063, 2967, 2926, 2844, 1626, 1605, 1492, 1470, 1397, 1242, 1206, 1192, 1166, 1117, 1064, 929 cm−1; anal. calcd for C20H24N2S: C, 74.03; H, 7.46; N, 8.63; S, 9.88. Found: C, 74.13; H, 7.44; N, 8.59; S, 9.84%.
9 ethyl acetate–hexane Rf = 0.58; colorless solid; yield 79% (128 mg); mp 69–70 °C; 1H NMR (400 MHz, CDCl3) δ 7.38 (d, J = 7.2 Hz, 2H), 7.32–7.25 (m, 3H), 6.94 (s, 1H), 6.89 (d, J = 7.6 Hz, 1H), 6.76 (d, J = 7.6 Hz, 1H), 4.68–4.61 (m, 2H), 3.85 (dd, J = 9.2, 7.6 Hz, 1H), 3.52 (t, J = 8.4 Hz, 1H), 2.24 (s, 3H), 2.17 (s, 3H), 1.28 (d, J = 6.8 Hz, 3H), 1.24 (d, J = 6.8 Hz, 3H); 13C NMR (150 MHz, CDCl3) δ 157.8, 148.5, 139.7, 132.4, 131.0, 130.2, 128.8, 128.1, 127.5, 126.9, 121.2, 53.2, 46.6, 46.5, 21.0, 19.9, 19.1, 18.1; FT-IR (KBr) 3063, 2967, 2926, 2844, 1626, 1605, 1492, 1470, 1397, 1242, 1206, 1192, 1166, 1117, 1064, 929 cm−1; anal. calcd for C20H24N2S: C, 74.03; H, 7.46; N, 8.63; S, 9.88. Found: C, 74.13; H, 7.44; N, 8.59; S, 9.84%.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 ethyl acetate–hexane Rf = 0.56; colorless solid; yield 81% (131 mg); mp 96–97 °C; 1H NMR (400 MHz, CDCl3) δ 7.41 (d, J = 7.6 Hz, 2H), 7.34–7.27 (m, 3H), 7.02 (d, J = 8.0 Hz, 1H), 6.77 (s, 1H), 6.73 (d, J = 8.0 Hz, 1H), 4.71–4.63 (m, 2H), 3.84 (dd, J = 10.0, 6.8 Hz, 1H), 3.53 (dd, J = 9.6, 7.6 Hz, 1H), 2.21 (s, 3H), 2.19 (s, 3H), 1.27 (d, J = 6.8 Hz, 3H), 1.23 (d, J = 6.8 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 157.8, 150.0, 139.3, 136.8, 130.8, 129.9, 128.7, 128.0, 127.5, 123.4, 119.1, 52.8, 46.6, 46.2, 20.0, 19.9, 19.2, 19.0; FT-IR (KBr) 3060, 3025, 2973, 2852, 1618, 1594, 1493, 1467, 1454, 1234, 1180, 1149, 1060, 966, 827 cm−1; anal. calcd for C20H24N2S: C, 74.03; H, 7.46; N, 8.63; S, 9.88. Found: C, 74.11; H, 7.44; N, 8.60; S, 9.85%.
9 ethyl acetate–hexane Rf = 0.56; colorless solid; yield 81% (131 mg); mp 96–97 °C; 1H NMR (400 MHz, CDCl3) δ 7.41 (d, J = 7.6 Hz, 2H), 7.34–7.27 (m, 3H), 7.02 (d, J = 8.0 Hz, 1H), 6.77 (s, 1H), 6.73 (d, J = 8.0 Hz, 1H), 4.71–4.63 (m, 2H), 3.84 (dd, J = 10.0, 6.8 Hz, 1H), 3.53 (dd, J = 9.6, 7.6 Hz, 1H), 2.21 (s, 3H), 2.19 (s, 3H), 1.27 (d, J = 6.8 Hz, 3H), 1.23 (d, J = 6.8 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 157.8, 150.0, 139.3, 136.8, 130.8, 129.9, 128.7, 128.0, 127.5, 123.4, 119.1, 52.8, 46.6, 46.2, 20.0, 19.9, 19.2, 19.0; FT-IR (KBr) 3060, 3025, 2973, 2852, 1618, 1594, 1493, 1467, 1454, 1234, 1180, 1149, 1060, 966, 827 cm−1; anal. calcd for C20H24N2S: C, 74.03; H, 7.46; N, 8.63; S, 9.88. Found: C, 74.11; H, 7.44; N, 8.60; S, 9.85%.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 ethyl acetate–hexane Rf = 0.58; colorless solid; yield 85% (138 mg); mp 74–75 °C; 1H NMR (400 MHz, CDCl3) δ 7.45–7.34 (m, 1H), 7.38–7.31 (m, 5H), 6.72 (d, J = 8.4 Hz, 1H), 6.68 (s, 1H), 4.78–4.71 (m, 1H), 4.68 (t, J = 7.6 Hz, 1H), 3.87 (dd, J = 9.6, 6.8 Hz, 1H), 3.57 (dd, J = 9.6, 8.0 Hz, 1H), 2.33 (s, 6H), 1.32 (d, J = 6.8 Hz, 3H), 1.29 (d, J = 6.8 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 157.5, 152.0, 139.2, 138.1, 128.7, 128.0, 127.4, 124.6, 119.7, 52.7, 46.6, 46.2, 21.3, 19.9, 18.9; FT-IR (KBr) 3029, 2971, 2927, 2867, 1614, 1455, 1402, 1362, 1289, 1240, 1210, 1143, 1068, 1027, 953 cm−1; anal. calcd for C20H24N2S: C, 74.03; H, 7.46; N, 8.63; S, 9.88. Found: C, 73.95; H, 7.45; N, 8.67; S, 9.93%.
9 ethyl acetate–hexane Rf = 0.58; colorless solid; yield 85% (138 mg); mp 74–75 °C; 1H NMR (400 MHz, CDCl3) δ 7.45–7.34 (m, 1H), 7.38–7.31 (m, 5H), 6.72 (d, J = 8.4 Hz, 1H), 6.68 (s, 1H), 4.78–4.71 (m, 1H), 4.68 (t, J = 7.6 Hz, 1H), 3.87 (dd, J = 9.6, 6.8 Hz, 1H), 3.57 (dd, J = 9.6, 8.0 Hz, 1H), 2.33 (s, 6H), 1.32 (d, J = 6.8 Hz, 3H), 1.29 (d, J = 6.8 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 157.5, 152.0, 139.2, 138.1, 128.7, 128.0, 127.4, 124.6, 119.7, 52.7, 46.6, 46.2, 21.3, 19.9, 18.9; FT-IR (KBr) 3029, 2971, 2927, 2867, 1614, 1455, 1402, 1362, 1289, 1240, 1210, 1143, 1068, 1027, 953 cm−1; anal. calcd for C20H24N2S: C, 74.03; H, 7.46; N, 8.63; S, 9.88. Found: C, 73.95; H, 7.45; N, 8.67; S, 9.93%.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 ethyl acetate–hexane Rf = 0.49; pale yellow liquid; yield 70% (113 mg); 1H NMR (400 MHz, CDCl3) δ 7.42–7.37 (m, 4H), 7.35–7.25 (m, 5H), 7.21–7.16 (m, 1H), 4.63–4.56 (m, 2H), 4.29 (q, J = 6.4 Hz, 1H), 3.69 (dd, J = 9.6, 6.8 Hz, 1H), 3.41 (dd, J = 9.6, 7.2 Hz, 1H), 1.46 (d, J = 6.8 Hz, 3H), 1.18 (d, J = 6.8 Hz, 6H); 13C NMR (100 MHz, CDCl3) δ 156.2, 147.2, 140.1, 128.8, 128.1, 128.0, 127.5, 126.5, 126.3, 64.1, 52.7, 46.6, 46.2, 25.9, 19.7, 18.9; FT-IR (neat) 3049, 3029, 2917, 2858, 1633, 1588, 1489, 1451, 1312, 1258, 1183, 1074, 1025, 908 cm−1; anal. calcd for C20H24N2S: C, 74.03; H, 7.46; N, 8.63; S, 9.88. Found: C, 74.14; H, 7.44; N, 8.59; S, 9.83%.
9 ethyl acetate–hexane Rf = 0.49; pale yellow liquid; yield 70% (113 mg); 1H NMR (400 MHz, CDCl3) δ 7.42–7.37 (m, 4H), 7.35–7.25 (m, 5H), 7.21–7.16 (m, 1H), 4.63–4.56 (m, 2H), 4.29 (q, J = 6.4 Hz, 1H), 3.69 (dd, J = 9.6, 6.8 Hz, 1H), 3.41 (dd, J = 9.6, 7.2 Hz, 1H), 1.46 (d, J = 6.8 Hz, 3H), 1.18 (d, J = 6.8 Hz, 6H); 13C NMR (100 MHz, CDCl3) δ 156.2, 147.2, 140.1, 128.8, 128.1, 128.0, 127.5, 126.5, 126.3, 64.1, 52.7, 46.6, 46.2, 25.9, 19.7, 18.9; FT-IR (neat) 3049, 3029, 2917, 2858, 1633, 1588, 1489, 1451, 1312, 1258, 1183, 1074, 1025, 908 cm−1; anal. calcd for C20H24N2S: C, 74.03; H, 7.46; N, 8.63; S, 9.88. Found: C, 74.14; H, 7.44; N, 8.59; S, 9.83%.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 ethyl acetate–hexane Rf = 0.56; colorless solid; yield 82% (142 mg); mp 102–103 °C; 1H NMR (600 MHz, CDCl3) δ 8.06 (d, J = 3.6 Hz, 1H), 7.69–7.68 (m, 1H), 7.42 (d, J = 7.8 Hz, 1H), 7.35–7.33 (m, 2H), 7.28–7.25 (m, 3H), 7.19 (t, J = 7.2 Hz, 2H), 7.15–7.12 (m, 1H), 6.95 (d, J = 6.6 Hz, 2H), 4.80–4.78 (m, 1H), 4.55 (t, J = 7.2 Hz, 1H), 3.81 (dd, J = 9.6, 6.6 Hz, 1H), 3.49 (dd, J = 9.6, 7.8 Hz, 1H), 1.27 (d, J = 6.6 Hz, 3H), 1.23 (d, J = 6.6 Hz, 3H); 13C NMR (150 MHz, CDCl3) δ 158.2, 148.9, 139.4, 134.5, 128.9, 128.2, 127.9, 127.5, 126.0, 125.1, 124.1, 123.0, 116.2, 53.2, 46.8, 46.6, 20.2, 19.4; FT-IR (KBr) 3050, 3020, 2969, 2916, 2870, 2848, 1604, 1568, 1501, 1456, 1386, 1276, 1242, 1215, 1174, 1063, 1038, 926 cm−1; anal. calcd for C22H22N2S: C, 76.26; H, 6.40; N, 8.08; S, 9.26. Found: C, 76.20; H, 6.41; N, 8.11; S, 9.28%.
9 ethyl acetate–hexane Rf = 0.56; colorless solid; yield 82% (142 mg); mp 102–103 °C; 1H NMR (600 MHz, CDCl3) δ 8.06 (d, J = 3.6 Hz, 1H), 7.69–7.68 (m, 1H), 7.42 (d, J = 7.8 Hz, 1H), 7.35–7.33 (m, 2H), 7.28–7.25 (m, 3H), 7.19 (t, J = 7.2 Hz, 2H), 7.15–7.12 (m, 1H), 6.95 (d, J = 6.6 Hz, 2H), 4.80–4.78 (m, 1H), 4.55 (t, J = 7.2 Hz, 1H), 3.81 (dd, J = 9.6, 6.6 Hz, 1H), 3.49 (dd, J = 9.6, 7.8 Hz, 1H), 1.27 (d, J = 6.6 Hz, 3H), 1.23 (d, J = 6.6 Hz, 3H); 13C NMR (150 MHz, CDCl3) δ 158.2, 148.9, 139.4, 134.5, 128.9, 128.2, 127.9, 127.5, 126.0, 125.1, 124.1, 123.0, 116.2, 53.2, 46.8, 46.6, 20.2, 19.4; FT-IR (KBr) 3050, 3020, 2969, 2916, 2870, 2848, 1604, 1568, 1501, 1456, 1386, 1276, 1242, 1215, 1174, 1063, 1038, 926 cm−1; anal. calcd for C22H22N2S: C, 76.26; H, 6.40; N, 8.08; S, 9.26. Found: C, 76.20; H, 6.41; N, 8.11; S, 9.28%.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 ethyl acetate–hexane Rf = 0.54; yellow liquid; yield 70% (113 mg); 1H NMR (400 MHz, CDCl3) δ 7.40 (d, J = 7.2 Hz, 2H), 7.32–7.26 (m, 3H), 7.00 (t, J = 7.2 Hz, 1H), 6.93 (d, J = 7.2 Hz, 1H), 6.87 (d, J = 6.4 Hz, 2H), 5.99–5.89 (m, 1H), 5.27–5.19 (m, 2H), 4.71 (t, J = 7.2 Hz, 1H), 4.21–4.18 (m, 2H), 3.86–3.84 (m, 1H), 3.81 (s, 3H), 3.57 (t, J = 8.4 Hz, 1H); 13C NMR (150 MHz, CDCl3) δ 159.4, 151.8, 141.4, 139.2, 133.0, 128.7, 128.1, 127.6, 124.1, 122.9, 120.8, 118.0, 111.8, 58.2, 55.9, 49.3, 46.7; FT-IR (neat) 3064, 3025, 3006, 2952, 2924, 2832, 1627, 1586, 1493, 1466, 1453, 1399, 1247, 1173, 1110, 1046, 1027, 1005, 927 cm−1; anal. calcd for C19H20N2OS: C, 70.34; H, 6.21; N, 8.63; S, 9.88. Found: C, 70.42; H, 6.19; N, 8.60; S, 9.85%.
9 ethyl acetate–hexane Rf = 0.54; yellow liquid; yield 70% (113 mg); 1H NMR (400 MHz, CDCl3) δ 7.40 (d, J = 7.2 Hz, 2H), 7.32–7.26 (m, 3H), 7.00 (t, J = 7.2 Hz, 1H), 6.93 (d, J = 7.2 Hz, 1H), 6.87 (d, J = 6.4 Hz, 2H), 5.99–5.89 (m, 1H), 5.27–5.19 (m, 2H), 4.71 (t, J = 7.2 Hz, 1H), 4.21–4.18 (m, 2H), 3.86–3.84 (m, 1H), 3.81 (s, 3H), 3.57 (t, J = 8.4 Hz, 1H); 13C NMR (150 MHz, CDCl3) δ 159.4, 151.8, 141.4, 139.2, 133.0, 128.7, 128.1, 127.6, 124.1, 122.9, 120.8, 118.0, 111.8, 58.2, 55.9, 49.3, 46.7; FT-IR (neat) 3064, 3025, 3006, 2952, 2924, 2832, 1627, 1586, 1493, 1466, 1453, 1399, 1247, 1173, 1110, 1046, 1027, 1005, 927 cm−1; anal. calcd for C19H20N2OS: C, 70.34; H, 6.21; N, 8.63; S, 9.88. Found: C, 70.42; H, 6.19; N, 8.60; S, 9.85%.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 ethyl acetate–hexane Rf = 0.58; colorless solid; yield 78% (117 mg); mp 87–88 °C; 1H NMR (400 MHz, CDCl3) δ 7.46 (d, J = 7.6 Hz, 2H), 7.38–7.35 (m, 4H), 7.31–7.25 (m, 4H), 7.05–7.02 (m, 2H), 7.00–6.91 (m, 2H), 4.90 (d, J = 14.8, 1H), 4.80 (d, J = 14.8, 1H), 4.70 (t, J = 7.6, 1H), 3.88 (s, 3H), 3.78 (dd, J = 10.0, 7.6 Hz, 1H), 3.52 (dd, J = 10.0, 8.4 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ 159.6, 151.9, 141.5, 139.1, 137.2, 128.8, 128.7, 128.6, 128.1, 127.6, 127.5, 124.1, 122.9, 120.9, 111.8, 57.9, 56.0, 50.2, 46.8; FT-IR (KBr) 3080, 3061, 3025, 2990, 2960, 2916, 2867, 1624, 1585, 1493, 1452, 1407, 1360, 1240, 1213, 1152, 1110, 1023, 920 cm−1; anal. calcd for C23H22N2OS: C, 73.76; H, 5.92; N, 7.48; S, 8.56. Found: C, 73.69; H, 5.94; N, 7.51; S, 8.53%.
9 ethyl acetate–hexane Rf = 0.58; colorless solid; yield 78% (117 mg); mp 87–88 °C; 1H NMR (400 MHz, CDCl3) δ 7.46 (d, J = 7.6 Hz, 2H), 7.38–7.35 (m, 4H), 7.31–7.25 (m, 4H), 7.05–7.02 (m, 2H), 7.00–6.91 (m, 2H), 4.90 (d, J = 14.8, 1H), 4.80 (d, J = 14.8, 1H), 4.70 (t, J = 7.6, 1H), 3.88 (s, 3H), 3.78 (dd, J = 10.0, 7.6 Hz, 1H), 3.52 (dd, J = 10.0, 8.4 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ 159.6, 151.9, 141.5, 139.1, 137.2, 128.8, 128.7, 128.6, 128.1, 127.6, 127.5, 124.1, 122.9, 120.9, 111.8, 57.9, 56.0, 50.2, 46.8; FT-IR (KBr) 3080, 3061, 3025, 2990, 2960, 2916, 2867, 1624, 1585, 1493, 1452, 1407, 1360, 1240, 1213, 1152, 1110, 1023, 920 cm−1; anal. calcd for C23H22N2OS: C, 73.76; H, 5.92; N, 7.48; S, 8.56. Found: C, 73.69; H, 5.94; N, 7.51; S, 8.53%.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 ethyl acetate–hexane Rf = 0.61; colorless solid; yield 83% (141 mg); mp 71–72 °C; 1H NMR (400 MHz, CDCl3) δ 7.41 (d, J = 7.6 Hz, 2H), 7.33–7.26 (m, 3H), 7.02–6.98 (m, 1H), 6.94 (d, J = 7.2 Hz, 1H), 6.87 (t, J = 6.4 Hz, 2H), 4.67 (t, J = 7.6 Hz, 1H), 3.88 (dd, J = 9.2, 6.8 Hz, 1H), 3.81 (s, 3H), 3.63–3.57 (m, 3H), 1.68–1.63 (m, 2H), 1.42 (q, J = 7.6 Hz, 2H), 0.97 (t, J = 7.2 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 159.2, 151.8, 141.8, 139.5, 128.7, 128.0, 127.5, 123.9, 123.0, 120.7, 111.7, 58.6, 55.8, 46.7, 46.2, 29.3, 20.2, 14.0; FT-IR (KBr) 3064, 3001, 2956, 2924, 2857, 2829, 1628, 1582, 1495, 1457, 1403, 1293, 1248, 1225, 1176, 1101, 1046, 1027, 988 cm−1; anal calcd for C20H24N2OS: C, 70.55; H, 7.10; N, 8.23; S, 9.42. Found: C, 70.65; H, 7.09; N, 8.20; S, 9.38%.
9 ethyl acetate–hexane Rf = 0.61; colorless solid; yield 83% (141 mg); mp 71–72 °C; 1H NMR (400 MHz, CDCl3) δ 7.41 (d, J = 7.6 Hz, 2H), 7.33–7.26 (m, 3H), 7.02–6.98 (m, 1H), 6.94 (d, J = 7.2 Hz, 1H), 6.87 (t, J = 6.4 Hz, 2H), 4.67 (t, J = 7.6 Hz, 1H), 3.88 (dd, J = 9.2, 6.8 Hz, 1H), 3.81 (s, 3H), 3.63–3.57 (m, 3H), 1.68–1.63 (m, 2H), 1.42 (q, J = 7.6 Hz, 2H), 0.97 (t, J = 7.2 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 159.2, 151.8, 141.8, 139.5, 128.7, 128.0, 127.5, 123.9, 123.0, 120.7, 111.7, 58.6, 55.8, 46.7, 46.2, 29.3, 20.2, 14.0; FT-IR (KBr) 3064, 3001, 2956, 2924, 2857, 2829, 1628, 1582, 1495, 1457, 1403, 1293, 1248, 1225, 1176, 1101, 1046, 1027, 988 cm−1; anal calcd for C20H24N2OS: C, 70.55; H, 7.10; N, 8.23; S, 9.42. Found: C, 70.65; H, 7.09; N, 8.20; S, 9.38%.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 ethyl acetate–hexane Rf = 0.59; yellow liquid; yield 69% (126 mg); 1H NMR (400 MHz, CDCl3) δ 7.41 (d, J = 7.2 Hz, 2H), 7.32–7.26 (m, 3H), 7.00 (t, J = 7.2 Hz, 1H), 6.93 (d, J = 7.2 Hz, 1H), 6.88–6.85 (m, 2H), 4.61 (t, J = 6.8 Hz, 1H), 4.36–4.31 (m, 1H), 3.91 (t, J = 8.8 Hz, 1H), 3.81 (s, 3H), 3.59 (t, J = 8.0 Hz, 1H), 2.03–1.65 (m, 5H), 1.48–1.06 (m, 5H); 13C NMR (100 MHz, CDCl3) δ 159.3, 151.8, 141.8, 139.8, 128.7, 128.0, 127.4, 123.9, 123.3, 120.8, 111.8, 55.9, 54.5, 54.3, 46.6, 30.5, 29.7, 25.8, 25.7, 25.6; FT-IR (neat) 3060, 3020, 2937, 2851, 2829, 1628, 1583, 1493, 1466, 1401, 1302, 1231, 1173, 1111, 1046, 1028, 910, 891, 806 cm−1; anal. calcd for C22H26N2OS: C, 72.09; H, 7.15; N, 7.64; S, 8.75. Found: C, 72.00; H, 7.13; N, 7.66; S, 8.71%.
9 ethyl acetate–hexane Rf = 0.59; yellow liquid; yield 69% (126 mg); 1H NMR (400 MHz, CDCl3) δ 7.41 (d, J = 7.2 Hz, 2H), 7.32–7.26 (m, 3H), 7.00 (t, J = 7.2 Hz, 1H), 6.93 (d, J = 7.2 Hz, 1H), 6.88–6.85 (m, 2H), 4.61 (t, J = 6.8 Hz, 1H), 4.36–4.31 (m, 1H), 3.91 (t, J = 8.8 Hz, 1H), 3.81 (s, 3H), 3.59 (t, J = 8.0 Hz, 1H), 2.03–1.65 (m, 5H), 1.48–1.06 (m, 5H); 13C NMR (100 MHz, CDCl3) δ 159.3, 151.8, 141.8, 139.8, 128.7, 128.0, 127.4, 123.9, 123.3, 120.8, 111.8, 55.9, 54.5, 54.3, 46.6, 30.5, 29.7, 25.8, 25.7, 25.6; FT-IR (neat) 3060, 3020, 2937, 2851, 2829, 1628, 1583, 1493, 1466, 1401, 1302, 1231, 1173, 1111, 1046, 1028, 910, 891, 806 cm−1; anal. calcd for C22H26N2OS: C, 72.09; H, 7.15; N, 7.64; S, 8.75. Found: C, 72.00; H, 7.13; N, 7.66; S, 8.71%.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 ethyl acetate–hexane Rf = 0.57; colorless solid; yield 85% (172 mg); mp 79–80 °C; 1H NMR (400 MHz, CDCl3) δ 7.44 (d, J = 8.0 Hz, 2H), 7.30 (d, J = 8.4 Hz, 2H), 7.01–6.98 (m, 1H), 6.92–6.85 (m, 3H), 4.73–4.70 (m, 1H), 4.56 (t, J = 6.8 Hz, 1H), 3.87 (dd, J = 10.0, 7.2 Hz, 1H), 3.81 (s, 3H), 3.50 (dd, J = 9.2, 6.4 Hz, 1H), 1.28 (d, J = 6.8 Hz, 3H), 1.22 (d, J = 6.8 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 158.8, 151.7, 141.6, 139.1, 131.8, 129.2, 124.1, 123.0, 121.9, 120.8, 111.7, 55.9, 53.2, 46.5, 45.8, 19.7, 19.3; FT-IR (KBr) 3050, 3004, 2968, 2927, 2845, 2823, 1627, 1585, 1490, 1467, 1399, 1275, 1260, 1235, 1177, 1110, 1061, 934 cm−1; anal. calcd for C19H21BrN2OS: C, 56.30; H, 5.22; N, 6.91; S, 7.91. Found: C, 56.38; H, 5.20; N, 6.89; S, 7.87%.
9 ethyl acetate–hexane Rf = 0.57; colorless solid; yield 85% (172 mg); mp 79–80 °C; 1H NMR (400 MHz, CDCl3) δ 7.44 (d, J = 8.0 Hz, 2H), 7.30 (d, J = 8.4 Hz, 2H), 7.01–6.98 (m, 1H), 6.92–6.85 (m, 3H), 4.73–4.70 (m, 1H), 4.56 (t, J = 6.8 Hz, 1H), 3.87 (dd, J = 10.0, 7.2 Hz, 1H), 3.81 (s, 3H), 3.50 (dd, J = 9.2, 6.4 Hz, 1H), 1.28 (d, J = 6.8 Hz, 3H), 1.22 (d, J = 6.8 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 158.8, 151.7, 141.6, 139.1, 131.8, 129.2, 124.1, 123.0, 121.9, 120.8, 111.7, 55.9, 53.2, 46.5, 45.8, 19.7, 19.3; FT-IR (KBr) 3050, 3004, 2968, 2927, 2845, 2823, 1627, 1585, 1490, 1467, 1399, 1275, 1260, 1235, 1177, 1110, 1061, 934 cm−1; anal. calcd for C19H21BrN2OS: C, 56.30; H, 5.22; N, 6.91; S, 7.91. Found: C, 56.38; H, 5.20; N, 6.89; S, 7.87%.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 ethyl acetate–hexane Rf = 0.55; colorless solid; yield 74% (127 mg); mp 120–121 °C; 1H NMR (600 MHz, CDCl3) δ 7.40–7.38 (m, 2H), 7.02–6.99 (m, 3H), 6.92 (dd, J = 8.4, 1.2 Hz, 1H), 6.87–6.85 (m, 2H), 4.74–4.72 (m, 1H), 4.62 (t, J = 6.6 Hz, 1H), 3.87 (dd, J = 9.6, 6.6 Hz, 1H), 3.82 (s, 3H), 3.51 (dd, J = 9.6, 7.2 Hz, 1H), 1.28 (d, J = 6.6 Hz, 3H), 1.23 (d, J = 6.6 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 163.7, 161.2 (d, 1JCF = 215.3 Hz), 151.8, 141.6, 135.7, 135.6, 129.3 (d, 3JCF = 8.0 Hz), 124.1, 123.1, 120.9, 115.8 (d, 2JCF = 21.3 Hz), 111.8, 56.0, 53.5, 46.5, 45.9, 19.9, 19.3; FT-IR (KBr) 3053, 3034, 2965, 2927, 2861, 2834, 1625, 1585, 1510, 1493, 1466, 1240, 1224, 1174, 1114, 1026, 939 cm−1; anal. calcd for C19H21FN2OS: C, 66.25; H, 6.15; N, 8.13; S, 9.31. Found: C, 66.32; H, 6.13; N, 8.16; S, 9.35%.
9 ethyl acetate–hexane Rf = 0.55; colorless solid; yield 74% (127 mg); mp 120–121 °C; 1H NMR (600 MHz, CDCl3) δ 7.40–7.38 (m, 2H), 7.02–6.99 (m, 3H), 6.92 (dd, J = 8.4, 1.2 Hz, 1H), 6.87–6.85 (m, 2H), 4.74–4.72 (m, 1H), 4.62 (t, J = 6.6 Hz, 1H), 3.87 (dd, J = 9.6, 6.6 Hz, 1H), 3.82 (s, 3H), 3.51 (dd, J = 9.6, 7.2 Hz, 1H), 1.28 (d, J = 6.6 Hz, 3H), 1.23 (d, J = 6.6 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 163.7, 161.2 (d, 1JCF = 215.3 Hz), 151.8, 141.6, 135.7, 135.6, 129.3 (d, 3JCF = 8.0 Hz), 124.1, 123.1, 120.9, 115.8 (d, 2JCF = 21.3 Hz), 111.8, 56.0, 53.5, 46.5, 45.9, 19.9, 19.3; FT-IR (KBr) 3053, 3034, 2965, 2927, 2861, 2834, 1625, 1585, 1510, 1493, 1466, 1240, 1224, 1174, 1114, 1026, 939 cm−1; anal. calcd for C19H21FN2OS: C, 66.25; H, 6.15; N, 8.13; S, 9.31. Found: C, 66.32; H, 6.13; N, 8.16; S, 9.35%.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 ethyl acetate–hexane Rf = 0.51; yellow liquid; yield 89% (159 mg); 1H NMR (600 MHz, CDCl3) δ 7.34 (d, J = 8.4 Hz, 2H), 7.01 (t, J = 7.2 Hz, 1H), 6.95 (d, J = 7.2 Hz, 1H), 6.87–6.83 (m, 4H), 4.76–4.72 (m, 1H), 4.64 (t, J = 7.2 Hz, 1H), 3.86 (dd, J = 10.2, 7.2 Hz, 1H), 3.81 (s, 3H), 3.79 (s, 3H), 3.54 (dd, J = 9.6, 7.8 Hz, 1H), 1.29 (d, J = 6.6 Hz, 3H), 1.25 (d, J = 6.6 Hz, 3H); 13C NMR (150 MHz, CDCl3) δ 159.5, 152.1, 131.4, 128.8, 124.3, 123.4, 120.9, 120.8, 114.2, 111.9, 56.1, 55.5, 53.8, 46.7, 46.4, 20.1, 19.2; FT-IR (neat) 3061, 2969, 2932, 2834, 1623, 1585, 1512, 1493, 1463, 1355, 1254, 1177, 1029, 928 cm−1; anal. calcd for C20H24N2O2S: C, 67.38; H, 6.79; N, 7.86; S, 8.99. Found: C, 67.30; H, 6.81; N, 7.83; S, 9.03%.
9 ethyl acetate–hexane Rf = 0.51; yellow liquid; yield 89% (159 mg); 1H NMR (600 MHz, CDCl3) δ 7.34 (d, J = 8.4 Hz, 2H), 7.01 (t, J = 7.2 Hz, 1H), 6.95 (d, J = 7.2 Hz, 1H), 6.87–6.83 (m, 4H), 4.76–4.72 (m, 1H), 4.64 (t, J = 7.2 Hz, 1H), 3.86 (dd, J = 10.2, 7.2 Hz, 1H), 3.81 (s, 3H), 3.79 (s, 3H), 3.54 (dd, J = 9.6, 7.8 Hz, 1H), 1.29 (d, J = 6.6 Hz, 3H), 1.25 (d, J = 6.6 Hz, 3H); 13C NMR (150 MHz, CDCl3) δ 159.5, 152.1, 131.4, 128.8, 124.3, 123.4, 120.9, 120.8, 114.2, 111.9, 56.1, 55.5, 53.8, 46.7, 46.4, 20.1, 19.2; FT-IR (neat) 3061, 2969, 2932, 2834, 1623, 1585, 1512, 1493, 1463, 1355, 1254, 1177, 1029, 928 cm−1; anal. calcd for C20H24N2O2S: C, 67.38; H, 6.79; N, 7.86; S, 8.99. Found: C, 67.30; H, 6.81; N, 7.83; S, 9.03%.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 ethyl acetate–hexane Rf = 0.56; colorless solid; yield 77% (131 mg); mp 117–118 °C; 1H NMR (400 MHz, CDCl3) δ 7.29 (d, J = 8.0 Hz, 2H), 7.11 (d, J = 7.6 Hz, 2H), 6.98 (t, J = 6.8 Hz, 1H), 6.91 (d, J = 6.8 Hz, 1H), 6.85 (d, J = 6.8 Hz, 2H), 4.72–4.69 (m, 1H), 4.61 (t, J = 6.8 Hz, 1H), 3.84 (dd, J = 9.6, 7.2 Hz, 1H), 3.80 (s, 3H), 3.51 (dd, J = 9.2, 8.0 Hz, 1H), 2.30 (s, 3H), 1.26 (d, J = 6.8 Hz, 3H), 1.22 (d, J = 6.8 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 159.5, 151.9, 141.7, 137.9, 136.5, 129.5, 127.5, 124.0, 123.1, 120.8, 111.8, 56.0, 53.5, 46.6, 46.5, 21.2, 20.0, 19.2; FT-IR (KBr) 3040, 3009, 2973, 2923, 2848, 2834, 1629, 1586, 1492, 1469, 1450, 1402, 1260, 1236, 1220, 1180, 1158, 1111, 1062, 1045, 1025, 820 cm−1; anal. calcd for C20H24N2OS: C, 70.55; H, 7.10; N, 8.23; S, 9.42. Found: C, 70.65; H, 7.09; N, 8.20; S, 9.38%.
9 ethyl acetate–hexane Rf = 0.56; colorless solid; yield 77% (131 mg); mp 117–118 °C; 1H NMR (400 MHz, CDCl3) δ 7.29 (d, J = 8.0 Hz, 2H), 7.11 (d, J = 7.6 Hz, 2H), 6.98 (t, J = 6.8 Hz, 1H), 6.91 (d, J = 6.8 Hz, 1H), 6.85 (d, J = 6.8 Hz, 2H), 4.72–4.69 (m, 1H), 4.61 (t, J = 6.8 Hz, 1H), 3.84 (dd, J = 9.6, 7.2 Hz, 1H), 3.80 (s, 3H), 3.51 (dd, J = 9.2, 8.0 Hz, 1H), 2.30 (s, 3H), 1.26 (d, J = 6.8 Hz, 3H), 1.22 (d, J = 6.8 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 159.5, 151.9, 141.7, 137.9, 136.5, 129.5, 127.5, 124.0, 123.1, 120.8, 111.8, 56.0, 53.5, 46.6, 46.5, 21.2, 20.0, 19.2; FT-IR (KBr) 3040, 3009, 2973, 2923, 2848, 2834, 1629, 1586, 1492, 1469, 1450, 1402, 1260, 1236, 1220, 1180, 1158, 1111, 1062, 1045, 1025, 820 cm−1; anal. calcd for C20H24N2OS: C, 70.55; H, 7.10; N, 8.23; S, 9.42. Found: C, 70.65; H, 7.09; N, 8.20; S, 9.38%.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 ethyl acetate–hexane Rf = 0.57; colorless solid; yield 73% (129 mg); mp 84–85 °C; 1H NMR (600 MHz, CDCl3) δ 7.52 (d, J = 7.8 Hz, 1H), 7.03–6.98 (m, 2H), 6.95 (s, 1H), 6.93 (d, J = 7.8 Hz, 1H), 6.87 (d, J = 7.8 Hz, 2H), 4.88 (t, J = 7.2 Hz, 1H), 4.76–4.74 (m, 1H), 3.85 (dd, J = 9.6, 7.2 Hz, 1H), 3.82 (s, 3H), 3.57 (dd, J = 9.6, 7.8 Hz, 1H), 2.29 (s, 3H), 2.28 (s, 3H), 1.30 (d, J = 6.6 Hz, 3H), 1.27 (d, J = 6.6 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 159.5, 151.9, 141.7, 137.5, 135.5, 134.4, 131.3, 127.3, 126.7, 123.9, 123.1, 120.8, 111.7, 55.9, 52.1, 46.5, 42.3, 21.1, 19.9, 19.6, 19.2; FT-IR (KBr) 3058, 2970, 2924, 2859, 2831, 1624, 1585, 1493, 1464, 1402, 1362, 1257, 1236, 1177, 1113, 1046, 1028, 934 cm−1; anal. calcd for C21H26N2OS: C, 71.15; H, 7.39; N, 7.90; S, 9.04. Found: C, 71.22; H, 7.37; N, 7.93; S, 9.00%.
9 ethyl acetate–hexane Rf = 0.57; colorless solid; yield 73% (129 mg); mp 84–85 °C; 1H NMR (600 MHz, CDCl3) δ 7.52 (d, J = 7.8 Hz, 1H), 7.03–6.98 (m, 2H), 6.95 (s, 1H), 6.93 (d, J = 7.8 Hz, 1H), 6.87 (d, J = 7.8 Hz, 2H), 4.88 (t, J = 7.2 Hz, 1H), 4.76–4.74 (m, 1H), 3.85 (dd, J = 9.6, 7.2 Hz, 1H), 3.82 (s, 3H), 3.57 (dd, J = 9.6, 7.8 Hz, 1H), 2.29 (s, 3H), 2.28 (s, 3H), 1.30 (d, J = 6.6 Hz, 3H), 1.27 (d, J = 6.6 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 159.5, 151.9, 141.7, 137.5, 135.5, 134.4, 131.3, 127.3, 126.7, 123.9, 123.1, 120.8, 111.7, 55.9, 52.1, 46.5, 42.3, 21.1, 19.9, 19.6, 19.2; FT-IR (KBr) 3058, 2970, 2924, 2859, 2831, 1624, 1585, 1493, 1464, 1402, 1362, 1257, 1236, 1177, 1113, 1046, 1028, 934 cm−1; anal. calcd for C21H26N2OS: C, 71.15; H, 7.39; N, 7.90; S, 9.04. Found: C, 71.22; H, 7.37; N, 7.93; S, 9.00%.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 ethyl acetate–hexane Rf = 0.67; yellow liquid; yield 91% (156 mg); 1H NMR (400 MHz, CDCl3) δ 7.41 (d, J = 7.2 Hz, 2H), 7.31–7.22 (m, 5H), 7.01 (t, J = 7.2 Hz, 1H), 6.96 (d, J = 7.2 Hz, 2H), 4.78–4.72 (m, 2H), 3.85 (dd, J = 10.4, 6.4 Hz, 1H), 3.65 (dd, J = 10.4, 8.0 Hz, 1H), 1.26 (t, J = 6.8 Hz, 6H); 13C NMR (100 MHz, CDCl3) δ 155.8, 153.1, 140.0, 128.5, 128.3, 127.4, 127.2, 122.8, 121.3, 53.4, 46.8, 41.6, 19.6, 19.0; FT-IR (neat) 3058, 3027, 2971, 2929, 2869, 1614, 1590, 1489, 1454, 1402, 1363, 1243, 1205, 1185, 1163, 1126, 1069, 1024 cm−1; anal. calcd for C18H20N2Se: C, 62.97; H, 5.87; N 8.16. Found: C, 63.07; H, 5.83; N, 8.19%.
9 ethyl acetate–hexane Rf = 0.67; yellow liquid; yield 91% (156 mg); 1H NMR (400 MHz, CDCl3) δ 7.41 (d, J = 7.2 Hz, 2H), 7.31–7.22 (m, 5H), 7.01 (t, J = 7.2 Hz, 1H), 6.96 (d, J = 7.2 Hz, 2H), 4.78–4.72 (m, 2H), 3.85 (dd, J = 10.4, 6.4 Hz, 1H), 3.65 (dd, J = 10.4, 8.0 Hz, 1H), 1.26 (t, J = 6.8 Hz, 6H); 13C NMR (100 MHz, CDCl3) δ 155.8, 153.1, 140.0, 128.5, 128.3, 127.4, 127.2, 122.8, 121.3, 53.4, 46.8, 41.6, 19.6, 19.0; FT-IR (neat) 3058, 3027, 2971, 2929, 2869, 1614, 1590, 1489, 1454, 1402, 1363, 1243, 1205, 1185, 1163, 1126, 1069, 1024 cm−1; anal. calcd for C18H20N2Se: C, 62.97; H, 5.87; N 8.16. Found: C, 63.07; H, 5.83; N, 8.19%.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 ethyl acetate–hexane Rf = 0.60; colorless solid; yield 80% (149 mg); mp 107–108 °C; 1H NMR (400 MHz, CDCl3) δ 7.40 (d, J = 7.6 Hz, 2H), 7.27–7.18 (m, 3H), 6.99 (t, J = 8.0 Hz, 1H), 6.93 (d, J = 7.2 Hz, 1H), 6.84 (t, J = 7.6 Hz, 2H), 4.83–4.80 (m, 1H), 4.72 (t, J = 7.2 Hz, 1H), 3.83 (dd, J = 10.4, 6.4 Hz, 1H), 3.76 (s, 3H), 3.63 (dd, J = 10.4, 7.6 Hz, 1H), 1.26 (t, J = 7.6 Hz, 6H); 13C NMR (100 MHz, CDCl3) δ 157.2, 151.6, 142.7, 140.2, 128.4, 127.5, 127.4, 123.9, 122.0, 120.6, 111.5, 55.6, 54.0, 47.1, 41.8, 19.8, 19.1; FT-IR (KBr) 3026, 2970, 2923, 2870, 1623, 1586, 1493, 1464, 1400, 1363, 1236, 1204, 1184, 1112, 1047, 1027 cm−1; anal. calcd for C19H22N2OSe: C, 61.12; H, 5.94; N, 7.50. Found: C, 61.19; H, 5.90; N, 7.47%.
9 ethyl acetate–hexane Rf = 0.60; colorless solid; yield 80% (149 mg); mp 107–108 °C; 1H NMR (400 MHz, CDCl3) δ 7.40 (d, J = 7.6 Hz, 2H), 7.27–7.18 (m, 3H), 6.99 (t, J = 8.0 Hz, 1H), 6.93 (d, J = 7.2 Hz, 1H), 6.84 (t, J = 7.6 Hz, 2H), 4.83–4.80 (m, 1H), 4.72 (t, J = 7.2 Hz, 1H), 3.83 (dd, J = 10.4, 6.4 Hz, 1H), 3.76 (s, 3H), 3.63 (dd, J = 10.4, 7.6 Hz, 1H), 1.26 (t, J = 7.6 Hz, 6H); 13C NMR (100 MHz, CDCl3) δ 157.2, 151.6, 142.7, 140.2, 128.4, 127.5, 127.4, 123.9, 122.0, 120.6, 111.5, 55.6, 54.0, 47.1, 41.8, 19.8, 19.1; FT-IR (KBr) 3026, 2970, 2923, 2870, 1623, 1586, 1493, 1464, 1400, 1363, 1236, 1204, 1184, 1112, 1047, 1027 cm−1; anal. calcd for C19H22N2OSe: C, 61.12; H, 5.94; N, 7.50. Found: C, 61.19; H, 5.90; N, 7.47%.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 ethyl acetate–hexane Rf = 0.62; colorless liquid; yield 83% (148 mg); 1H NMR (400 MHz, CDCl3) δ 7.40 (d, J = 7.2 Hz, 2H), 7.29–7.19 (m, 3H), 7.14 (t, J = 7.6 Hz, 1H), 6.85–6.80 (m, 3H), 4.81–4.77 (m, 1H), 4.72 (t, J = 6.8 Hz, 1H), 3.80 (dd, J = 10.4, 6.4 Hz, 1H), 3.62 (dd, J = 10.4, 8.0 Hz, 1H), 2.30 (s, 3H), 1.28 (d, J = 4.8 Hz, 3H), 1.26 (d, J = 4.4 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 156.0, 153.2, 140.1, 138.3, 128.5, 127.6, 127.5, 123.8, 122.3, 118.3, 53.6, 47.0, 41.8, 21.3, 19.9, 19.2; FT-IR (neat) 3030, 2971, 2927, 2862, 1613, 1593, 1455, 1400, 1362, 1282, 1207, 1185, 1069 cm−1; anal. calcd for C19H22N2Se: C, 63.86; H, 6.21; N, 7.84. Found: C, 63.87; H, 6.23; N, 7.80%.
9 ethyl acetate–hexane Rf = 0.62; colorless liquid; yield 83% (148 mg); 1H NMR (400 MHz, CDCl3) δ 7.40 (d, J = 7.2 Hz, 2H), 7.29–7.19 (m, 3H), 7.14 (t, J = 7.6 Hz, 1H), 6.85–6.80 (m, 3H), 4.81–4.77 (m, 1H), 4.72 (t, J = 6.8 Hz, 1H), 3.80 (dd, J = 10.4, 6.4 Hz, 1H), 3.62 (dd, J = 10.4, 8.0 Hz, 1H), 2.30 (s, 3H), 1.28 (d, J = 4.8 Hz, 3H), 1.26 (d, J = 4.4 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 156.0, 153.2, 140.1, 138.3, 128.5, 127.6, 127.5, 123.8, 122.3, 118.3, 53.6, 47.0, 41.8, 21.3, 19.9, 19.2; FT-IR (neat) 3030, 2971, 2927, 2862, 1613, 1593, 1455, 1400, 1362, 1282, 1207, 1185, 1069 cm−1; anal. calcd for C19H22N2Se: C, 63.86; H, 6.21; N, 7.84. Found: C, 63.87; H, 6.23; N, 7.80%.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 ethyl acetate–hexane Rf = 0.58; yellow liquid; yield 89% (168 mg); 1H NMR (400 MHz, CDCl3) δ 7.39 (d, J = 8.0 Hz, 2H), 7.30–7.22 (m, 3H), 7.18 (d, J = 8.4 Hz, 2H), 6.87 (d, J = 8.4 Hz, 2H), 4.78–4.68 (m, 2H), 3.84 (dd, J = 10.4, 6.8 Hz, 1H), 3.65 (dd, J = 10.4, 8.0 Hz, 1H), 1.24 (t, J = 6.4 Hz, 6H); 13C NMR (100 MHz, CDCl3) δ 156.8, 151.8, 139.8, 128.7, 128.6, 128.0, 127.8, 127.4, 123.0, 53.8, 47.2, 42.2, 19.8, 19.2; FT-IR (neat) 3061, 3029, 2972, 2930, 2870, 1614, 1585, 1485, 1404, 1364, 1274, 1243, 1205, 1185, 1162, 1088, 1068, 1009 cm−1; anal. calcd for C18H19ClN2Se: C, 57.23; H, 5.07; N, 7.42. Found: C, 57.31; H, 5.08; N, 7.39%.
9 ethyl acetate–hexane Rf = 0.58; yellow liquid; yield 89% (168 mg); 1H NMR (400 MHz, CDCl3) δ 7.39 (d, J = 8.0 Hz, 2H), 7.30–7.22 (m, 3H), 7.18 (d, J = 8.4 Hz, 2H), 6.87 (d, J = 8.4 Hz, 2H), 4.78–4.68 (m, 2H), 3.84 (dd, J = 10.4, 6.8 Hz, 1H), 3.65 (dd, J = 10.4, 8.0 Hz, 1H), 1.24 (t, J = 6.4 Hz, 6H); 13C NMR (100 MHz, CDCl3) δ 156.8, 151.8, 139.8, 128.7, 128.6, 128.0, 127.8, 127.4, 123.0, 53.8, 47.2, 42.2, 19.8, 19.2; FT-IR (neat) 3061, 3029, 2972, 2930, 2870, 1614, 1585, 1485, 1404, 1364, 1274, 1243, 1205, 1185, 1162, 1088, 1068, 1009 cm−1; anal. calcd for C18H19ClN2Se: C, 57.23; H, 5.07; N, 7.42. Found: C, 57.31; H, 5.08; N, 7.39%.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 ethyl acetate–hexane Rf = 0.57; yellow liquid; yield 85% (159 mg); 1H NMR (400 MHz, CDCl3) δ 7.44 (d, J = 7.2 Hz, 2H), 7.33–7.25 (m, 3H), 6.97 (d, J = 7.2 Hz, 2H), 6.85 (d, J = 8.8 Hz, 2H), 4.82–4.75 (m, 2H), 3.86 (dd, J = 10.4, 6.4 Hz, 1H), 3.73 (s, 3H), 3.67 (dd, J = 10.4, 7.6 Hz, 1H), 1.31 (d, J = 4.8 Hz, 3H), 1.29 (d, J = 4.4 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 156.8, 155.7, 146.8, 140.2, 128.6, 127.6, 127.5, 122.3, 113.9, 55.1, 53.7, 47.1, 41.8, 19.8, 19.2; FT-IR (neat) 3031, 2971, 2870, 2833, 1614, 1504, 1464, 1401, 1363, 1294, 1239, 1205, 1185, 1067, 1035, 981 cm−1; anal. calcd for C19H22N2OSe: C, 61.12; H, 5.94; N, 7.50. Found: C, 61.05; H, 5.96; N, 7.54%.
9 ethyl acetate–hexane Rf = 0.57; yellow liquid; yield 85% (159 mg); 1H NMR (400 MHz, CDCl3) δ 7.44 (d, J = 7.2 Hz, 2H), 7.33–7.25 (m, 3H), 6.97 (d, J = 7.2 Hz, 2H), 6.85 (d, J = 8.8 Hz, 2H), 4.82–4.75 (m, 2H), 3.86 (dd, J = 10.4, 6.4 Hz, 1H), 3.73 (s, 3H), 3.67 (dd, J = 10.4, 7.6 Hz, 1H), 1.31 (d, J = 4.8 Hz, 3H), 1.29 (d, J = 4.4 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 156.8, 155.7, 146.8, 140.2, 128.6, 127.6, 127.5, 122.3, 113.9, 55.1, 53.7, 47.1, 41.8, 19.8, 19.2; FT-IR (neat) 3031, 2971, 2870, 2833, 1614, 1504, 1464, 1401, 1363, 1294, 1239, 1205, 1185, 1067, 1035, 981 cm−1; anal. calcd for C19H22N2OSe: C, 61.12; H, 5.94; N, 7.50. Found: C, 61.05; H, 5.96; N, 7.54%.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 ethyl acetate–hexane Rf = 0.56; colorless liquid; yield 73% (87 mg); 1H NMR (400 MHz, CDCl3) δ 7.36–7.25 (m, 5H), 5.21–5.14 (m, 1H), 4.79 (dd, J = 7.6, 6.8 Hz, 1H), 4.28 (dd, J = 11.6, 8.4 Hz, 1H), 3.94 (dd, J = 11.6, 6.8 Hz, 1H), 1.25 (d, J = 6.8 Hz, 3H), 1.19 (d, J = 6.8 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 194.7, 138.9, 129.1, 128.5, 127.2, 58.4, 49.6, 47.2, 19.6, 19.2; FT-IR (neat) 3056, 3031, 2972, 2930, 2873, 1668, 1601, 1472, 1430, 1367, 1300, 1275, 1239, 1201, 1184, 1076, 1029, 973 cm−1; anal. calcd for C12H15NS2: C, 60.72; H, 6.37; N, 5.90; S, 27.02. Found: C, 60.63; H, 6.38; N, 5.93; S, 27.06%.
9 ethyl acetate–hexane Rf = 0.56; colorless liquid; yield 73% (87 mg); 1H NMR (400 MHz, CDCl3) δ 7.36–7.25 (m, 5H), 5.21–5.14 (m, 1H), 4.79 (dd, J = 7.6, 6.8 Hz, 1H), 4.28 (dd, J = 11.6, 8.4 Hz, 1H), 3.94 (dd, J = 11.6, 6.8 Hz, 1H), 1.25 (d, J = 6.8 Hz, 3H), 1.19 (d, J = 6.8 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 194.7, 138.9, 129.1, 128.5, 127.2, 58.4, 49.6, 47.2, 19.6, 19.2; FT-IR (neat) 3056, 3031, 2972, 2930, 2873, 1668, 1601, 1472, 1430, 1367, 1300, 1275, 1239, 1201, 1184, 1076, 1029, 973 cm−1; anal. calcd for C12H15NS2: C, 60.72; H, 6.37; N, 5.90; S, 27.02. Found: C, 60.63; H, 6.38; N, 5.93; S, 27.06%.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 ethyl acetate–hexane Rf = 0.52; colorless solid; yield 85% (134 mg); mp 69–70 °C; 1H NMR (400 MHz, CDCl3) 7.46 (d, J = 8.4 Hz, 2H), 7.22 (d, J = 8.4 Hz, 2H), 5.18–5.11 (m, 1H), 4.71 (dd, J = 8.0, 6.4 Hz, 1H), 4.26 (dd, J = 11.6, 8.4 Hz, 1H), 3.87 (dd, J = 11.6, 6.4 Hz, 1H), 1.23 (d, J = 7.2 Hz, 3H), 1.17 (d, J = 6.8 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 194.1, 138.1, 132.1, 128.8, 122.3, 58.2, 49.6, 46.4, 19.4, 19.2; FT-IR (KBr) 3042, 2970, 2923, 2867, 1663, 1610, 1582, 1510, 1481, 1432, 1369, 1301, 1241, 1199, 1182, 1072, 1029, 1008, 823 cm−1; anal. calcd for C12H14BrNS2: C, 45.57; H, 4.46; N, 4.43; S, 20.28. Found: C, 45.65; H, 4.44; N, 4.40; S, 20.32%.
9 ethyl acetate–hexane Rf = 0.52; colorless solid; yield 85% (134 mg); mp 69–70 °C; 1H NMR (400 MHz, CDCl3) 7.46 (d, J = 8.4 Hz, 2H), 7.22 (d, J = 8.4 Hz, 2H), 5.18–5.11 (m, 1H), 4.71 (dd, J = 8.0, 6.4 Hz, 1H), 4.26 (dd, J = 11.6, 8.4 Hz, 1H), 3.87 (dd, J = 11.6, 6.4 Hz, 1H), 1.23 (d, J = 7.2 Hz, 3H), 1.17 (d, J = 6.8 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 194.1, 138.1, 132.1, 128.8, 122.3, 58.2, 49.6, 46.4, 19.4, 19.2; FT-IR (KBr) 3042, 2970, 2923, 2867, 1663, 1610, 1582, 1510, 1481, 1432, 1369, 1301, 1241, 1199, 1182, 1072, 1029, 1008, 823 cm−1; anal. calcd for C12H14BrNS2: C, 45.57; H, 4.46; N, 4.43; S, 20.28. Found: C, 45.65; H, 4.44; N, 4.40; S, 20.32%.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 ethyl acetate–hexane Rf = 0.40; colorless solid; yield 81% (108 mg); mp 62–63 °C; 1H NMR (400 MHz, CDCl3) 7.27 (d, J = 8.8 Hz, 2H), 6.86 (d, J = 8.8 Hz, 2H), 5.23–5.13 (m, 1H), 4.74 (t, J = 8.0 Hz, 1H), 4.22 (dd, J = 11.6, 8.0 Hz, 1H), 3.88 (dd, J = 11.6, 6.8 Hz, 1H), 3.77 (s, 3H), 1.24 (d, J = 6.8 Hz, 3H), 1.18 (d, J = 6.8 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 194.3, 159.3, 130.5, 128.2, 114.7, 58.3, 55.1, 49.3, 46.5, 19.3, 19.0; FT-IR (KBr) 3009, 2975, 2948, 2925, 2898, 2828, 1662, 1607, 1509, 1480, 1465, 1433, 1369, 1303, 1280, 1250, 1200, 1175, 1075, 1033, 831 cm−1; anal. calcd for C13H17NOS2: C, 58.39; H, 6.41; N, 5.24; S, 23.98. Found: C, 58.50; H, 6.40; N, 5.21; S, 23.94%.
9 ethyl acetate–hexane Rf = 0.40; colorless solid; yield 81% (108 mg); mp 62–63 °C; 1H NMR (400 MHz, CDCl3) 7.27 (d, J = 8.8 Hz, 2H), 6.86 (d, J = 8.8 Hz, 2H), 5.23–5.13 (m, 1H), 4.74 (t, J = 8.0 Hz, 1H), 4.22 (dd, J = 11.6, 8.0 Hz, 1H), 3.88 (dd, J = 11.6, 6.8 Hz, 1H), 3.77 (s, 3H), 1.24 (d, J = 6.8 Hz, 3H), 1.18 (d, J = 6.8 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 194.3, 159.3, 130.5, 128.2, 114.7, 58.3, 55.1, 49.3, 46.5, 19.3, 19.0; FT-IR (KBr) 3009, 2975, 2948, 2925, 2898, 2828, 1662, 1607, 1509, 1480, 1465, 1433, 1369, 1303, 1280, 1250, 1200, 1175, 1075, 1033, 831 cm−1; anal. calcd for C13H17NOS2: C, 58.39; H, 6.41; N, 5.24; S, 23.98. Found: C, 58.50; H, 6.40; N, 5.21; S, 23.94%.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 ethyl acetate–hexane Rf = 0.49; colorless liquid; yield 77% (97 mg); 1H NMR (400 MHz, CDCl3) 7.25 (d, J = 8.0 Hz, 2H), 7.14 (d, J = 7.6 Hz, 2H), 5.20–5.13 (m, 1H), 4.77 (t, J = 8.0 Hz, 1H), 4.26 (dd, J = 11.6, 8.4 Hz, 1H), 3.91 (dd, J = 11.6, 6.8 Hz, 1H), 2.31 (s, 3H), 1.24 (d, J = 6.8 Hz, 3H), 1.19 (d, J = 6.8 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 194.6, 138.2, 135.7, 129.6, 127.0, 58.3, 49.5, 46.9, 21.0, 19.5, 19.1; FT-IR (neat) 3017, 2972, 2927, 2873, 1654, 1610, 1512, 1472, 1431, 1360, 1297, 1239, 1185, 1076, 1036, 816 cm−1; anal. calcd for C13H17NS2: C, 62.11; H, 6.82; N, 5.57; S, 25.50. Found: C, 62.21; H, 6.80; N, 5.54; S, 25.45%.
9 ethyl acetate–hexane Rf = 0.49; colorless liquid; yield 77% (97 mg); 1H NMR (400 MHz, CDCl3) 7.25 (d, J = 8.0 Hz, 2H), 7.14 (d, J = 7.6 Hz, 2H), 5.20–5.13 (m, 1H), 4.77 (t, J = 8.0 Hz, 1H), 4.26 (dd, J = 11.6, 8.4 Hz, 1H), 3.91 (dd, J = 11.6, 6.8 Hz, 1H), 2.31 (s, 3H), 1.24 (d, J = 6.8 Hz, 3H), 1.19 (d, J = 6.8 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 194.6, 138.2, 135.7, 129.6, 127.0, 58.3, 49.5, 46.9, 21.0, 19.5, 19.1; FT-IR (neat) 3017, 2972, 2927, 2873, 1654, 1610, 1512, 1472, 1431, 1360, 1297, 1239, 1185, 1076, 1036, 816 cm−1; anal. calcd for C13H17NS2: C, 62.11; H, 6.82; N, 5.57; S, 25.50. Found: C, 62.21; H, 6.80; N, 5.54; S, 25.45%.Acknowledgements
We thank Science and Engineering Research Board (SR/S1/OC-55/2011) and Council of Scientific and Industrial Research (02(0088)/12/EMR-II) for financial Support. M.S. is grateful to UGC, New Delhi, for SRF fellowship. Central instruments facility (CIF) IIT Guwahati, for NMR analysis is thankfully acknowledged.Notes and references
- (a) Organic Synthesis in Water, ed. P. A. Grieco, Springer, New York, 1997 Search PubMed; (b) Clean Solvents: Alternative Media for Chemical Reactions and Processing, ed. M. A. Abraham and L. Moens, ACS Symp. Ser., 2002, p. 819 Search PubMed; (c) Organic Reactions in Water, ed. U. M. Lindstroem, Blackwell, Oxford, UK, 2007 Search PubMed; (d) H. C. Hailes, Org. Process Res. Dev., 2007, 11, 114 CrossRef CAS; (e) Handbook of Green Chemistry, ed. P. T. Anastas, Wiley-VCH, Weinheim, 2010, vol. 5 Search PubMed; (f) P. Anastas and N. Eghbali, Chem. Soc. Rev., 2010, 39, 301 RSC.
- For examples of aqueous organic reactions, see: (a) A. Lubineau, J. Org. Chem., 1986, 51, 2142 CrossRef CAS; (b) R. Breslow, Acc. Chem. Res., 1991, 24, 159 CrossRef CAS; (c) W. Blokzijl and J. B. F. N. Engberts, J. Am. Chem. Soc., 1992, 114, 5440 CrossRef CAS; (d) U. M. Lindstroem, Chem. Rev., 2002, 102, 2751 CrossRef CAS PubMed; (e) C. J. Li, Chem. Rev., 2005, 105, 3095 CrossRef CAS PubMed; (f) I. Vilotijevic and T. F. Jamison, Science, 2007, 317, 1189 CrossRef CAS PubMed; (g) L. Rout, P. Saha, S. Jammi and T. Punniyamurthy, Eur. J. Org. Chem., 2008, 640 CrossRef CAS; (h) E. Coutouli-Argyropoulou, P. Sarridis and P. Gkizis, Green Chem., 2009, 11, 1906 RSC; (i) S. Kobayashi, T. Endo and M. Ueno, Angew. Chem., Int. Ed., 2011, 50, 12262 CrossRef CAS PubMed; (j) S. Kobayashi, P. Xu, T. Endo, M. Ueno and T. Kitanosono, Angew. Chem., Int. Ed., 2012, 51, 12763 CrossRef CAS PubMed; (k) M.-O. Simon and C.-J. Li, Chem. Soc. Rev., 2012, 41, 1415 RSC; (l) S. Kobayashi, Pure Appl. Chem., 2013, 85, 1089 CrossRef CAS PubMed; (m) T. Kitanosono, P. Xu and S. Kobayashi, Chem.–Asian J., 2014, 9, 179 CrossRef CAS PubMed.
- For examples of reactions “on water”, see: (a) D. C. Rideout and R. Breslow, J. Am. Chem. Soc., 1980, 102, 7816 CrossRef CAS; (b) J. E. Klijn and J. B. F. N. Engberts, Nature, 2005, 435, 746 CrossRef CAS PubMed; (c) S. Narayan, J. Muldoon, M. G. Finn, V. V. Fokin, H. C. Kolb and K. B. Sharpless, Angew. Chem., Int. Ed., 2005, 44, 3275 CrossRef CAS PubMed; (d) D. González-Cruz, D. Tejedor, P. de Armas, E. Q. Morales and F. García-Tellado, Chem. Commun., 2006, 2798 RSC; (e) P. G. Cozzi and L. Zoli, Green Chem., 2007, 9, 1292 RSC; (f) M. Y. Liao and J. B. Wang, Green Chem., 2007, 9, 184 RSC; (g) D. González-Cruz, D. Tejedo, P. de Armas and F. Garcia-Tellado, Chem.–Asian J., 2007, 13, 4823 Search PubMed; (h) Y. S. Jung and R. A. Marcus, J. Am. Chem. Soc., 2007, 129, 5492 CrossRef CAS PubMed; (i) N. Shapiro and A. Vigalok, Angew. Chem., Int. Ed., 2008, 47, 2849 CrossRef CAS PubMed; (j) M. C. Pirrung, K. D. Sarma and J. M. Wang, J. Org. Chem., 2008, 73, 8723 CrossRef CAS PubMed; (k) P. G. Cozzi and L. Zoli, Angew. Chem., Int. Ed., 2008, 47, 4162 CrossRef CAS PubMed; (l) S. Jammi, M. A. Ali, S. Sakthivel, L. Rout and T. Punniyamurthy, Chem.–Asian J., 2009, 4, 314 CrossRef CAS PubMed; (m) S. Jammi and T. Punniyamurthy, Eur. J. Inorg. Chem., 2009, 2508 CrossRef CAS; (n) C. Duplais, A. Krasovskiy, A. Wattenberg and B. H. Lipshutz, Chem. Commun., 2010, 46, 562 RSC; (o) N. Mase and C. F. Barbas III, Org. Biomol. Chem., 2010, 8, 4043 RSC; (p) M. J. Raihan, V. Kavala, C.-W. Kuo, B. R. Raju and C.-F. Yao, Green Chem., 2010, 12, 1090 RSC; (q) V. Chudasama, J. M. Ahern, D. V. Dhokia, R. J. Fitzmaurice and S. Caddick, Chem. Commun., 2011, 47, 3269 RSC; (r) S. Melloui, L. Bousekkine, A. B. Theberge and W. T. S. Huck, Angew. Chem., Int. Ed., 2012, 51, 7981 CrossRef PubMed; (s) M. Sengoden and T. Punniyamurthy, Angew. Chem., Int. Ed., 2013, 52, 572 CrossRef CAS PubMed.
- For the biological properties of five membered heterocycles, see: (a) M. E. Dyer and D. Swern, Chem. Rev., 1967, 67, 197 CrossRef; (b) T. Fujinami, T. Suziki, M. Kamiya, S. Fukuzawa and S. Sakai, Chem. Lett., 1985, 199 CrossRef CAS; (c) H. Kisch, R. Millini and I. Wang, Chem. Ber., 1986, 119, 1090 CrossRef CAS; (d) I. Shibata, A. Baba, H. Iwasaki and H. Matsuda, J. Org. Chem., 1986, 51, 2177 CrossRef CAS; (e) B. M. Trost and A. R. Sudhakar, J. Am. Chem. Soc., 1987, 109, 3792 CrossRef CAS; (f) M. Fujiwara, A. Baba and H. Matsuda, J. Heterocycl. Chem., 1988, 25, 1351 CrossRef CAS; (g) M. Fujiwara, M. Imada, A. Baba and H. Matsuda, J. Org. Chem., 1988, 53, 5974 CrossRef CAS; (h) A. L. Cardoso and T. M. V. D. Pinhoe Melo, Eur. J. Org. Chem., 2012, 6479 CAS; (i) Ö. Poyraz, V. U. Jeankumar, S. Saxena, R. Schnell, M. Haraldsson, P. Yogeeswari, D. Sriram and G. Schneider, J. Med. Chem., 2013, 56, 6457 CrossRef PubMed; (j) D. Havrylyuk, B. Zimenkovsky, O. Vasylenko, C. W. Day, D. F. Smee, P. Grellier and R. Lesyk, Eur. J. Med. Chem., 2013, 66, 228 CrossRef CAS PubMed.
- For examples, see: (a) J.-O. Baeg and H. Alper, J. Am. Chem. Soc., 1994, 116, 1220 CrossRef CAS; (b) J.-O. Baeg and H. Alper, J. Org. Chem., 1992, 57, 157 CrossRef CAS; (c) J.-O. Baeg, C. Bensimon and H. Alper, J. Am. Chem. Soc., 1995, 117, 4700 CrossRef CAS; (d) C. D. Butler, A. G. Inman and H. Alper, J. Org. Chem., 2000, 65, 5887 CrossRef PubMed; (e) B. M. Trost and D. R. Fandrick, J. Am. Chem. Soc., 2003, 125, 11836 CrossRef CAS PubMed; (f) A. Okano, S. Oishi, T. Tanaka, N. Fujii and H. Ohno, J. Org. Chem., 2010, 75, 3396 CrossRef CAS PubMed.
- (a) T. Munegumi, I. Azumaya, T. Kato, H. Masu and S. Saito, Org. Lett., 2006, 8, 379 CrossRef CAS PubMed; (b) K. Zhang, P. R. Chopade and J. Louie, Tetrahedron Lett., 2008, 49, 4306 CrossRef CAS PubMed.
- R. A. Craig, N. R. O'Connor, A. F. G. Goldberg and B. M. Stoltz, Chem.–Asian J., 2014, 20, 4806 CAS.
- J.-Y. Wu, Z.-B. Luo, L.-X. Dai and X.-L. Hou, J. Org. Chem., 2008, 73, 9137 CrossRef CAS PubMed.
- (a) U. K. Nadir and N. Basu, Tetrahedron Lett., 1992, 33, 7949 CrossRef CAS; (b) U. K. Nadir and N. Basu, J. Org. Chem., 1995, 60, 1458 CrossRef CAS.
- E. Pfeil and K. Milzner, Angew. Chem., Int. Ed., 1966, 5, 667 CrossRef CAS.
- R. Nomura, T. Nakano, Y. Nishio, S. Ogawa, A. Ninagawa and H. Matsuda, Chem. Ber., 1989, 122, 240 CrossRef.
- For examples of pyrrolidine catalyzed reactions, see: (a) F. Li, J. Nie, J.-W. Wu, Y. Zheng and J.-A. Ma, J. Org. Chem., 2012, 77, 2398 CrossRef CAS PubMed; (b) F.-Z. Bao, X.-B. Wang and L.-Y. Kong, Tetrahedron Lett., 2013, 54, 1405 CrossRef CAS PubMed; (c) S. Morales, F. G. Guijarro, J. L. G. Ruano and M. B. Cid, J. Am. Chem. Soc., 2014, 136, 1082 CrossRef CAS PubMed.
- (a) Z.-Z. Yang, L.-N. He, S.-Y. Peng and A.-H. Liu, Green Chem., 2010, 12, 1850 RSC; (b) I. Saikia, B. Kashyap and P. Phukan, Chem. Commun., 2011, 47, 2967 RSC.
- M. Dai, C. Wang, G. Dong, J. Xiang, T. Luo, B. Liang, J. Chen and Z. Yang, Eur. J. Org. Chem., 2003, 4346 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: NMR (1H, 13C) spectra of the products. CCDC 1025804. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c4ra08902b |
| This journal is © The Royal Society of Chemistry 2014 |