Engineered nanoporous gold microspheres for stochastic sensing
Abstract
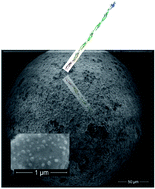
Engineered nanoporous gold microspheres were designed and used as new materials for stochastic microsensors. Carcinoembryonic antigen was used as model analyte to prove the stochastic sensing capabilities of the new material. A new sensor based on gold particles was used for the assay of the carcinoembryonic antigen in biological fluids. Stochastic sensing was used to determine the antigen in the samples. The working concentration range of the stochastic microsensor (1.6 × 10−8 to 1.6 × 10−5 mg mL−1) as well as its sensitivity (3.0 × 103 s mg mL−1) and limit of quantification (16 ng mL−1) made possible its reliable utilization for screening tests of whole blood samples for the carcinoembryonic antigen.

 Please wait while we load your content...
Please wait while we load your content...