A novel ultra-thin catalyst layer based on wheat ear-like catalysts for polymer electrolyte membrane fuel cells†
Abstract
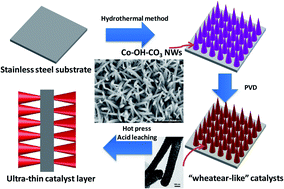
Wheat ear-like catalysts were prepared on Co–OH–CO3 nanowires to design an ultra-thin catalyst layer (UTCL). Without any ionomers, the UTCL exhibited a maximum power density of 481 mW cm−2 at an ultra-low Pt loading of 43 μg cm−2Pt, resulting in a relatively high Pt utilization of 11.2 kW g−1Pt. It is expected that the nanostructured thin film materials will lead to further technological advancements in fuel cells and other applications.

 Please wait while we load your content...
Please wait while we load your content...