An electrogenerated chemiluminescent biosensor based on a g-C3N4–hemin nanocomposite and hollow gold nanoparticles for the detection of lactate†
Abstract
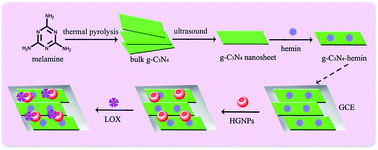
In this article, a new electrochemiluminescent (ECL) biosensor based on a g-C3N4–hemin nanocomposite and hollow gold nanoparticles (HGNPs) was constructed to detect lactate. Firstly, the g-C3N4 nanosheets were prepared through ultrasonication-assisted liquid exfoliation of bulk g-C3N4, which was obtained through polymerizing melamine under 600 °C. Then, the nanocomposites of g-C3N4 nanosheets and hemin were prepared to modify a glassy carbon electrode. Subsequently, HGNPs were self-assembled onto the electrode for adsorbing lactate oxidase to achieve a lactate biosensor. Due to the excellent catalytic effect of g-C3N4–hemin and HGNPs on the luminol/H2O2 ECL system, the as-prepared biosensor exhibited a good response performance to lactate with a linear range of 1.7 × 10−8 to 5.0 × 10−4 M and a detection limit of 5.5 × 10−9 M. In addition, the prepared ECL biosensor exhibited satisfying reproducibility and stability. The g-C3N4–hemin nanocomposite might have great potential application in a luminol/H2O2 ECL system.

 Please wait while we load your content...
Please wait while we load your content...