Synthesis, characterization and degradation of organic dye over Co3O4 nanoparticles prepared from new binuclear complex precursors
Mojgan Goudarzia,
Mehdi Bazarganipourb and
Masoud Salavati-Niasari*a
aInstitute of Nano Science and Nano Technology, University of Kashan, Kashan, P. O. Box 87317-51167, Islamic Republic of Iran. E-mail: salavati@kashanu.ac.ir; Fax: +98 361 555 29 30; Tel: +98 361 5555 333
bNanotechnology and Advanced Materials Institute, Isfahan University of Technology, P. O. Box 84156-83111, Isfahan, Islamic Republic of Iran
First published on 15th September 2014
Abstract
The synthesis and characterization of Co3O4 nanoparticles via solid state thermal decomposition of a binuclear cobalt(III) complex, as a new precursor is reported. Solid state thermal decomposition of the complex at 450 °C for 3 h produces Co3O4 nanoparticles which are characterized by FT-IR spectroscopy, X-ray diffraction (XRD), scanning electron microscopy (SEM), and transmission electron microscopy (TEM). SEM and TEM images exhibit the quasi-spherical shape of the Co3O4 nanoparticles of between 20 and 30 nm. Moreover, adsorption of methylene orange dye on Co3O4 nanoparticles was investigated and the uptake% was determined to be >45% in 200 min.
1. Introduction
In recent years, the preparation of transition metal oxide nanoparticles has been widely studied due to their versatile applications, such as chemical sensors, catalysts, antistatic coating, radioactive waste management, etc.1,2 The synthesis and characterization of different transition metal oxide nanoparticles with specific sizes and morphology have been reported.3–8Co3O4 is a significant antiferromagnetic p-type semiconductor with outstanding properties such as gas-sensing, catalytic and electrochemical properties, and has been investigated greatly for applications in solid-state sensors, electrochromic devices and heterogeneous catalysts as well as lithium batteries.9,10 When decreased down to the nanometer scale, Co3O4 were found to have interesting magnetic, optical, field emission and electrochemical properties that are interesting in device applications.11,12 Thus enormous attempts have been guided in recent years to the synthesis and investigation of properties of Co3O4 nanostructures.
To the present time, various approaches have been presented in order to prepare Co3O4 nanoparticles, involving sol–gel approach,13 hydrothermal approach,14,15 combustion approach,16 microemulsion approach,17 chemical spray pyrolysis,18 chemical vapor deposition,19,20 thermal decomposition of cobalt precursors,21–27 sonochemical approach,28,29 co-precipitation,30 microwave irradiation,31 and mechanochemical processing.32
Between the numerous procedures developed for preparing metal oxides nanoparticles, the thermal decomposition molecular precursor method has been considered as one of the most useful and applicable techniques, because it not only capable to prevent special instruments and complicated procedures and severe preparation conditions, but also supplies good control over purity, composition, homogeneity, phase and microstructure of the resultant products.33,34
In our group, we have been fascinated for a few years in the synthesis of metal, metal oxide and magnetic nanoparticles, utilizing new inorganic precursor, taking profit of the tools of organometallic chemistry.35–38 A main fascinate at this time is in the evolvement of organometallic or inorganic compound for preparation of nanoparticles. Utilizing of the novel compound can be useful and open a new method for synthesizing nanoparticles to control nanoparticle size, shape and distribution size.
Herein, we report on the preparation of Co3O4 nanoparticles by direct solid-state thermal decomposition of the solid molecular precursor; [(NH3)5Co(O2)Co(NH3)5](NO3)4; complex as a new precursor for the first time.
2. Experimental
2.1. Materials and characterization
All the chemical reagents used in our experiments were of analytical grade and were used as received without further purification. Cobalt(II) nitrate hexahydrate, ammonia and sodium nitrate were obtained from Merck Co. XRD patterns were recorded by a Rigaku D-max C III, X-ray diffractometer using Ni-filtered Cu Kα radiation. Scanning electron microscopy (SEM) images were obtained on Philips XL-30ESEM. Transmission electron microscopy (TEM) images were obtained on a Philips EM208 transmission electron microscope with an accelerating voltage of 100 kV. Fourier transform infrared (FT-IR) spectra were recorded on Shimadzu Varian 4300 spectrophotometer in KBr pellets. The magnetic measurement was carried out in a vibrating sample magnetometer (VSM) (BHV-55, Riken, Japan) at room temperature.2.2. Synthesis of binuclear precursor; [(NH3)5Co(O2)Co (NH3)5](NO3)4 (ref. 39)
5 g quantity of cobalt(II) nitrate hexahydrate is dissolved in 100 ml of water and filtered. Aqueous ammonia (15 M, 25 ml) is added, and after the mixture has been cooled to 5–15 °C, a current of oxygen (1000 ml min−1, i.e., a brisk stream) is passed through the cooled solution for an hour, the mixture being stirred with a magnetic stirrer. Oxygen may be replaced by air, in which case longer bubbling (2–3 h) at 0–5 °C is recommended. Sodium nitrate (2 g) dissolved in 5 ml of water is then added; oxygen (or air) is passed through the solution for another hour, with the mixture cooled in ice toward the end of this period. The dark brown crystals are gathered on a glass filter and washed with a small quantity of 15 M aqueous ammonia and then with ethanol.2.3. Synthesis of Co3O4 nanoparticles
Black Co3O4-based nanocomposites were produced by subjecting 0.01 mol of the as-prepared binuclear complexes Co(III); [(NH3)5Co(O2)Co(NH3)5](NO3)4; powders to heat treatment at a relatively low temperature (773 K) in air. An average temperature increase of 303 K every minute was selected before the temperature reached 773 K, and after keeping the thermal treatment at 773 K for 3 h, it was allowed to cool to the room temperature naturally.3. Results and discussion
Fig. 1a–c reveal a typical XRD pattern of the sample obtained by thermal decomposition of binuclear complexes Co(III). All the reflection peaks in this pattern could be readily indexed to crystalline Co3O4 nanoparticles (JCPDS Card file no. 78-1970). All of the reflection peaks could be readily indexed to crystalline cubic phase Co3O4 with a lattice constant of a = 8.065 Å. The crystallite sizes of the as-synthesized Co3O4 nanoparticles, Dc, was calculated from the major diffraction peaks of the base of (1 1 1) using the Scherrer formula (eqn (1)),40
Dc = Kλ/(β![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cos cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ) θ)
| (1) |
 | ||
| Fig. 1 XRD patterns of Co3O4 nanoparticles obtained by the thermal decomposition of the precursor at (a) 250, (b) 300, (c) 400 °C for 5 h. | ||
FT-IR spectroscopy is an applicable device to understand the functional group of any organic molecule (Fig. 2). For the complex, the characteristic bands of NH3 are observed at approximately 3438, 1638, 1054 cm−1 and band of the NO3 appeared at 790 cm−1.27 Metal oxides; Co3O4; generally give absorption bands below 1000 cm−1 resulting from interatomic vibrations. The spectra (Fig. 2) also included two strong absorption bands at ∼790 and 470 cm−1 which verify the spinel structure of Co3O4. The former peak at ∼790 cm−1 is ascribed to the stretching vibration mode of M–O in which M is Co+2 and is tetrahedrally coordinated. The band at ∼470 cm−1 can be assigned to the M–O in which M is Co+3 and so coordinates octahedrally.41 This result further approved the formation of Co3O4 nanoparticles by the above-mentioned thermal decomposition.
 | ||
| Fig. 2 IR spectra of the product obtained on thermal decomposition of the precursor at (a) 400, (b) 500, (c) 700 and (d) 900 °C for 5 h. | ||
The morphology of the Co3O4 nanoparticles is investigated by SEM (Fig. 3a–c). The SEM micrograph of the Co3O4 nanoparticles treated at 400 °C exhibits that the sample includes of agglomerated particles and the grains are approximately spherical with nearly uniform particle-size (Fig. 3a). By enhancing the treatment temperature to 600 °C, the primary particles and the aggregates tend to become larger, as can be seen from the SEM image (Fig. 3b and c).
The TEM images (Fig. 4) exhibit the presence of dense agglomerates. The particles have a spherical shape, and their distribution, likewise, is not uniformed (10–15 nm).
Fig. 5 exhibits the magnetic properties of the Co3O4 nanoparticles. The fine shape of the hysteresis loops is attributing to a weak ferromagnetic behavior, although bulk Co3O4 is antiferromagnetic.42 From the inset, the coercive field (Hc) and the remanent magnetization (Mr) are estimated to be 0.010 kOe and 0.001 emu g−1, respectively. The low coercive fields and remanent magnetizations approve that the Co3O4 nanoparticles have weak ferromagnetic properties. Co3O4 nanoparticles comprise of small magnetic domains, each characterized by its own randomly oriented magnetic moment. The total magnetic moment of the nanoparticles is the sum of these magnetic domains coupled by dipolar interactions. The change from an antiferromagnetic state for bulk Co3O4 to a weakly ferromagnetic state for Co3O4 nanoparticles can be assigned to uncompensated surface spins and/or finite size effects.43,44
The photocatalytic activity of the Co3O4 nanoparticles was evaluated by monitoring the degradation of methyl orange (MO) in an aqueous solution, under irradiation with visible light (Fig. 6, Scheme 1). Without light or nanoparticles, nearly no MO was break down after 200 min, revealing that the contribution of self-degradation was insignificant. However, Co3O4 nanoparticles exposed high photocatalytic activity. The heterogeneous photocatalytic processes include many steps, such as diffusion, adsorption and reaction, appropriate distribution of the pore is advantageous to diffusion of reactants and products, which prefer the photocatalytic reaction. In this paper, the enhanced photocatalytic activity may be ascribed to suitable distribution of the pore, high hydroxyl content and high separation rate of photo induced charge carriers.45
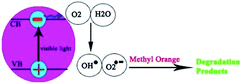 | ||
| Scheme 1 Reaction mechanism of MO photodegradation over Co3O4 nanoparticles under visible light irradiation. | ||
4. Conclusion
Adopting the self-prepared binuclear precursor Co(III) as precursor, Co3O4 nanoparticles have been synthesized by thermal decomposition method. The proposed methods to Co3O4 nanoparticles were simple, mild and cheap, which makes them very suitable for scale-up production. We have presented a simple method to synthesize Co3O4 nanoparticles through simple thermal decomposition of the [(NH3)5Co(O2)Co(NH3)5](NO3)4 complex as a new precursor for the first time. The synthesized Co3O4 nanoparticles can be used in some analytical applications such as removal of methylene orange dye, since the %uptake was found to be >45% within 200 min.Acknowledgements
The authors are grateful to University of Kashan for supporting this work by Grant no. (159271/195).References
- R. Xu, J. Wang, Q. Li, G. Sun, E. Wang and S. Li, J. Solid State Chem., 2009, 182, 3177 CrossRef CAS.
- A. El-Trass, H. Elshamy, I. El-Mehasseb and M. El-Kemary, Appl. Surf. Sci., 2012, 258, 2997 CrossRef CAS.
- A. Mehrani and A. Morsali, J. Inorg. Organomet. Polym. Mater., 2011, 21, 476 CrossRef CAS.
- M. Salavati-Niasari, F. Davar and M. Mazaheri, Polyhedron, 2008, 27, 3467 CrossRef CAS.
- S. Farhadi and J. Safabakhsh, J. Alloys Compd., 2012, 515, 180 CrossRef CAS.
- W. Jia, E. Reitz, P. Shimpi, E. G. Rodriguez, P. X. Gao and Y. Lei, Mater. Res. Bull., 2009, 44, 1681 CrossRef CAS.
- G. Sharma and P. Jeevanandam, Appl. Surf. Sci., 2012, 258, 3679 CrossRef CAS.
- A. D. Khalaji, J. Clust. Sci., 2013, 24, 185–189 CrossRef.
- M. Ando, T. Kobayashi, S. Iijima and M. Haruta, J. Mater. Chem., 1997, 7, 1779 RSC.
- Z. L. Zhang, H. R. Geng, L. S. Zheng and B. Du, J. Alloys Compd., 2005, 392, 317 CrossRef CAS.
- R. M. Wang, C. M. Liu, H. Z. Zhang, C. P. Chen and L. Guo, Appl. Phys. Lett., 2004, 85, 2080 CrossRef CAS.
- A. Khansari, M. Salavati-Niasari and A. KazemiBabaheydari, J. Cluster Sci., 2012, 23, 557 CrossRef CAS.
- M. E. Baydi, G. Poillerat, J. L. Rehspringer, J. L. Gautier, J. F. Koenig and P. Chartier, J. Solid State Chem., 1994, 109, 281 CrossRef.
- Y. Chen, Y. Zhang and S. Fu, Mater. Lett., 2007, 61, 701 CrossRef CAS.
- L. Li, Y. Chu, Y. Liu, J. L. Song, D. Wang and X. W. Du, Mater. Lett., 2008, 62, 1507 CrossRef CAS.
- F. Gu, C. Li, Y. Hu and L. Zhang, J. Cryst. Growth, 2007, 304, 369 CrossRef CAS.
- R. M. Wang, C. M. Liu, H. Z. Zhang, C. P. Chen, L. Guo, H. B. Xu and S. H. Yang, Appl. Phys. Lett., 2004, 85, 2080 CrossRef CAS.
- D. Y. Kim, S. H. Ju, H. Y. Koo, S. K. Hong and Y. C. J. Kangf, J. Alloys Compd., 2006, 417, 254 CrossRef CAS.
- A. U. Mane, K. Shalini, A. Wohlfart, A. Devi and S. A. Shivashankar, J. Cryst. Growth, 2002, 240, 157 CrossRef CAS.
- Y. Xuan, R. Liu and Y. Q. Jia, Mater. Chem. Phys., 1998, 53, 256 CrossRef CAS.
- A. Rumplecker, F. Kleitz, E. L. Salabas and F. Schüth, Chem. Mater., 2007, 19, 485 CrossRef CAS.
- W. W. Wang and Y. J. Zhu, Mater. Res. Bull., 2005, 40, 1929–1935 CrossRef CAS.
- L. X. Yang, Y. J. Zhu, L. Li, L. Zhang, H. Tong and W. W. Wang, Eur. J. Inorg. Chem., 2006, 23, 4787 CrossRef.
- F. Mohandes, F. Davar and M. Salavati-Niasari, J. Magn. Magn. Mater., 2010, 322, 872 CrossRef CAS.
- J. Jiang and L. Li, Mater. Lett., 2007, 61, 4894 CrossRef CAS.
- L. Ren, P. Wang, Y. Han, C. Hu and B. Wei, Mater. Phys. Lett., 2009, 476, 78 CAS.
- S. Farhadi, K. Pourzare and S. Sadeghinejad, J. Nanostructure Chem., 2013, 3, 16 CrossRef.
- R. V. Kumar, Y. Diamant and A. Gedanken, Chem. Mater., 2000, 12, 2301 CrossRef CAS.
- S. W. Oh, H. J. Bang, Y. C. Bae and Y.-K. Sun, J. Power Sources, 2007, 173, 502 CrossRef CAS.
- T. Lai, Y. Lai, C. Lee, Y. Shu and C. Wang, Catal. Today, 2008, 131, 105 CrossRef CAS.
- A. S. Bhatt, D. K. Bhat, C.-W. Tai and M. S. Santosh, Mater. Chem. Phys., 2011, 125, 347 CrossRef CAS.
- X. Wang, X. Y. Chen, L. S. Gao, H. G. Zheng, Z. Zhang and Y. T. Qian, J. Phys. Chem. B, 2004, 108, 16401 CrossRef CAS.
- J. Teichgräber, S. Dechert and F. Meyer, J. Organomet. Chem., 2005, 690, 5255 CrossRef.
- M. Veith, A. Altherr and N. Lecerf, Nanostruct. Mater., 1999, 12, 191 CrossRef.
- Z. Shahri, M. Bazarganipour and M. Salavati-Niasari, Superlattices Microstruct., 2013, 63, 258 CrossRef CAS.
- M. Salavati-Niasari, B. Shoshtari-Yeganeh and M. Bazarganipour, Superlattices Microstruct., 2013, 58, 20 CrossRef CAS.
- T. Gholami, M. Salavati-Niasari, M. Bazarganipour and E. Noori, Superlattices Microstruct., 2013, 61, 33 CrossRef CAS.
- E. Noori, M. Bazarganipour, M. Salavati-Niasari and T. Gholami, J. Cluster Sci., 2013, 24, 1171 CrossRef CAS.
- R. W. Parry, Inorganic Syntheses, McGraw-Hill Book Company, 1970, Vol. XII Search PubMed.
- R. Jenkins and R. L. Snyder, Chemical Analysis: Introduction to X-ray Powder Diffractometry, John Wiley & Sons, Inc., New York, 1996, p. 90 Search PubMed.
- M. Bazarganipour and M. Salavati-Niasari, Micro Nano Lett., 2012, 7, 388 Search PubMed.
- Y. Ichiyanagi, Y. Kimishima and S. Yamada, J. Magn. Magn. Mater., 2004, 272–276, e1245 CrossRef CAS.
- R. H. Kodama, S. A. Makhlouf and A. E. Berkowitz, Phys. Rev. Lett., 1997, 79, 1393 CrossRef CAS.
- T. Ozkaya, A. Baykal, M. S. Toprak, Y. Koseoglu and Z. Durmus, J. Magn. Magn. Mater., 2009, 321, 2145 CrossRef CAS.
- J. Zhong, J. Li, F. Feng, Y. Lu, J. Zeng, W. Hu and Z. Tang, J. Mol. Catal. A: Chem., 2012, 357, 101 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2014 |




