One-pot synthesis of a highly active, non-spherical PdPt@Pt core–shell nanospike electrocatalyst exhibiting a thin Pt shell with multiple grain boundaries†
Jisun Yoon‡
a,
Sungwon Kang‡a,
Hionsuck Baik*b,
Yong Soo Choic,
Seong Jung Kwon*c and
Kwangyeol Lee*a
aDepartment of Chemistry and Research Institute for Natural Sciences, Korea University, Seoul 136-701, Korea. E-mail: kylee1@korea.ac.kr; Tel: +82-2-3290-3139
bKorea Basic Science Institute (KBSI), Seoul 136-713, Korea. E-mail: baikhs@kbsi.re.kr; Tel: +82-2-6943-4117
cDepartment of Chemistry, Konkuk University, Seoul, 143-701, Korea. E-mail: sjkwon@konkuk.ac.kr; Tel: +82-2-450-3629
First published on 16th September 2014
Abstract
Co-decomposition of Pd and Pt precursors in the presence of trioctylphosphine and stearic acid gives a unique non-spherical PdPt@Pt core–shell nanospike with multiple grain boundaries in a facile one-pot synthesis. The difference in the metal–P bond strengths causes the disparate precursor decomposition kinetics, which in turn positions the Pt content on the nanoparticle surface. The core–shell composition, crystallinity, and shell thickness are conveniently controlled by simple variations in the amount of precursors and surfactants. The PdPt@Pt core–shell nanospike shows a high electrocatalytic activity toward methanol oxidation reaction. The excellent catalytic performance seems to originate from (1) the existence of multiple, surface energy-elevating grain boundaries, (2) roughened surface, and (3) lattice mismatch between the core and shell.
Recent advances in nanoparticle-based catalysis have been possible by establishing relationships between the catalytic activity and relevant structural features.1–5 Major breakthroughs in this field include the synthesis of facet-controlled alloy nanoparticles6–8 and heterostructured nanoparticles such as core–shell nanoparticles.9–13 Efforts to prepare nanoparticles to exhibit high energy surface features such as steps, edges, and twinning boundaries4,12,14–18 have also been fruitful in preparing active nanocatalysts. It is also greatly advantageous to prepare nonspherical nanoparticles with high surface area per mass because nanoparticle catalysis is an inherently surface area dependent phenomenon. We have been interested in the preparation of core–shell nanostructures with above mentioned surface energy elevating structural features. Herein, we report a facile one step synthesis of catalytically highly active PdPt@Pt core–shell nanoparticles with (i) nonspherical core shape, (ii) roughened surface, and (iii) grain boundaries, which all contribute to enhancing the nanoparticle electrocatalytic activity, by understanding the decomposition kinetics of Pd and Pt precursors.
The Pd complex with trioctylphosphine (TOP) ligands has been used in the preparation of monodisperse Pd nanoparticles.19 On the other hand, Pt–P bond is much stronger than Pd–P bond,20,21 and therefore it is reasonable to attempt the one step synthesis of a phase-segregated Pd@Pt core–shell nanoparticle, involving fast decomposition of Pd precursors, followed by decomposition of Pt precursors. However, we and others have found independently that co-decomposition of TOP complexes of Pt and Pd in the presence of TOP proceeds only to form completely mixed phase PdPt alloy nanoparticles.22 Also, the decomposition of Pt is very sluggish in the presence of TOP, leading to incomplete decomposition of the precursor, and therefore less than expected Pt content is found in PdPt alloy nanoparticles. In order to form phase segregated core–shell nanoparticles with a Pt shell, decomposition kinetics of Pd and Pt should be further differentiated and a thorough decomposition of Pt should be accomplished at the same time. We have found that the usage of excess stearic acid (SA) fulfils these requirements, while not much affecting the decomposition kinetics of Pd precursors, thereby forming novel PdPt@Pt core–shell nanospikes.
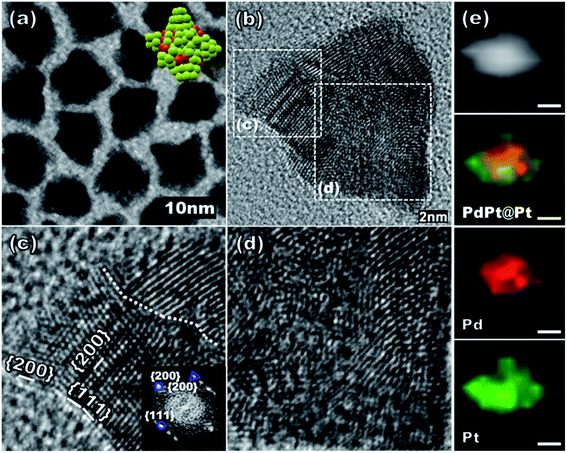
In a typical synthesis, a slurry of Pt(acac)2 (0.2 mmol), Pd(acac)2 (0.05 mmol), TOP (0.11 mmol), SA (8 mmol), 1,2-hexadecanediol (0.4 mmol), and octadecylamine (7.4 mmol) was prepared in a two-neck round bottom flask with a magnetic stirring. Resulting reaction mixture was placed in an oil bath preheated to 180 °C and was heated for 1 h at that temperature to give PdPt@Pt core–shell nanoparticles with a roughened surface as shown in Fig. 1. The transmission electron microscopy (TEM), high resolution TEM (HRTEM) images and elemental analysis are shown in Fig. 1 for monodisperse PdPt@Pt core–shell nanospikes with an average size of about 11.5 nm (±1.1) (see ESI Fig. S1†). The core–shell structure with a PdPt core and a Pt shell is identified by elemental mapping analysis (Fig. 1e). The protruded spike parts are more enriched by the Pt component, but the thickness of Pt-only shell seems to be very small below <2.5 nm. Notably, the Pt shell thickness is not homogeneous and some parts of the PdPt core are exposed. The HRTEM images of a nanospike in Fig. 1b–d show a very poorly crystalline and non-spherical PdPt core and a highly crystalline Pt shell, but with multiple grain boundaries. The thickness of polycrystalline Pt shell as judged by the elemental mapping analysis seems to be in a good accordance with the dimension of highly crystalline domain in HRTEM images of Fig. 1b–d. Therefore, the nanospike structure is the best described as having a non-spherical and poorly crystalline PdPt core and a thin, non-uniform, and highly crystalline, but polycrystalline, Pt shell.
The structural evolution of nanospikes was analysed by temporal images of reaction intermediates (Fig. 2). Initially, very small spherical nanoparticles (<3.9 nm (±0.3)) were formed, which kept the same morphology until they reached the size of 9.7 nm (±0.6) as shown in Fig. 2c. The elemental mapping and HRTEM analysis of nanoparticles in Fig. 2c (Fig. S2†) reveal the composition of thoroughly mixed PdPt phase with a Pd/Pt ratio of 7/3 and a very poor crystallinity. The nanoparticles became multi-faceted at the nanoparticle size of 9.7 nm (±0.6), and then spikes appeared on them gradually. After reaction time of 30 min, the nanospike morphology did not change much. Therefore, it appears that the Pt shell starts to grow after using up all Pd precursors in forming the poorly crystalline PdPt alloy core part and the crystallinity starts to emerge only with a sufficient Pt phase build-up afterwards. The non-epitaxial growth of Pt phase on the poorly crystalline PdPt core results in the simultaneous formation of multiple crystals, which lead to the generation of multiple grain boundaries.
The Pd/Pt ratio and amount of SA were found to be critical in determining the core–shell composition as well as the overall morphology and crystallinity. When the ratio of Pd(acac)2/Pt(acac)2 was varied as 1/1, 1/4, and 1/5.7 without changing the quantity of SA, which was fixed at 2 equiv. of the Pd precursor, spherical PdPt nanoparticles of a poor crystallinity were formed (Fig. 3a–f). No evidence of core–shell structure was found for these nanoparticles although the crystallinity seems to be enhanced with larger Pt contents incorporated (Fig. 4a–c, vide infra). The elemental analysis of the nanoparticles reveals that the decomposition of Pt precursor is mostly incomplete under the investigated reaction conditions (Fig. S4, S6 and S8†). However, with 16 equiv. SA, a thorough decomposition of Pt precursor could be accomplished. While the core–shell structure is still not formed at the Pd/Pt ratio of 1/1, the crystallinity is far enhanced as compared to nanoparticles formed at 2 equiv. SA (Fig. 3j vs. Fig. 3d). The core–shell structure and spike formation are evident for Pd/Pt ratios of 1/4 and 1/5.5 (Fig. 3h and i). In the case of Pd/Pt ratio of 1/5.5, nanospikes with a size of 25.5 (±2.1) nm are formed with a Pt shell thickness reaching up to 6.1 nm and with highly crystalline spikes (Fig. 3i).
Energy dispersive X-ray spectroscopy (EDS) was used to analyse the composition of PdPt nanostructures (Fig. S4–S9†). As the amount of Pt(acac)2 or SA increased, increase in the Pt content was found in the formed nanoparticles. The compositions of nanoparticles prepared with 2 equiv. SA were found as Pd0.65Pt0.35 (Fig. 3a and d), Pd0.52Pt0.48 (Fig. 3b and e), and Pd0.41Pt0.59 (Fig. 3c and f) for the Pd/Pt precursor ratios of 1/1, 1/4 and 1/5.7, respectively. With 16 equiv. SA, the compositions of Pd0.56Pt0.44 (Fig. 3g and j), Pd0.32Pt0.68 (Fig. 3h and k), and Pd0.17Pt0.83 (Fig. 3i and l) were obtained with the Pd/Pt precursor ratios of 1/1, 1/4 and 1/5.7, respectively. It should be emphasized again that, with a small incorporated SA amount, the Pt content in the nanoparticles does not represent the initial Pd/Pt ratio due to the incomplete decomposition of the Pt precursor. Only at high SA content of 16 equiv., the Pt content in the formed nanoparticles approaches the initial Pd/Pt precursor ratio. Furthermore, at this high SA condition, the core–shell morphology with a PdPt core and a Pt shell is generated.
The crystallinity of nanoparticles was enhanced by the increased amount of added SA and by the increased Pt precursor content as shown in Fig. 4. It has been reported that the strongly surface-bound TOP ligands disrupt the underlying Pd atom ordering and cause a poor crystallinity of Pd nanoparticles.19 The deterioration of crystallinity induced by TOP coordination is obvious for all the PdPt phase either as the core or as the alloy particle (Fig. 3). Also, it has been reported that the Pd nanoparticles in TOP at elevated temperatures can experience dissolution of surface Pd atoms.18 Therefore, it might be feasible that Pd or Pt atoms in the initially formed alloy nanoparticle are also destabilized by the TOP at elevated temperatures and the TOP-induced destabilization of the PdPt alloy nanoparticles leads to a size homogenization and also to a poor crystallinity. Therefore, it is understandable that, under high SA condition, the smaller degree of metal–TOP interaction, due to competing SA molecules, would result in mitigated effect on the underlying core atoms, thus higher crystallinity; there are a few reports for the synthesis of stable and highly crystalline Pt nanostructures in the presence of organic acids,23,24 and therefore, it is reasonable that SA competes strongly with TOP for coordination with surface Pt atoms. Interestingly, the size analysis of formed nanoparticles reveals very similar nanoparticle sizes for all three samples prepared with 2 equiv. SA and the 1/1 Pd/Pt sample with 16 equiv. SA (Fig. S1†). Only the two samples with core–shell spike morphologies with Pt spike shells, where the nanospike size increases without much difference in the core size, are bigger than the PdPt alloy nanoparticles. This higher surface crystallinity and lessened metal–TOP interaction of the core PdPt alloy nanoparticles, prepared under higher SA content, might have caused the more facilitated decomposition of Pt precursor and thereby Pt shell growth. It is interesting to note that the crystallinity of Pt shell seems to be rather unperturbed by TOP in spite of stronger Pt–P bond strength. Again, the mitigated TOP interaction with the surface Pt phase in the presence of SA might have contributed to the higher crystallinity of the surface Pt phase in the final PdPt@Pt core–shell nanoparticles.
The electrocatalytic properties of surfactant-removed PdPt nanostructures toward methanol oxidation reaction (MOR) were examined as shown in Fig. 5. The electrochemically active surface area (ECSA) was calculated by a method described in ESI.† The catalytic activity for the MOR was normalized to the surface area of Pt. The current densities, the mass activity, and ECSA are summarized in Table S1.†
 | ||
| Fig. 5 Cyclic voltammograms of commercial Pt/C and PdPt nanostructures-modified glassy carbon electrodes (GCEs) in 0.5 M MeOH + 0.5 M H2SO4 electrolyte solution. Scan rate was 50 mV s−1. | ||
Two notable observations are that in the case of PdPt alloy nanoparticles, the higher Pt content results in a better catalytic performance and that the PdPt@Pt core–shell morphology obtained with 1/4 Pd/Pt ratio, namely, Pd0.32Pt0.68 core–shell, is the most superior among studied nanoparticles. The Pd0.32Pt0.68 core–shell nanoparticle shows an even higher catalytic activity than all Pt commercial nanocatalyst. This latter observation is particularly noteworthy in that the catalytic performance is not directly related to a higher Pt content and that the other structural features should be accountable for the high catalytic activity. Obviously, the crystallinity on the nanoparticle surface is very important in determining the catalytic activity. The Pd0.65Pt0.35 alloy nanoparticle obtained with 1/1 Pd/Pt and at 2 equiv. SA has a very poor crystallinity and the worst catalytic performance, while other alloy nanoparticles exhibit some highly crystalline regions on the nanoparticle surface. The core–shell nanoparticles prepared from 1/4 and 1/5.7 Pd/Pt ratios and 16 equiv. SA also exhibit high crystallinity on the nanoparticle surface. However, the nanoparticle crystallinity or higher Pt surface content cannot explain the observed catalytic performance completely, because the best performing PdPt@Pt core–shell nanospike obtained with 1/4 Pd/Pt ratio exhibits poorer crystallinity and lower Pt content than the PdPt@Pt core–shell nanoparticle with longer spikes obtained with 1/5.7 Pd/Pt ratio.
The non-spherical nanospike structure appears to exhibit the following additional structural advantages over alloy nanoparticles and the core–shell nanoparticles with thick and well developed Pt spikes. First, the PdPt@Pt core–shell morphology obtained with 1/4 Pd/Pt ratio shows a core–shell structure with a thin (<2.5 nm) but highly crystalline Pt shell. The thickness of shell in core–shell structure is very important to determining catalytic activities.11,26 A thick Pt shell would render a core–shell nanoparticle to behave like Pt-only catalyst and therefore it would not utilize the lattice mismatch between the core and shell to improve the electro-catalytic activity, and this explains the low catalytic activity of the large core–shell nanospikes obtained with 1/5.5 Pd/Pt ratio. Also, a high catalytic activity was recently demonstrated for a very thin Pt shell on a core–shell morphology, obtained by thermal treatment at very high temperature.13 Secondly, the surface Pt layer exhibit multiple grain boundaries, which have been correlated with superior catalytic activity.4,25 Finally, the surface of the nanospike is severely roughened so that the increase in the active surface area is accomplished over conventional spherical nanoparticles.
The methanol oxidation peaks for the most PdPt nanostructures except Pd0.32Pt0.68 core–shell modified GCE appeared at ∼0.8 V (forward scan) and at ∼0.6 V (backward scan). The Pd0.32Pt0.68 core–shell modified GCE with the highest currents has 0.1 V positive shifts for each peak. However, this additional over-potential does not seem to indicate thermodynamic difficulty of MOR in Pd0.32Pt0.68 core–shell because the shift would be due to the polarization effect of huge raw currents. Therefore, all the peaks including Pd0.32Pt0.68 core–shell are consistent with typical features of the methanol oxidation at Pt surface.4,12,25
The forward peak represents the methanol oxidation, and the backward peak occurs by the oxidation of surface poisoning CO or CO-like species.17 Therefore, the peak current ratio of the forward scan (iF) to backward scan (iB), iF/iB indicates the resistance to the poisoning or the efficiency of methanol oxidation.25 While the Pd0.65Pt0.35 alloy has a high iF/iB of 4.61 but the iF/iB is unreliable due to the very small absolute currents. Other PdPt nanostructures have the ratio of iF/iB ∼ 1, which is similar to the previous study results of PdPt alloy electrocatalyst.27,28 Specifically, the iF/iB for the other PdPt nanostructures are 0.89 for Pd0.32Pt0.68 core–shell, 1.31 for Pd0.17Pt0.83 core–shell, 1.17 for Pd0.56Pt0.44 alloy, 1.36 for Pd0.52Pt0.48 alloy, and 1.19 for Pd0.41Pt0.59 alloy.
Chronoamperometry (CA) experiments at 0.84 V were performed for 1000 s in order to test the electrochemical stability of PdPt nanostructures toward MOR (Fig. S10†). The initial high current of CA is caused by the charging currents and the initial high concentration of methanol at the surface of the nanostructures. Later on, the current decays rapidly due to the decrease of the concentration gradient and poisoning of Pt surface by the intermediate species. The long-term poisoning rate of PdPt nanostructure was calculated by the following equation:27
The value (dI/dt) t > 500 s is the slope of the linear portion of the current decay, and I0 is the current at the start of polarization back extrapolated from the linear current decay. The calculated long-term poisoning rates of Pd0.65Pt0.35 alloy, Pd0.32Pt0.68 core–shell, Pd0.17Pt0.83 core–shell, Pd0.56Pt0.44 alloy, Pd0.41Pt0.59 alloy, and Pd0.52Pt0.48 alloy are 0.05% s−1, 0.03% s−1, 0.04% s−1, 0.04% s−1, 0.05% s−1, and 0.05% s−1 respectively. The nanospike, Pd0.32Pt0.68 core–shell exhibits the best resistance toward CO poisoning than the others. The stability of nanospike might be due to two major reasons. First, the alloy core is likely to affect the modification of Pt shell electronic band structure, so it might render the mitigated CO poisoning of the Pt shell.27,29 Second, the partially exposed PdPt alloy core might facilitate the formation of Pd–OH from the dissociated water molecules, which might participate in the CO oxidation process, thus recovering the Pt active sites from CO poisoning.27,30
In summary, we have demonstrated that the formation of ultra-thin Pt shell in a PdPt@Pt core–shell structure can be accomplished by a facile one pot decomposition of Pd and Pt precursors in the presence of TOP and SA. While TOP is accountable for the disparate decomposition kinetics of Pd and Pt precursors, the usage of stearic acid accomplishes both further differentiated decompositions of Pd and Pt precursors and a thorough decomposition of Pt precursor to form a Pt shell. Most notably, a PdPt@Pt core–shell nanoparticle with a roughened surface, multiple grain boundaries, and a very thin Pt layer exhibited both the best catalytic activity and electrochemical stability among the examined nanoparticles, thereby demonstrating that the surface structural effects are more important than simple alloy compositions and nanoparticle crystallinity. Specifically, increased activity of the core–shell nanoparticle is induced from multiple grain boundaries, roughened surface, and lattice mismatch between the core and the shell. Furthermore, the existence of alloy core underneath Pt shell and the partially exposed Pd atoms in the alloy core, not totally covered by surface Pt atoms, led to weakened Pt–CO bonding and thus less severe CO poisoning. We believe that other interesting core–shell nanoparticles with different compositions might be also prepared by the developed synthetic strategy, and a related further study is under way.
Acknowledgements
This work was supported by NRF-2013R1A2A2A 01015168, NRF 2010-0020209, MIHWAF(HI 10C 1911), NRF-2012R1A1A1040285, KBSI project T34520. We thank KBSI for allowing the usage of their HRTEM instruments.Notes and references
- N. Tian, Z. Y. Zhou, S. G. Sun, Y. Ding and Z. L Wang, Science, 2007, 316, 732 CrossRef CAS PubMed.
- K. M. Bratlie, H. Lee, K. Komvopoulos, P. Yang and G. A. Somorjai, Nano Lett., 2007, 7, 3097 CrossRef CAS PubMed.
- H. Kim, N. T. Khi, J. Yoon, H. Yang, Y. Chae, H. Baik, H. Lee, J.-H. Sohn and K. Lee, Chem. Commun., 2013, 49, 2225 RSC.
- J. Yoon, N. T. Khi, H. Kim, B. Kim, H. Baik, S. Back, S. Lee, S.-W. Lee, S. J. Kwon and K. Lee, Chem. Commun., 2013, 49, 573 RSC.
- B. Y. Xia, H. B. Wu, X. Wang and X. W. Lou, Angew. Chem., Int. Ed., 2013, 52, 12337 CrossRef CAS PubMed.
- J. Wu, A. Gross and H. Yang, Nano Lett., 2011, 11, 798 CrossRef CAS PubMed.
- Y. Jia, Y. Jiang, J. Zhang, L. Zhang, Q. Chen, Z. Xie and L. Zheng, J. Am. Chem. Soc., 2014, 136, 3748 CrossRef CAS PubMed.
- A. X. Yin, X. Q. Min, Y. W. Zhang and C. H. Yan, J. Am. Chem. Soc., 2011, 133, 3816 CrossRef CAS PubMed.
- S. E. Habas, H. Lee, V. Radmilovic and G. A. Somorjai, Nat. Mater., 2007, 6, 692 CrossRef CAS PubMed.
- S. Alayoglu, A. U. Nilekar, M. Mavrikakis and B. Eichhorn, Nat. Mater., 2008, 7, 333 CrossRef CAS PubMed.
- V. Mazumder, M. Chi, K. L. More and S. Sun, J. Am. Chem. Soc., 2010, 132, 7848 CrossRef CAS PubMed.
- N. T. Khi, J. Yoon, H. Kim, S. Lee, B. Kim, H. Baik, S. J. Kwon and K. Lee, Nanoscale, 2013, 5, 5738 RSC.
- D. Wang, H. L. Xin, R. Hovden, H. Wang, Y. Yu, D. A. Muller, F. J. DiSalvo and H. D. Abruna, Nat. Mater., 2013, 12, 81 CrossRef CAS PubMed.
- Y. Xiong, B. J. Wiley and Y. Xia, Angew. Chem., Int. Ed., 2007, 46, 7157 CrossRef CAS PubMed.
- Y. Yu, Q. Zhang, B. Liu and J. Y. Lee, J. Am. Chem. Soc., 2010, 132, 18258 CrossRef CAS PubMed.
- Z. Y. Zhou, Z. Z. Huang, D. J. Chen, Q. Wang, N. Tian and S. G. Sun, Angew. Chem., Int. Ed., 2010, 49, 411 CrossRef CAS PubMed.
- L. Ruan, E. Zhu, Y. Chen, Z. Lin, X. Huang, X. Duan and Y. Huang, Angew. Chem., Int. Ed., 2013, 52, 12577 CrossRef CAS PubMed.
- N. T. Khi, J. Yoon, H. Baik, S. Lee, D. J. Ahn, S. J. Kwon and K. Lee, CrystEngComm, 2014, 16, 8312 RSC.
- Y. Liu, C. Wang, Y. Wei, L. Zhu, D. Li, J. S. Jiang, N. M. Markovic, J. R. Stamenkovic and S. Sun, Nano Lett., 2011, 11, 1614 CrossRef CAS PubMed.
- B. L. Edelbach, R. J. Lachicotte and W. D. Jones, J. Am. Chem. Soc., 1998, 120, 2843 CrossRef CAS.
- R. Craciun, A. J. Vincent, K. H. Shaughnessy and D. A. Dixon, Inorg. Chem., 2010, 49, 5546 CrossRef CAS PubMed.
- G. Ren, H. Shi and Y. Xing, Nanotechnology, 2007, 18, 385604 CrossRef.
- C. Wang, H. Daimon, Y. Lee, J. Kim and S. Sun, J. Am. Chem. Soc., 2007, 129, 6947 Search PubMed.
- S. I. Lim, I. O. Jimenez, M. Varon, E. Casals, J. Arbiol and V. Puntes, Nano Lett., 2010, 10, 964 CrossRef CAS PubMed.
- J. Yoon, H. Baik, S. Lee, S. J. Kwon and K. Lee, Nanoscale, 2014, 6, 6434 RSC.
- J. X. Wang, H. Inada, L. Wu, Y. Zhu, Y. Choi, P. Liu, W. P. Zhou and R. R. Adzic, J. Am. Chem. Soc., 2009, 131, 17298 CrossRef CAS PubMed.
- H.-H. Li, S. Zhao, M. Gong, C.-H. Cui, D. He, H.-W. Liang, L. Wu and S.-H. Yu, Angew. Chem., Int. Ed., 2013, 52, 7472 CrossRef CAS PubMed.
- Y.-N. Wu, S.-J. Liao, H.-F. Guo and X.-Y. Hao, J. Power Sources, 2013, 235, 135 CrossRef CAS PubMed.
- R. Srivastava, P. Mani, N. Hahn and P. Strasser, Angew. Chem., Int. Ed., 2007, 46, 8988 CrossRef PubMed.
- Y. Liu, M. Chi, V. Mazumder, K. L. More, S. Soled, J. D. Henao and S. Sun, Chem. Mater., 2011, 23, 4199 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra09619c |
| ‡ These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2014 |