Two 3D photoluminescent Zn(II) complexes constructed from 5-amino-1-H-tetrazole with aromatic polycarboxylate ligands†
Fang-Hua Zhao and
Ji-Min Zheng*
Department of Chemistry, Nankai University, Tianjin, 300071, P. R. China. E-mail: jmzheng@nankai.edu.cn
First published on 8th October 2014
Abstract
Two new Zn(II) complexes, namely, [Zn2(BTEC)0.5(ATZ)2(H2O)3]·H2O (1) and [Zn1.5(2,5-PYDC)(ATZ)(H2O)]2 (2) have been assembled from 5-amino-1-H-tetrazole (HATZ) with two aromatic polycarboxylate ligands under hydrothermal conditions (H4BTEC = 1,2,4,5-benzenetetracarboxylic acid, 2,5-PYDC = 2,5-pyridinedicarboxylic acid). Single crystal X-ray analysis reveals that both complexes display three-dimensional (3D) structures. Complex 1 is a binodal (4,4)-connected bbf net with a (64·82)(66)2 topological symbol. Complex 2 displays a trinodal (3,3,4)-connected net with (4·102)2(102·12)2(42·102·122) topology. In the two structures, all the HATZ ligands are deprotonated and act as the anions, and the ATZ− anions all connected two Zn(II) centers with a μ2-mode. In addition, the two complexes display photoluminescence at 442 nm (λex = 342 nm) and 468 nm (λex = 374 nm), respectively. And their thermal stability has also been studied.
Introduction
Coordination polymers (CPs), as indicated by Batten et al.,1 are a subclass of coordination compounds with extended structures. They have been extensively studied for their intriguing topological structures2 and their potential applications as functional materials in many areas including gas storage,3 gas separation,4 catalysis,5 magnetism,6 drug delivery,7 nonlinear optics8 and luminescence.9 In the design and construction of CPs, the selection of organic ligands and metal ions are the most important, because the structure and properties of complex greatly depends upon the structure of the organic ligands, the coordinative geometry and electronic characteristics of metal ions. Control over the appropriate reaction conditions also plays a important role in the synthetic procedure, such as solvent, temperature, pH value and metal–ligand ratio.10 Therefore, this makes it difficult to predict the final structures, it is still a great challenge in crystal engineering to obtain designed and predictable frameworks with potential properties.Though it still is a challenge, researchers can also find some useful rules of assembly from numerous examples of previous work. One important strategy is to apply the mixed ligands to construct coordination polymers, which has been discussed in a recent review by Du et al.11 Compared with the single ligand, the combination of different ligands provides more possibilities to result in various structures, and there is a tendency to form polymer frameworks with high dimensionality. Among the mixed-ligand CPs systems, the most important branch is using the nitrogen-involving ligand and oxygen-involving carboxylate acid as mixed ligands. Through this approach, numerous coordination polymers have been successfully synthesized.
5-amino-1-H-tetrazolate (HATZ) is a good multidentate nitrogen-involving organic ligand which not only potentially possesses the capability to bridge metal centers in various coordination modes with the nitrogen atoms of the tetrazole ring, but also can form various hydrogen bonds with its amino group and nitrogen atoms of the tetrazole ring.12 In our previous work,13 we have successfully prepared a series of photoluminscent and ferroelectric complexes using HATZ ligand with metal inorganic salts only, which display various structures with unique molecule (0D), one- or three-dimensional structures, and it was proved that the HATZ ligand can lose the hydrogen atom of the tetrazolate ring to be an anionic ligand in higher temperature. If incorporated with polycarboxylate ligands, it is believed to be benefit to generate intriguing and multi-dimensional networks, and to date only few complexes construct from HATZ with polycarboxylate ligands have been reported.14 Both 1,2,4,5-benzenetetracarboxylic acid (H4BTEC) and 2,5-pyridinedicarboxylic acid (2,5-PYDC) are aromatic polycarboxylate ligands and possess multiple binding sites, which can form extended structures.15,16 Many CPs of them have been synthesized, however, to our best knowledge, no CPs of them mixed with HATZ have been reported.
In addition, the organic ligands of HATZ, H4BTEC and 2,5-PYDC all are typical aromatic ligands containing conjugated π moieties, which can give rise to optical emission or photoluminescence upon irradiation.9 The Zn(II) metal ion, as a d10 electronic configuration without unpaired electrons, can also contribute to photoluminescence,9 and it can present as four-, five- and six-coordinated geometries to generate various coordination structures.
On the basis of aforementioned points, herein, we selected the HATZ and two aromatic polycarboxylates as mixed ligands to construct multi-dimensional complexes under hydrothermal condition. Two new three-dimensional Zn(II) complexes have been successfully obtained, their topologies have been analyzed, and their phtoluminscent and thermal properties have also been investigated.
Experimental
Materials and general procedures
Solvents and starting materials for synthesis were purchased commercially and used as received without further purification. Elemental analysis for C, H, and N was performed on a Perkin-Elmer 240 analyzer. The IR spectrum was recorded as KBr pellets on a Nicolet Magna-FT-IR 560 spectrometer in the 4000–400 cm−1 region. The photoluminescence measurement was carried out on crystalline samples at room temperature, and the spectra were collected with a Hitachi F-2500FL spectrophotometer. The thermogravimetric analyses (TGA) were investigated on a standard TG-DTA analyzer under nitrogen flow at a heating rate of 5 °C min−1 for all measurements.Synthesis of complex [Zn2(BTEC)0.5(ATZ)2(H2O)3]·H2O (1)
A mixture of Zn(NO3)2·6H2O (148 mg, 0.5 mmol), H4BTEC (54 mg, 0.25 mmol), 5-ATZ (43 mg, 0.5 mmol) was dissolved in 8 mL distilled water. The pH value was adjusted to 6.0 with 1 M NaOH solution. The resulting mixture was sealed in a 25 mL Teflon-lined stainless steel vessel and heated at 180 °C for three days in an oven and then slowly cooled to room temperature. Colorless block-shaped crystals of 1 were obtained in 60% yield (based on Zn). Elemental analysis (%): calcd: C 16.95, H 2.64, N 28.24. Found: C 16.83, H 2.75, N 28.29. IR (KBr): ν (cm−1) = 3393w, 1629s, 1561m, 1474s, 1449w, 1376s, 1320s, 1275w, 1167w, 1091m, 1014w, 870m, 823w, 757w, 703w, 553w, 486m.Synthesis of complex [Zn1.5(2,5-PYDC)(ATZ)(H2O)]2 (2)
A mixture of ZnSO4·6H2O (216 mg, 0.75 mmol), 2,5-pydc (84 mg, 0.5 mmol), 5-ATZ (43 mg, 0.5 mmol) was dissolved in 8 mL distilled water. The pH value was adjusted to 6.0 with 1 M NaOH solution. The resulting mixture was sealed in a 25 mL Teflon-lined stainless steel vessel and heated at 180 °C for three days in an oven and then slowly cooled to room temperature. Colorless block-shaped crystals of 2 were obtained in 62% yield (based on Zn). Elemental analysis (%): calcd: C 26.31, H 1.93, N 23.01. Found: C 26.39, H 1.84, N 23.05. IR (KBr): ν (cm−1) = 3385w, 3320w, 3196w, 1638m, 1607m, 1584m, 1482m, 1411m, 1354s, 1290w, 1242w, 1177m, 1118w, 1088w, 1041m, 973w, 889w, 768w, 704w, 588w, 521w, 454w.X-ray crystallographic measurements for 1 and 2
Suitable single crystals of 1 and 2 were selected and mounted in air onto thin glass fibers. Accurate unit cell parameters of the two complexes were determined by a least-squares fit of 2θ values, and intensity data was measured on a Rigaku r-axis rapid IP area detector with Mo-Kα radiation (λ = 0.71073 Å). The intensity was corrected for Lorentz and polarization effects as well as for empirical absorption based on multi-scan technique.17 The structures were solved by direct method and refined by full-matrix least-squares fitting on F2 with the program SHELXL-97.18 All nonhydrogen atoms were refined anisotropically. The hydrogen atoms bond to carbon atoms were calculated theoretically and those to oxygen and nitrogen atoms were determined by difference fourier maps. Crystallographic data and structure refinement details are summarized in Table 1. Select bonds and angles are list in Table S1,† and those of the hydrogen bonds are listed in Table S2.†| Compound | 1 | 2 |
|---|---|---|
| a R1 = ∑(‖F0 − Fc‖)/∑|F0|; wR2 = [∑w(F02 − Fc2)2/∑w(F02)2]1/2. | ||
| Formula | C7H13N10O8Zn2 | C16H14N12O10Zn3 |
| Fw | 496.05 | 730.56 |
| T/K | 153(2) | 153(2) |
| Crystal system | Monoclinic | Monoclinic |
| Space group | P21/c | C2/c |
| a/Å | 12.511(3) | 19.474(4) |
| b/Å | 10.165(2) | 9.849(2) |
| c/Å | 12.487(3) | 12.064(2) |
| β/deg | 102.05(3) | 96.55(3) |
| V Å−3 | 1553.0(6) | 2298.8(8) |
| Z | 4 | 4 |
| D/g cm3 | 2.122 | 2.111 |
| F (000) | 996 | 1455 |
| R (int) | 0.1116 | 0.0423 |
| GOF | 1.029 | 1.052 |
| R1, wR2 [I > 2σ(I)]a | 0.0676, 0.1816 | 0.0268, 0.0620 |
Results and discussion
IR spectroscopy
In the IR spectra, the broad absorption bands at 3393 cm−1 in 1 and 3385 cm−1 in 2 can be assigined to the characteristic vibrations of O–H and N–H, suggesting the presence of water molecules and amino group of ATZ ligands. Compared with the free polycarboxylate acids, the absence of a strong absorption at around 1700 cm−1 in 1 and 2 suggests the complete deprotonation of the carboxylate coligands. The characteristic asymmetric and symmetric stretching vibrations bands of the carboxylate groups can be observed at 1629 and 1376 cm−1 for 1 and at 1607 and 1411 cm−1 for 2. The separation of νasym(CO2)–νsym(CO2) is 253 cm−1 for 1 suggests the monodentate bridging coordination mode of the carboxylate group in 1, and the separation of νasym(CO2)–νsym(CO2) is 194 cm−1 for 2 reveals a bidentate bridging mode of the carboxylate group in 2.19 The bands at 1561 cm−1 for 1 and 1584 cm−1 for 2 can be associated with ν(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N)/ring stretching vibrations plus δ(N–H)NH,NH2 of the ATZ ligand.20 The IR spectra of the two complexes are in good agreement with the single crystal X-ray diffraction results as following.
N)/ring stretching vibrations plus δ(N–H)NH,NH2 of the ATZ ligand.20 The IR spectra of the two complexes are in good agreement with the single crystal X-ray diffraction results as following.
Crystal structure of [Zn2(BTEC)0.5(ATZ)2(H2O)3]·H2O (1)
Single crystal X-ray analysis reveals that complex 1 crystallizes in the monoclinic space group P21/c. In the asymmetric unit, there are two independent Zn(II) ions, half BTEC4− anion, two ATZ− anions, three coordinated and one free water molecules. As shown in Fig. 1a, Zn1 is coordinated by one BTEC4− oxygen atom and three ATZ− nitrogen atoms to give the distorted ZnON3 tetrahedral geometry. Zn2 displays a distorted ZnO4N trigonal bipyramid geometry with the trigonality parameter τ = 0.87,21 completed by two coordinated water oxygen atoms (O5 and O7) and one ATZ− nitrogen atom (N9) on the equatorial plane, one BTEC4− oxygen atom (O3) and one coordinated water oxygen atom (O6) at the axial position. The Zn–O/N bond lengths in the range of 1.964(7)–2.114(6) Å (Table S1†) are comparable with those observed in other Zn-based complexes.22 The completely deprotonated BTEC4− ligands adopt the μ4-η1:η1:η1:η1 coordinated mode (Scheme 1) connecting two Zn1 and two Zn2 ions. The two deprotonated ATZ− ligands both coordinate to two Zn(II) ions with a μ2 mode, and one coordinates to two Zn1 ions via N1 and N4 atoms with the Zn1⋯Zn1 separation being 6.105(2) Å, and the other coordinates to one Zn1 and one Zn2 ions via N6 and N9 atoms with the Zn1⋯Zn2 separation being 5.986(2) Å. The Zn1 and Zn2 centers are connected by the two kinds of ligands to generate a 3D structure (Fig. 1b).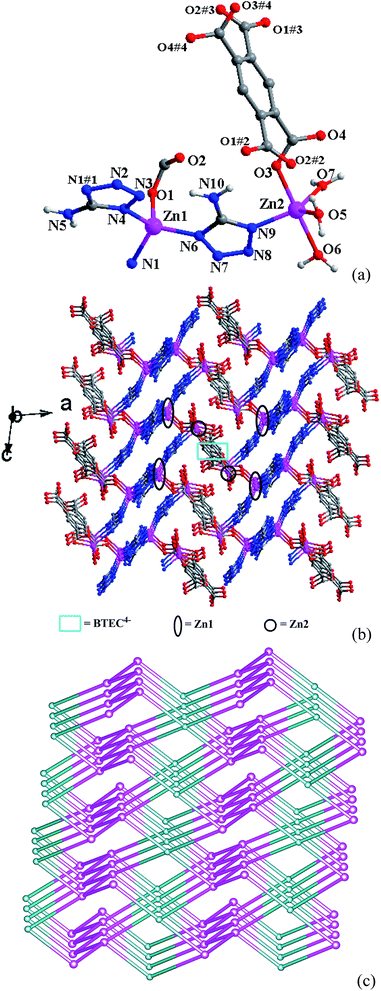 | ||
| Fig. 1 (a) The coordination environment of Zn(II) centers in 1, symmetry codes are as in Table S1;† (b) the 3D structure of 1; (c) the binodal (4,4)-connected bbf net with (64·82)(66)2 topological symbol for 1, constructed from 4-connected BTEC4− (light blue) and 4-connected Zn1 ion (pink). | ||
From further topological analysis (Fig. 1c), if the Zn1 center and the BTEC4− ligand can be simplified to nodes, the Zn2 center and ATZ− ligand act as the 2-connected spacers, then each Zn1 center is connected with two other Zn1 nodes and two BTEC4− nodes, so it can be considered as 4-connected node (Fig. 1c, pink nodes), and the BTEC4− is connected with four Zn nodes, so it act as an another 4-connected node (Fig. 1c, light blue nodes), then this 3D framework can be simplified to a binodal (4,4)-connected bbf net with (64·82)(66)2 topological symbol. Recently, a few examples with bbf net have been reported,23 which all display as 2- or 3-fold interpenetrating architectures, differently, complex 1 is a single net without interpenetration, this can be attributed to the shorter length of BTEC4− and ATZ− than the ligands used in reported structures. The topological index is computed via OLEX.24
Additional careful investigation for this 3D structure indicates that extensive O–H⋯O, O–H⋯N, N–H⋯O and N–H⋯N hydrogen bonds can be observed (Table S2†), which are formed among the water molecules with BTEC4− oxygens and ATZ− nitrogen atoms, and the amino group of ATZ− with BTEC4− oxygens and ATZ− nitrogen atoms, these hydrogen bonds further stabilize this structure.
Crystal structure of [Zn1.5(2,5-PYDC)(ATZ)(H2O)]2 (2)
Complex 2 crystallizes in the monoclinic space group C2/c. In the asymmetric unit, there are two independent Zn(II) ions, one 2,5-PYDC2− anion, one ATZ− anion and one coordinated water molecule. In complex 2 (Fig. 2a), Zn1 with the position occupation of 0.5 is coordinated by four O atoms and two N atoms from four different 2,5-PYDC2− anions to give the distorted ZnO4N2 octahedral geometry. Zn2 atom is coordinated by two ATZ− nitrogen atoms, one 2,5-PYDC2− oxygen atom and one coordinated water molecule to give the distorted ZnO2N2 tetrahedral geometry. The Zn–O/N bond lengths in the range of 1.9505(15)–2.1738(17) Å (Table S1†) are comparable with those of complex 1. The 2,5-PYDC2− anion adopts a μ3-η1:η1:η2 coordinated mode (Scheme 1) connecting two Zn1 and one Zn2 ions. The ATZ− anion also adopts a μ2 mode as that in 1 connecting two Zn2 ions with the Zn2⋯Zn2 separation being 6.134(3) Å. The Zn(II) centers are in turn connected by ATZ− and 2,5-PYDC2− anions to generate a 3D structure (Fig. 2b). | ||
| Fig. 2 (a) The coordination environment of Zn(II) centers in 2, symmetry codes are as in Table S1;† (b) the 3D structure of 2; (c) the trinodal (3,3,4)-connected net with (4·102)2(102·12)2(42·102·122) topological symbol for 2, constructed from 3-connected 2-PYDC2− (green), 3-connected Zn2 ion (blue) and 4-connected Zn1 ion (pink). | ||
From a topological view (Fig. 2c), each 2,5-PYDC2− anion connects three Zn(II) centers, and it can be considered as 3-connected node (Fig. 2c green node). Each Zn2 center are connected by one 2,5-PYDC2− and two ATZ− anions, so it can be considered as the 3-connected node (Fig. 2c blue node). Each Zn1 center are connected by four 2,5-PYDC2− ligands, so it can be simplified as the 4-connected node (Fig. 2c pink node). The ATZ− anions act as the 2-connected spacers, as a result, the 3D structure of 2 is a trinodal (3,3,4)-connected net with (4·102)2(102·12)2(42·102·122) topological symbol. This network is obviously different from the reported (3,3,4)-connected nets,25 which is a new type of 3D (3,3,4)-connected topological structure. In contrast to many CPs with single and double nodes, multinodal networks are still relatively less developed. Complex 2 is a new example of multinodal networks.
The N–H⋯O and O–H⋯O hydrogen bonds can be observed (Table S2†) among the coordinated water molecules with 2,5-PYDC2− oxygens, and the amino group of ATZ− with ATZ− nitrogen atoms, these hydrogen bonds further stabilize the structure of 2.
Compared with our previous structures based on HATZ ligand and metal inorganic salts,13 in this work, we successfully induced the organic polycarboxylate ligands to the structures. The previous six structures display as two unique molecules, two 1D chains and two 3D structures, respectively, in which the metal centers all show a six-coordinated geometry. While the metal centers in complexes 1 and 2 display diverse coordination geometries with four, five and six coordination number, and there are two different coordination geometries in each structure for both 1 and 2. Therefore, two more complicated 3D networks were constructed. This difference can be attributed to the incorporation of the auxiliary polycarboxylate ligands. This indicates that it is benefit to generate intriguing networks when using HATZ incorporated with polycarboxylate ligands.
Photoluminescent properties of 1 and 2
Considering complexes 1 and 2 as typical d10 transition-metal configuration,9 their photoluminescence experiments were performed at room temperature in the solid state. As shown in Fig. 3, complex 1 displays the emission maxima at 442 nm when excitated at 342 nm, complex 2 displays the emission maxima at 468 nm when excitated at 374 nm. According to the literatures, the HATZ ligand shows an emission band at 325 nm,26 the free H4BTEC ligand shows an emission band at 342 nm,27 and the 2,5-PYDC ligand shows an emission band at 392 nm.28 Complex 1 displays the red shift of ca. 117 nm and 100 nm relative to the free HATZ and H4BTEC ligands, and complex 2 displays the red shift of ca. 143 nm and 76 nm relative to the free HATZ and 2,5-PYDC ligands, which both could be assigned to the ligand-to-metal charge-transfer (LMCT).9 In addition, it can be seen from Fig. 3, the luminescence intensity of complex 1 is weaker than that of complex 2, this because there are much more hydrogen bonds in 1 than those in 2 (Table S2†), the hydrogen bonds formation can lead to the fluorescent quenching, which is normally found in supramolecular chemistry.29 The conformational rigidity of ligands and coordination environments of metal centers also affect the luminescence intensity.30 All carboxylate groups of BTEC4− ligand in 1 show obvious distortion with the benzene ring which reduces the “rigidity” of the ligand. While the carboxylate groups of 2,5-PYDC2− ligand in 2 are almost coplanar with the benzene ring. The Zn1 and Zn2 centers in 1 dispaly as four and five-coordinated environments, Zn1 is coordinated with four ligands, Zn2 is coordinated with two ligands and three terminal water molecules, such three terminal water molecules are unfavourable for the ligand-to-metal charge-transfer. The Zn1 and Zn2 centers in 2 dispaly as six and four-coordinated environments, Zn1 is coordinated with four ligands, Zn2 is coordinated with three ligands and one terminal water molecules, compared those of complex 1, the Zn centers of 2 connect with more ligands and less terminal water molecules, which is benefit to the ligand-to-metal charge-transfer. Therefore, on basis of aforementioned points, it is reasonable for that the luminescence intensity of complex 1 is weaker than that of complex 2.Thermal analyses
To study the stability of 1 and 2, thermogravimetric analyses (TGA) were performed on the single crystal samples under N2 atmosphere with a heating rate of 5 °C min−1. As shown in Fig. S1,† complex 1 shows a weight loss of 14.42% from 80 °C to 160 °C, corresponding to the loss of water molecules (calcd 14.53%), and there is almost no weight loss from 160 °C to 284 °C; and after that, a rapid weight loss is observed which can be attributed to the decomposition of the organic ligands. Finally, the remnants are 32.52% above 709 °C, which should be ZnO (calcd 32.82%). Complex 2 shows a weight loss of 4.72% from 50 °C to 167 °C, corresponding to the loss of water molecule (calcd 4.93%), and there is almost no weight loss from 167 °C to 370 °C, and then a rapid weight loss can be observed, which can be attributed to the decomposition of the framework, and it does not decompose completely until 800 °C.Conclusion
In summary, we have successfully prepared two new Zn(II) complexes based on 5-amino-1-H-tetrazole (HATZ) mixed with two aromatic polycarboxylate ligands. The two complexes both possess 3D structures and display as the binodal (4,4)-connected bbf net and trinodal (3,3,4)-connected net, respectively. In their structures, all the HATZ ligands are deprotonated and act as μ2-linker to connect with two Zn(II) centers. The two polycarboxylate ligands with multiple binding sites play the central role in the construction of the two 3D structures, such investigation demonstrates that it is benefit to generate intriguing and higher dimensional networks when using HATZ incorporated with polycarboxylate ligands. In addition, the two complexes both display intense photoluminescence in the solid state.Acknowledgements
This work was supported by the National Natural Science Foundation of China (no. 50872057).References
- (a) S. R. Batten, N. R. Champness, X. M. Chen, J. Garcia-Martinez, S. Kitagawa, L. Öhrström, M. O'Keeffe, M. P. Suh and J. Reedijk, CrystEngComm, 2012, 14, 3001 RSC; (b) S. R. Batten, N. R. Champness, X. M. Chen, J. Garcia-Martinez, S. Kitagawa, L. Öhrström, M. O'Keeffe, M. P. Suh and J. Reedijk, Pure Appl. Chem., 2013, 85, 1715 CrossRef CAS.
- (a) M. O'Keeffe and O. M. Yaghi, Chem. Rev., 2012, 112, 675 CrossRef PubMed; (b) H. L. Jiang, T. A. Makal and H. C. Zhou, Coord. Chem. Rev., 2013, 257, 2232 CrossRef CAS PubMed; (c) M. Li, D. Li, M. O'Keeffe and O. M. Yaghi, Chem. Rev., 2014, 114, 1343 CrossRef CAS PubMed.
- (a) L. J. Murray, M. Dinca and J. R. Long, Chem. Soc. Rev., 2009, 38, 1294 RSC; (b) M. P. Suh, H. J. Park, T. K. Prasad and D. W. Lim, Chem. Rev., 2012, 112, 782 CrossRef CAS PubMed; (c) J. A. Mason, M. Veenstra and J. R. Long, Chem. Sci., 2014, 5, 32 RSC.
- (a) J. R. Li, J. Sculley and H. C. Zhou, Chem. Soc. Rev., 2009, 38, 1477 RSC; (b) J. R. Li, J. Sculley and H. C. Zhou, Chem. Rev., 2012, 112, 869 CrossRef CAS PubMed.
- (a) M. Yoon, R. Srirambalaji and K. Kim, Chem. Rev., 2012, 112, 1196 CrossRef CAS PubMed; (b) H. R. Moon, D. W. Lim and M. P. Suh, Chem. Soc. Rev., 2013, 42, 1807 RSC; (c) J. Liu, L. Chen, H. Cui, J. Zhang, L. Zhang and C. Y. Su, Chem. Soc. Rev., 2014, 43, 6011 RSC.
- (a) P. Dechambenoit and J. R. Long, Chem. Soc. Rev., 2011, 40, 3249 RSC; (b) D. F. Weng, Z. M. Wang and S. Gao, Chem. Soc. Rev., 2011, 40, 3157 RSC.
- P. Horcajada, R. Gref, T. Baati, P. K. Allan, G. Maurin, P. Couvreur, G. Férey, R. E. Morris and C. Serre, Chem. Rev., 2012, 112, 1232–1268 CrossRef CAS PubMed.
- C. Wang, T. Zhang and W. Lin, Chem. Rev., 2012, 112, 1084 CrossRef CAS PubMed.
- (a) M. D. Allendorf, C. A. Bauer, R. K. Bhakta and R. J. T. Houk, Chem. Soc. Rev., 2009, 38, 1330 RSC; (b) Y. Cui, Y. Yue, G. Qian and B. Chen, Chem. Rev., 2012, 112, 1126 CrossRef CAS PubMed; (c) Z. Hu, B. J. Deibert and J. Li, Chem. Soc. Rev., 2014, 43, 5815 RSC.
- (a) N. Stock and S. Biswas, Chem. Rev., 2012, 112, 933 CrossRef CAS PubMed; (b) X. L. Wang, J. J. Huang, L. L. Liu, G. C. Liu, H. Y. Lin, J. W. Zhang, N. L. Chen and Y. Qu, RSC Adv., 2013, 3, 13944 RSC; (c) F. H. Zhao, S. Jing, Y. X. Che and J. M. Zheng, CrystEngComm, 2012, 14, 4478 RSC.
- M. Du, C. P. Li, C. S. Liu and S. M. Fang, Coord. Chem. Rev., 2013, 257, 1282 CrossRef CAS PubMed.
- X. He, C. Z. Lu and D. Q. Yuan, Inorg. Chem., 2006, 45, 5760 CrossRef CAS PubMed.
- (a) Y. L. Yao, L. Xue, Y. X. Che and J. M. Zheng, Cryst. Growth Des., 2009, 9, 606 CrossRef CAS; (b) F. H. Zhao, Y. X. Che, J. M. Zheng, F. Grandjean and G. J. Long, Inorg. Chem., 2012, 51, 4862 CrossRef CAS PubMed.
- (a) E. C. Yang, Y. L. Yang, Z. Y. Liu, K. S. Liu, X. Y. Wu and X. J. Zhao, CrystEngComm, 2011, 13, 2667 RSC; (b) E. C. Yang, Z. Y. Liu, X. Y. Wu, H. Chang, E. C. Wang and X. J. Zhao, Dalton Trans., 2011, 40, 10082 RSC; (c) Z. Y. Liu, H. A. Zou, Z. J. Hou, E. C. Yang and X. J. Zhao, Dalton Trans., 2013, 42, 15716 RSC; (d) D. S. Liu, J. Z. Yu, W. T. Chen, Y. Sui, L. M. Zhang and J. G. Huang, Inorg. Chim. Acta, 2014, 410, 131 CrossRef CAS PubMed.
- (a) Z. Su, M. Chen, T. Okamura, M. S. Chen, S. S. Chen and W. Y. Sun, Inorg. Chem., 2011, 50, 985 CrossRef CAS PubMed; (b) L. Wen, J. Zhao, K. Lv, Y. Wu, K. Deng, X. Leng and D. Li, Cryst. Growth Des., 2012, 12, 1603 CrossRef CAS.
- (a) R. Saha, S. K. Dey, S. Biswas, A. D. Jana and S. Kumar, Cryst. Growth Des., 2013, 13, 2135 CrossRef CAS; (b) X. L. Hu, C. Y. Sun, C. Qin, X. L. Wang, H. N. Wang, E. L. Zhou, W. E. Li and Z. M. Su, Chem. Commun., 2013, 49, 3564 RSC.
- CrystalClear, version 1.3.5., Rigaku Corp., Woodlands, TX, 1999 Search PubMed.
- G. M. Sheldrick, SHELX-97, Suite of programs for solution and refinement of crystal structures, University of Göttingen, Göttingen, Germany, 1997 Search PubMed.
- K. Nakamoto, Infrared and Raman Spectra of Inorganic and Coordination Compounds, Wiley, New York, 1986 Search PubMed.
- R. B. Zhang, Z. J. Li, Y. Y. Qin, J. K. Cheng, J. Zhang and Y. G. Yao, Inorg. Chem., 2008, 47, 4861 CrossRef CAS PubMed.
- A. W. Addison, T. N. Rao, J. Reedijk, J. van Rijn and G. C. Verschoor, J. Chem. Soc., Dalton Trans., 1984, 1349 RSC.
- (a) F. H. Zhao, Y. X. Che and J. M. Zheng, Inorg. Chim. Acta, 2012, 384, 170 CrossRef CAS PubMed; (b) F. H. Zhao, Y. X. Che and J. M. Zheng, Inorg. Chem. Commun., 2012, 17, 99 CrossRef CAS PubMed.
- (a) J. S. Hu, Y. J. Shang, X. Q. Yao, L. Qin, Y. Z. Li, Z. J. Guo, H. G. Zheng and Z. L. Xue, Cryst. Growth Des., 2010, 10, 2676 CrossRef CAS; (b) X. Zhang, L. Hou, B. Liu, L. Cui, Y. Y. Wang and B. Wu, Cryst. Growth Des., 2013, 13, 3177 CrossRef CAS; (c) X. He, X. P. Lu, Z. F. Ju, C. J. Li, Q. K. Zhang and M. X. Li, CrystEngComm, 2013, 15, 2731 RSC; (d) L. Fan, Y. Gao, G. Liu, W. Fan, W. Song, L. Sun, X. Zhao and X. Zhang, CrystEngComm, 2014, 16, 7649 RSC.
- O. V. Dolomanov, A. J. Blake, N. R. Champness and M. Schrõer, J. Appl. Crystallogr., 2003, 36, 1283 CrossRef CAS.
- (a) F. Nouar, J. F. Eubank, T. Bousquet, L. Wojtas, M. J. Zaworotko and M. Eddaoudi, J. Am. Chem. Soc., 2008, 130, 1833 CrossRef CAS PubMed; (b) Z. Guo, H. Wu, G. Srinivas, Y. Zhou, S. Xiang, Z. Chen, Y. Yang, W. Zhou, M. O'Keeffe and B. Chen, Angew. Chem., Int. Ed., 2011, 50, 3178 CrossRef CAS PubMed; (c) M. V. Kirillova, A. M. Kirillov, A. N. C. Martins, C. Graiff, A. Tiripicchio and A. J. L. Pombeiro, Inorg. Chem., 2012, 51, 5224 CrossRef CAS PubMed; (d) B. Li, S. Q. Zang, C. Ji, H. W. Hou and T. C. W. Mak, Cryst. Growth Des., 2012, 12, 1443 CrossRef CAS.
- X. W. Wang, J. Z. Chen and J. H. Liu, Cryst. Growth Des., 2007, 7, 1227 CAS.
- Y. Hou, S. T. Wang and E. H. Shen, Inorg. Chim. Acta, 2004, 357, 3155 CrossRef CAS PubMed.
- Y. S. Song, B. Yan and Z. X. Chen, J. Mol. Struct., 2005, 750, 101 CrossRef CAS PubMed.
- O. Nakagawa, S. Ono, Z. Li, A. Tsujimoto and S. Sasaki, Angew. Chem., Int. Ed., 2007, 46, 45 CrossRef PubMed.
- J. C. G. Bünzli and C. Piguet, Chem. Soc. Rev., 2005, 34, 1048 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1022209–1022210. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c4ra09588j |
| This journal is © The Royal Society of Chemistry 2014 |


