Extremely selective fluorescence detection of cysteine or superoxide with aliphatic ester hydrolysis†
Abstract
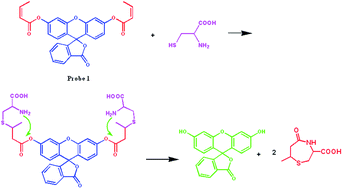
A novel fluorescence probe modality demonstrated with fluorescein affords a highly selective aqueous-based detection of cysteine over other biothiols, e.g. homocysteine, with a limit of detection of 11.3 μM.

 Please wait while we load your content...
Please wait while we load your content...