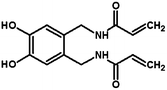
Size controllable synthesis and antimicrobial activity of poly-N,N′-[(4,5-dihydroxy-1,2-phenylene)bis(methylene)]bisacrylamide microspheres
Zhijia Zhangab,
Defeng Xinga,
Qing Lianga,
Daming Yongab and
Xiaojun Han*ab
aState Key Laboratory of Urban Water Resource and Environment, Harbin Institute of Technology, 150001, China. E-mail: hanxiaojun@hit.edu.cn
bSchool of Chemical Engineering and Technology, Harbin Institute of Technology, 150001, China
First published on 16th October 2014
Abstract
The monomer N,N′-[(4,5-dihydroxy-1,2-phenylene)bis(methylene)]bisacrylamide (OHABA) was successfully synthesized by the Friedel–Crafts reaction. Using precipitation polymerization, POHABA microspheres were easily obtained in a size-controlled manner. The microsphere diameters were controlled by the dosage of initiator (2,2′-azobisisobutyronitrile, AIBN). The minimum and maximum microsphere size are 0.83 ± 0.07 μm and 1.98 ± 0.13 μm, respectively. Antimicrobial activity test results showed that both OHABA and POHABA microspheres had a broad-spectrum inhibitory effect on both Gram-positive and Gram-negative bacteria, but POHABA performed better than the OHABA monomer. The inhibition rate of larger (1.98 μm in diameter) and smaller (1.00 μm in diameter) POHABA microspheres at the concentration of 0.238 mg mL−1 were 74.3% and 37.0% for soil microorganisms after a 48 hour reaction, respectively.
Introduction
From ancient times, people have used antimicrobial materials. It was found that silver and copper vessels retained water and did not decompose. At present, antimicrobial materials have wide applications in the food industry,1–3 in household appliances,4 sanitary ceramic products5 and in construction of steel, paint, and coatings.6 The facile production of antimicrobial materials has been the goal of a number of research groups for many decades. Antimicrobial agents are generally divided into three categories: inorganic materials, organic compounds and natural biological products. Silver ion is the most common type of antimicrobial agent,7–12 which can also be combined with glass,13 zirconium phosphate,14,15 zeolite,16 ceramics17 and activated carbon18–20 for antimicrobial purposes. In addition, zinc oxide,21 copper oxide22,23 and titanium dioxide24–26 are also inorganic antimicrobial agents. Organic antimicrobial agents, including varieties of vanillin and ethyl vanillin aldehyde compounds,27,28 acyl aniline,29 imidazoles,30–32 ketone of thiazole derivatives,33,34 quaternary ammonium salt35–37 and phenols,38 are considered to have antimicrobial effects. However, the safety of the organic antimicrobial agents is still under investigation. In this sense, the natural antimicrobial agents possess significant advantages. They are obtained from some living organisms38–42 such as chitin, castor oil, mustard, and horseradish. With the requirements for environmental protection, they have drawn a lot of attention from the scientists dealing with medical treatment, antimicrobial materials and biomedical production.Capsaicin (8-methyl-N-vanillyl-6-nonenamide), one of the pungent capsaicinoid compounds found in chili peppers, has already demonstrated a high degree of biological activity affecting the nervous, cardiovascular, and digestive systems. Recently, many efforts have focused on its possible antimicrobial effects.43–45 Capsaicins can prevent the growth of various bacteria, including Pseudomonas and Bacillus spp.44,46 However, the production of natural capsaicin extracts has to depend on the supply of raw material, which is very unreliable. Though N-vanillylnonanamide has been obtained by a chemical synthesis method and also has an inhibitory effect,47,48 its applications are limited due to its complex synthesis process.
Antimicrobial agents in liquid form suffer the inherent problem of residual toxicity, which can be solved by immobilizing them on solid surfaces. Antimicrobial agents in microspheric form have some advantages, including easy recycling, low residual toxicity, large contact area with microorganisms. Microspheres are considered to be one of the promising candidates as bioactive molecule carriers.49 To date, only a few reports have dealt with the antimicrobial effect of microspheres.49,50
Here, we presented a one-step process to produce a capsaicin analogue, i.e., N,N′-[(4,5-dihydroxy-1,2-phenylene)bis(methylene)]bisacrylamide (OHABA). OHABA has advantages over natural capsaicin extracts and N-vanillylnonanamide, because it is cheaper due to facile synthesis with higher purity and yield. We polymerized it into microspheres in a size-controlled manner. Both OHABA and POHABA microspheres had shown excellent efficacies in inactivating a wide range of microorganisms, including bacteria and fungi. They have great potential in various areas such as in antimicrobial agents, household appliances, sanitary products, and antifouling coating materials.
Experimental
Materials
The chemical reagents used for synthesis and polymerization, namely, pyrocatechol, concentrated sulfuric acid, absolute ethanol and 2,2′-azobisisobutyronitrile (AIBN), were obtained from Aladdin (China) and used without further purification. N-(hydroxymethyl) acrylamide (Aladdin) was recrystallized from ethanol before use.Monomer synthesis
The compound was synthesized by a classical synthetic procedure, the Friedel–Crafts alkylation reaction, which requires a Lewis acid or proton acid as catalysts.51 20.2 g (0.2 mol) N-(hydroxymethyl) acrylamide, 11.0 g (0.1 mol) pyrocatechol, and a certain volume of absolute ethanol were added into a 250 mL three-neck round-bottom flask. A clear solution was obtained after ultrasonication. The solution was easily mixed by stirring at room temperature, and then concentrated sulfuric acid was added dropwise to the solution at 35 °C until a white product precipitated. The product was recrystallized by ethanol, and then dried in a vacuum desiccator.The yield of monomer, i.e., N,N′-[(4,5-dihydroxy-1,2-phenylene)bis(methylene)]bisacrylamide (OHABA) was 73%. m.p. 206–207 °C. IR (KBr): ν = 3403 cm−1 (O–H), 3289 cm−1 (N–H), 1658 cm−1 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1611 cm−1 (C
O), 1611 cm−1 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 1563 cm−1, 1527 cm−1, 1459 cm−1 (C
C), 1563 cm−1, 1527 cm−1, 1459 cm−1 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, Ph), 1290 cm−1 (Ph–OH), 990 cm−1 (HC
C, Ph), 1290 cm−1 (Ph–OH), 990 cm−1 (HC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2); 1H NMR (600 MHz, DMSO-d6, δ): 8.82 (1H, s, O–H), 8.41 (1H, t, N–H), 6.64 (1H, s, Ph–H), 6.26 (1H, q,
CH2); 1H NMR (600 MHz, DMSO-d6, δ): 8.82 (1H, s, O–H), 8.41 (1H, t, N–H), 6.64 (1H, s, Ph–H), 6.26 (1H, q, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–), 6.11 (1H, q,
CH–), 6.11 (1H, q, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–), 5.60 (1H, q,
CH–), 5.60 (1H, q, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–), 4.21 (2H, d, –CH2–). The compound structure was confirmed and is shown in Scheme 1.
CH–), 4.21 (2H, d, –CH2–). The compound structure was confirmed and is shown in Scheme 1.
Polymerization of OHABA
A certain amount of OHABA and 10 mL absolute ethanol were added into a dry glass bottle. The bottle was then sealed and placed in an oil bath at 70 °C under vigorous magnetic stirring for 30 min. The OHABA solids were completely dissolved and a transparent solution was obtained. Under a constant magnetic stirring rate, a certain amount of AIBN was added into the reaction system for further 10 h at 70 °C. In order to remove impurities, such as unreacted OHABA, the resulting particles were purified by repeated centrifugation (7000 rpm) and redispersion in ethanol by ultrasonication.Characterizations
The morphology and size were observed using a field emission gun scanning electron microscope (SEM; XL 30 Philips, Philips, lnc., Netherlands). Fourier transformation infrared (FT-IR) spectra were collected with a Thermo Scientific Nicolet iS10 spectrophotometer scanning from 500 to 4000 cm−1 with a resolution of 2 cm−1 for 20 scans. 1H NMR spectroscopy of OHABA was carried out using JEOL JNM ECP 600 MHz superconducting nuclear magnetic resonance spectrometer (Japanese Electronics Co., LTD).Stains and antimicrobial activity test
A fungus Rhizoctonia solani AG1-IB (R. solani), and the Gram-positive Bacillus subtilis SF4-3 (B. subtilis) and Gram-negative Escherichia coli DH5α (E. coli) bacteria were obtained from Northeast Agricultural University. The microbes were pre-cultured routinely prior to antimicrobial tests. E. coli and B. subtilis were inoculated to LB medium (pH 7.4)52 (including peptone of 10 g, yeast extract of 5 g, NaCl of 10 g in one liter of distilled water), and cultivated for 72 hours at 37 ± 1 °C.The fungus R. solani was inoculated into a potato dextrose agar medium53 (PDA, containing per liter: 250 g potato extract, 20 g dextrose, distilled water, autoclaving for twenty minutes at 121 °C) and cultivated for 72 hours at 30 ± 1 °C.
The antimicrobial activity tests were performed by adding OHABA and POHABA microspheres into culture medium, then using Bioscreen C (Labsystems, Helsinki, Finland), an automated optical density-monitoring system, to monitor microbial growth in 100-well honeycomb plates at 37 °C with shaking. All cultures were performed in a final volume of 400 μL. The turbidity of culture solution was detected at 540 nm, and all tests were performed in triplicate.
The minimum inhibitory concentration (MIC) was determined spectrophotometrically at 540 nm according to the agar dilution method.54 A series of OHABA/POHABA microspheres samples with the concentration ranging from 0.025 to 12.8 mg mL−1 were prepared in 10 mL experimental tubes. After autoclaving, 1 mL of each OHABA/POHABA microspheres sample and 100 μL bacterial suspension (105 CFU mL−1) were placed aseptically in 900 μL of LB medium and incubated for 24 h at 37 °C. The lowest concentration of the test sample showing no visible growth of bacterium when compared with the control sample was recorded as the MIC.
Results and discussion
Size controllable synthesis of POHABA microspheres
Fig. 1 shows the SEM image and FT-IR spectra of typical POHABA microsphere samples prepared by mixing 0.1 g OHABA precursor and 0.1 g AIBN initiator in 10 mL absolute ethanol at 70 °C under vigorous magnetic stirring for 10 h. The POHABA microspheres are uniform in size with smooth surface (Fig. 1(a)). The mean diameter of the monodisperse spheres is 1.00 ± 0.07 μm. It should be noted that the uniformity in size/shape could be easily accomplished by our method.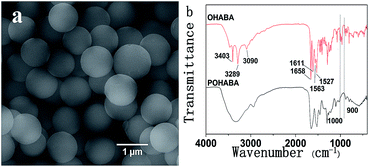 | ||
| Fig. 1 (a) SEM image of POHABA microspheres; and (b) infrared spectra of the OHABA precursor and the POHABA microspheres. | ||
The chemical structures of POHABA microspheres and OHABA monomer were studied by FT-IR (Fig. 1(b)). The spectra in 3090 cm−1 and 1611 cm−1 bands correspond to the broadening C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–H stretching vibration and the C
C–H stretching vibration and the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching vibration, respectively. In Fig. 1(b) red curve, the peaks at ∼900–1000 cm−1 are ascribed to C
C stretching vibration, respectively. In Fig. 1(b) red curve, the peaks at ∼900–1000 cm−1 are ascribed to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–H deformation vibration. All aforementioned peaks in the red curve in Fig. 1(b) confirm the existence of vinyl group in OHABA. The absence of those peaks at ∼900–1000 cm−1 in the black curve of Fig. 1(b) indicates that vinyl groups were opened after polymerization. The polymerization process is proposed and shown in Scheme 2.
C–H deformation vibration. All aforementioned peaks in the red curve in Fig. 1(b) confirm the existence of vinyl group in OHABA. The absence of those peaks at ∼900–1000 cm−1 in the black curve of Fig. 1(b) indicates that vinyl groups were opened after polymerization. The polymerization process is proposed and shown in Scheme 2.
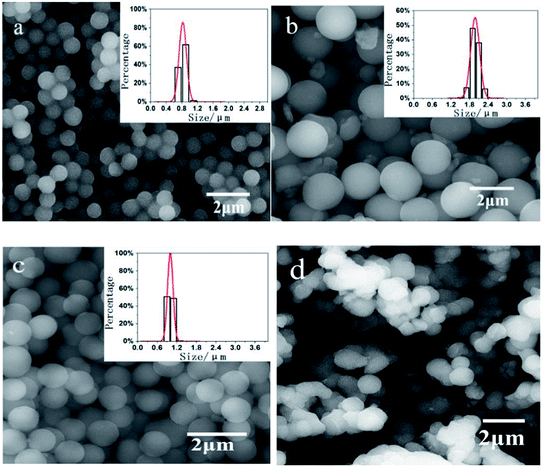
The size of the microspheres can be easily adjusted by maintaining the dosage of OHABA (0.1 g in 10 mL ethanol) constant whilst varying the dosage of AIBN. Fig. 2 shows the SEM images of the microspheres obtained at the dosage of AIBN of 0.005 g in 10 mL ethanol (a), 0.01 g in 10 mL ethanol (b), and 0.1 g in 10 mL ethanol (c), respectively. Their corresponding sizes are 0.83 ± 0.07 μm, 1.98 ± 0.13 μm and 1.00 ± 0.07 μm in diameter, respectively. All these spheres have good monodispersity. It should also be noted that the size peaks at the AIBN dosage of 0.01 g in 10 mL ethanol.
As the dosage of AIBN was elevated, the microsphere size increased and reached its maximum at first, and then a further increase of the dosage of AIBN resulted in the decrease of the microsphere size. Obviously, the AIBN was a key parameter here. It is well known that microsphere formation mechanism includes two stages, i.e. a nucleation stage and a growth stage. At the second stage, the residual double bonds hanging on the surface of microspheres continue to capture monomers from the solution to make microspheres gradually grow.55 If there is not enough AIBN, the growth stage can terminate earlier, resulting in a small size. If there is too much AIBN, the chance of the new emerging nucleation sites becomes larger, consequently producing the small size microspheres. When the AIBN dosage was over 0.15 g in 10 mL ethanol, the clusters of the microsphere were obtained, as shown in Fig. 2(d).
A significant part of previous publications have been related to the use of atom transfer radical polymerization (ATRP).56–59 A large variety of monomers can be polymerized and copolymerized radically under relatively simple experimental conditions.60–62 However, free radical polymerization processes often yield polymers with uncontrolled molecular weights, morphology and high polydispersities. In the current study, we demonstrated the monodispersed microsphere could be fabricated in a size-controlled manner by varying the initiator via atom transfer radical polymerization. Such an approach will help define the appropriate requirements to produce a direct synthesis of large monodisperse particles without using any seeds and time-consuming multistep reaction scheme.
Antimicrobial activity of OHABA
In order to investigate the antimicrobial activity of OHABA, we chose a wide range of microorganisms, including E. coli, B. subtilis, R. solani and soil microorganisms. Fig. 3 shows the growth curves of different concentration of OHABA mixed together with E. coli (a), B. subtilis (b), R. solani (c) and soil microorganisms (d), respectively. Each curve is the average of three parallel experiments. From Fig. 3, we can see that the growth curves show a similar “classical” growth pattern for different microbial genera. The exponential phase of growth generally occurred from 1 to 3 hour incubation, whereas the stationary phase was reached between 11 and 13 hours after culturing. With increase in OHABA concentration from 0.475 mg mL−1 to 1.9 mg mL−1, the antimicrobial activity became more pronounced. We did not detect an inhibition to the growth of E. coli, B. subtilis, R. solani with the OHABA concentration less than 0.475 mg mL−1.In order to measure the antimicrobial activity quantitatively, we calculated the inhibition rate of all microorganisms with the OHABA of 1.9 mg mL−1 at 24 h, 36 h, 48 h, and 60 h respectively, as shown in Fig. 3(e). The inhibition rates of E. coli, B. subtilis, R. solani and soil microorganisms are 46.0%, 53.6%, 23.8%, 89.6% at 24 h, respectively, which proves the broad-spectrum antimicrobial effect of OHABA. The inhibition rate fells slightly afterwards, which may be due to the consumption of OHABA or drug resistance of microorganisms. The antibacterial effect of the OHABA monomer towards B. subtilis is superior to E. coli. Moreover, the OHABA monomer has lowest inhibition effect to R. solani at the same concentration. Like capsaicin,63 the OHABA is toxic to the microorganism possibly due to its influence on the membrane structure or osmotic stress by entering into the cells.
Antimicrobial activity of POHABA microspheres
A wide type of microorganisms, including E. coli, B. subtilis, R. solani and soil microorganisms, were also used to investigate the antimicrobial activity of POHABA microspheres with different sizes. In order to simplify the expression, POHABA microspheres of 1.00 ± 0.07 μm and 1.98 ± 0.13 μm in diameters designated named POHABA (1 μm) and POHABA (2 μm), respectively. Fig. 4 shows the growth curves of OHABA, POHABA (1 μm), and POHABA (2 μm) with the same concentration of 0.238 mg mL−1 mixed with E. coli (a), B. subtilis (b), R. solani (c) and soil microorganisms (d), respectively. It should be noted that OHABA at concentration of 0.238 mg mL−1 has no inhibitory effect to E. coli, B. subtilis, and R. solani. On the contrary, at the same concentration, the POHABA microsphere shows an obvious antimicrobial effect, which proves the better antimicrobial effect of the microsphere over its monomer. This is probably due to the surface of POHABA microspheres having more phenol groups than OHABA, and thus a greater opportunity to contact with the microorganisms due to their large surface areas. The sites and the numbers of phenol groups are thought to correspond to their toxicity to microorganism.39,64 The POHABA microspheres have broad-spectrum antimicrobial effects. In terms of soil microorganisms, both the monomer and the microsphere showed antimicrobial activities, which indicates that the soil microorganisms contain some OHABA-sensitive microorganisms.Another conclusion we can draw from Fig. 4 is that POHABA microspheres (2 μm) have a better antimicrobial activity than POHABA microspheres (1 μm). The inhibition rates for E. coli and B. subtilis are 66.9% and 76.9%, respectively, from 2 μm microspheres, and 54.2% and 49.4% from 1 μm microspheres at the concentration of 0.238 mg mL−1 at 24 hours, respectively. These values are much better than 1.6% for E. coli and 10.1% for B. subtilis from the antimicrobial agent of ascopyrone P (APP, a secondary metabolite formed by the fungi) at the concentration of 0.25 mg mL−1 at 24 h.41 For the soil microorganisms, the antimicrobial effect of POHABA (2 μm) is the best with the inhibition rate over 70% after 48 h at the concentration of 0.238 mg mL−1, as shown in Fig. 4(e).
The mechanisms responsible for the toxicity of phenol functionalized microspheres may be the enzyme inhibition by the reaction with sulfhydryl groups or by the nonspecific interactions with the proteins,65 and the influence on membrane structures of the microorganisms. A more detailed study of the action mechanism is still under investigation.
In order to study the morphology change of the microsphere after antimicrobial activity test, the SEM technique was used to image the samples after mixing with R. solani and E. coli for 48 hours. From red circle areas in Fig. 5, we can see that the microsphere has still a smooth spherical shape with the same size as the fresh sample, which indicates that POHABA microspheres probably have a permanent antimicrobial effect. These dead R. solani and E. coli are shown in the black circle in Fig. 5(a and b), respectively.
Determination of OHABA/POHABA microsphere MIC
The minimum inhibitory concentration (MIC) is a common parameter used to evaluate the activity of new antimicrobial agents. The MIC values of OHABA/POHABA microspheres were obtained using a spectrophotometric method and are listed in Table 1. The MIC values confirmed the effectiveness of the OHABA, POHABA (1 μm) and POHABA (2 μm) against E. coli and B. subtilis. Table 1 shows that the MIC values of OHABA for E. coli and B. subtilis were 6.4 and 3.2 mg mL−1, respectively. Therefore, the Gram-positive bacteria have a higher sensitivity to OHABA compared to Gram-negative bacteria. The MIC values of POHABA (1 μm) and POHABA (2 μm) for E. coli and B. subtilis were all 0.4 mg mL−1. Comparing the MIC values of microspheres with that of OHABA, it is concluded that the microspheres have better antibacterial performance than OHABA. The MIC value of OHABA (3.2 mg mL−1) is 10 times lower than that of crude extract of Capsicum Chinense (40 mg mL−1) for E. coli at 24 h.66 Both OHABA and POHABA microspheres are much more effective for antibacterial inhibition than capsaicin because the MIC values of capsaicin for E. coli and B. subtilis were 100 mg mL−1 and 25 mg mL−1, respectively.67| Microorganisms | MIC (mg mL−1) | ||
|---|---|---|---|
| OHABA | POHABA (1 μm) | POHABA (2 μm) | |
| E. coli | 6.4 | 0.4 | 0.4 |
| B. subtilis | 3.2 | 0.4 | 0.4 |
Conclusions
In summary, we have successfully prepared OHABA by a one-step reaction. Using precipitation polymerization, POHABA microspheres were successfully obtained, which are highly monodisperse with a smooth surface. The sizes of POHABA microspheres could be systematically controlled by varying the dosage of AIBN. Both OHABA and POHABA exhibit broad-spectrum antimicrobial activities. The antimicrobial effect of POHABA microspheres is better than that of OHABA monomer. The larger size microsphere has the better antimicrobial effect. The broad-spectrum antimicrobial property of OHABA and POHABA gives these microspheres great potential in the fields of antimicrobial agents, household appliances, sanitary products, and antifouling coating materials.Acknowledgements
This work was supported by the National Natural Science Foundation of China (grant no. 21273059, 21003032), the State Key Laboratory of Urban Water Resource and Environment (Harbin Institute of Technology) (grant no. 2014DX09), the Fundamental Research Funds for the Central Universities (grant no. HIT. KISTP. 201407, HIT. NSRIF. 201501508376), and the Harbin Science and Technology Research Council (grant no. 2014RFXXJ063).Notes and references
- A. Alonso, X. Munoz-Berbel, N. Vigues, R. Rodriguez-Rodriguez, J. Macanas, M. Munoz, J. Mas and D. N. Muraviev, Adv. Funct. Mater., 2013, 23, 2450–2458 CrossRef CAS.
- A. L. Brody, B. Bugusu, J. H. Han, C. K. Sand and T. H. Mchugh, J. Food Sci., 2008, 73, R107–R116 CrossRef CAS PubMed.
- P. M. Davidson, J. N. Sofos and A. L. Branen, Antimicrobials in food, CRC Press, 2010 Search PubMed.
- L. Bizhong, J. Junhui, D. Xiaoxu, W. Jin and V. Qing, Eng. Plast. Appl., 1999, 2, 006 Search PubMed.
- M. Machida, K. Norimoto and T. Kimura, J. Am. Ceram. Soc., 2005, 88, 95–100 CrossRef CAS PubMed.
- J. Lee, S. Mahendra and P. J. Alvarez, ACS Nano, 2010, 4, 3580–3590 CrossRef CAS PubMed.
- S. Ghosh, A. Saraswathi, S. S. Indi, S. L. Hoti and H. N. Vasan, Langmuir, 2012, 28, 8550–8561 CrossRef CAS PubMed.
- A. Ivask, A. ElBadawy, C. Kaweeteerawat, D. Boren, H. Fischer, Z. X. Ji, C. H. Chang, R. Liu, T. Tolaymat, D. Telesca, J. I. Zink, Y. Cohen, P. A. Holden and H. A. Godwin, ACS Nano, 2014, 8, 374–386 CrossRef CAS PubMed.
- Z. M. Davoudi, A. E. Kandjani, A. I. Bhatt, I. L. Kyratzis, A. P. O'Mullane and V. Bansal, Adv. Funct. Mater., 2014, 24, 1047–1053 CrossRef CAS.
- M. Rai, A. Yadav and A. Gade, Biotechnol. Adv., 2009, 27, 76–83 CrossRef CAS PubMed.
- V. K. Sharma, R. A. Yngard and Y. Lin, Adv. Colloid Interface Sci., 2009, 145, 83–96 CrossRef CAS PubMed.
- J. S. Kim, E. Kuk, K. N. Yu, J. H. Kim, S. J. Park, H. J. Lee, S. H. Kim, Y. K. Park, Y. H. Park, C. Y. Hwang, Y. K. Kim, Y. S. Lee, D. H. Jeong and M. H. Cho, J. Nanomed. Nanotechnol., 2007, 3, 95–101 CrossRef CAS PubMed.
- M. Bellantone, H. D. Williams and L. L. Hench, Antimicrob. Agents Chemother., 2002, 46, 1940–1945 CrossRef CAS PubMed.
- N. Biswal, S. Martha, U. Subudhi and K. Parida, Ind. Eng. Chem. Res., 2011, 50, 9479–9486 CrossRef CAS.
- M. Turkoz, A. O. Atilla and Z. Evis, Ceram. Int., 2013, 39, 8925–8931 CrossRef CAS PubMed.
- H. Y. Shi, F. Liu and L. X. Xue, J. Membr. Sci., 2013, 437, 205–215 CrossRef CAS PubMed.
- Y. H. Lv, H. Liu, Z. Wang, S. J. Liu, L. J. Hao, Y. H. Sang, D. Liu, J. Y. Wang and R. I. Boughton, J. Membr. Sci., 2009, 331, 50–56 CrossRef CAS PubMed.
- M. J. Chen, Y. N. Zhao, W. T. Yang and M. Z. Yin, Langmuir, 2013, 29, 16018–16024 CrossRef CAS PubMed.
- Z. J. Fan, B. Liu, J. Q. Wang, S. Y. Zhang, Q. Q. Lin, P. W. Gong, L. M. Ma and S. R. Yang, Adv. Funct. Mater., 2014, 24, 3933–3943 CrossRef CAS.
- I. Ocsoy, M. L. Paret, M. A. Ocsoy, S. Kunwar, T. Chen, M. X. You and W. H. Tan, ACS Nano, 2013, 7, 8972–8980 CrossRef CAS PubMed.
- K. R. Raghupathi, R. T. Koodali and A. C. Manna, Langmuir, 2011, 27, 4020–4028 CrossRef CAS PubMed.
- M. Eshed, J. Lellouche, A. Gedanken and E. Banin, Adv. Funct. Mater., 2014, 24, 1382–1390 CrossRef CAS.
- K. Giannousi, K. Lafazanis, J. Arvanitidis, A. Pantazaki and C. Dendrinou-Samara, J. Inorg. Biochem., 2014, 133, 24–32 CrossRef CAS PubMed.
- S. L. Mei, H. Y. Wang, W. Wang, L. P. Tong, H. B. Pan, C. S. Ruan, Q. L. Ma, M. Y. Liu, H. L. Yang, L. Zhang, Y. C. Cheng, Y. M. Zhang, L. Z. Zhao and P. K. Chu, Biomaterials, 2014, 35, 4255–4265 CrossRef CAS PubMed.
- T. Paul, P. L. Miller and T. J. Strathmann, Environ. Sci. Technol., 2007, 41, 4720–4727 CrossRef CAS.
- W. Kangwansupamonkon, V. Lauruengtana, S. Surassmo and U. Ruktanonchai, J. Nanomed. Nanotechnol., 2009, 5, 240–249 CrossRef CAS PubMed.
- J. Sun, Y. Yin, G. H. Sheng, Z. B. Yang and H. L. Zhu, J. Mol. Struct., 2013, 1039, 214–218 CrossRef CAS PubMed.
- D. J. Fitzgerald, M. Stratford and A. Narbad, Int. J. Food Microbiol., 2003, 86, 113–122 CrossRef CAS.
- R. A. Coburn, A. J. Batista, R. T. Evans and R. J. Genco, J. Med. Chem., 1981, 24, 1245–1249 CrossRef CAS.
- N. N. Al-Mohammed, Y. Alias, Z. Abdullah, R. M. Shakir, E. M. Taha and A. A. Hamid, Molecules, 2013, 18, 11978–11995 CrossRef CAS PubMed.
- Y. T. Duan, Z. C. Wang, Y. L. Sang, X. X. Tao and H. L. Zhu, Curr. Top. Med. Chem., 2013, 13, 3118–3130 CrossRef CAS.
- D. Sharma, B. Narasimhan, P. Kumar, V. Judge, R. Narang, E. De Clercq and J. Balzarini, Eur. J. Med. Chem., 2009, 44, 2347–2353 CrossRef CAS PubMed.
- S. Bondock, W. Fadaly and M. A. Metwally, Eur. J. Med. Chem., 2010, 45, 3692–3701 CrossRef CAS PubMed.
- S. Bondock, W. Khalifa and A. A. Fadda, Eur. J. Med. Chem., 2007, 42, 948–954 CrossRef CAS PubMed.
- J. H. Wynne, P. A. Fulmer, D. M. McCluskey, N. M. Mackey and J. P. Buchanan, ACS Appl. Mater. Interfaces, 2011, 3, 2005–2011 CAS.
- A. A. Fadda and R. E. El-Mekawy, Dyes Pigm., 2013, 99, 512–519 CrossRef CAS PubMed.
- C. Z. Chen, N. C. Beck-Tan, P. Dhurjati, T. K. van Dyk, R. A. LaRossa and S. L. Cooper, Biomacromolecules, 2000, 1, 473–480 CrossRef CAS.
- T. S. Sileika, D. G. Barrett, R. Zhang, K. H. Lau and P. B. Messersmith, Angew. Chem., Int. Ed., 2013, 52, 10766–10770 CrossRef CAS PubMed.
- M. M. Cowan, Clin. Microbiol. Rev., 1999, 12, 564–582 CAS.
- L. V. Thomas, J. W. Wimpenny and A. C. Peters, Int. J. Food Microbiol., 1991, 14, 261–275 CrossRef CAS.
- L. V. Thomas, S. Yu, R. E. Ingram, C. Refdahl, D. Elsser and J. Delves-Broughton, J. Appl. Microbiol., 2002, 93, 697–705 CrossRef CAS.
- I. Hernandez, L. Marquez, I. Martinez, R. Dieguez, C. Delporte, S. Prieto, J. Molina-Torres and G. Garrido, J. Ethnopharmacol., 2009, 124, 649–652 CrossRef CAS PubMed.
- R. H. Cichewicz and P. A. Thorpe, J. Ethnopharmacol., 1996, 52, 61–70 CrossRef CAS.
- J. Molina-Torres, A. Garcia-Chavez and E. Ramirez-Chavez, J. Ethnopharmacol., 1999, 64, 241–248 CrossRef CAS.
- R. Martini, L. Serrano, S. Barbosa and J. Labidi, Cellulose, 2014, 21, 1909–1919 CrossRef CAS.
- L. Dorantes, R. Colmenero, H. Hernandez, L. Mota, M. E. Jaramillo, E. Fernandez and C. Solano, Int. J. Food Microbiol., 2000, 57, 125–128 CrossRef.
- F. Villa, L. Giacomucci, A. Polo, P. Principi, L. Toniolo, M. Levi, S. Turri and F. Cappitelli, Biotechnol. Lett., 2009, 31, 1407–1413 CrossRef CAS PubMed.
- T. Satoh, H. Miyoshi, K. Sakamoto and H. Iwamura, Biochim. Biophys. Acta, Bioenerg., 1996, 1273, 21–30 CrossRef.
- Z. P. Cheng, X. L. Zhu, Z. L. Shi, K. G. Neoh and E. T. Kang, Ind. Eng. Chem. Res., 2005, 44, 7098–7104 CrossRef CAS.
- L. H. Xiao, T. Wang, T. Y. Zhao, X. Zheng, L. Y. Sun, P. Li, F. Q. Liu, G. Gao and A. Dong, Int. J. Mol. Sci., 2013, 14, 7391–7404 CrossRef CAS PubMed.
- H. Xu, L. Yu, C. Li and Z. Zhang, Chin. J. Appl. Chem., 2007, 24, 916 Search PubMed.
- J. Sambrook, E. F. Fritsch and T. Maniatis, Molecular cloning, Cold spring harbor laboratory press, New York, 1989 Search PubMed.
- A. Ingle, M. Rai, A. Gade and M. Bawaskar, J. Nanopart. Res., 2009, 11, 2079–2085 CrossRef CAS.
- I. Wiegand, K. Hilpert and R. E. Hancock, Nat. Protoc., 2008, 3, 163–175 CrossRef CAS PubMed.
- J. S. Downey, R. S. Frank, W. H. Li and H. D. H. Stover, Macromolecules, 1999, 32, 2838–2844 CrossRef CAS.
- P. Alexander, R. Black and A. Charlesby, Proc. R. Soc. London, Ser. A, 1955, 232, 31–48 CrossRef CAS.
- K. Matyjaszewski and J. H. Xia, Chem. Rev, 2001, 101, 2921–2990 CrossRef CAS PubMed.
- T. E. Patten and K. Matyjaszewski, Adv. Mater., 1998, 10, 901–915 CrossRef CAS.
- S. Coca, C. B. Jasieczek, K. L. Beers and K. Matyjaszewski, J. Polym. Sci., Part A: Polym. Chem., 1998, 36, 1417–1424 CrossRef CAS.
- K. L. Beers, S. G. Gaynor, K. Matyjaszewski, S. S. Sheiko and M. Moller, Macromolecules, 1998, 31, 9413–9415 CrossRef CAS.
- K. Matyjaszewski, P. J. Miller, N. Shukla, B. Immaraporn, A. Gelman, B. B. Luokala, T. M. Siclovan, G. Kickelbick, T. Vallant, H. Hoffmann and T. Pakula, Macromolecules, 1999, 32, 8716–8724 CrossRef CAS.
- V. Coessens, T. Pintauer and K. Matyjaszewski, Prog. Polym. Sci., 2001, 26, 337–377 CrossRef CAS.
- S. Kurita, E. Kitagawa, C. H. Kim, Y. Momose and H. Iwahashi, Biosci., Biotechnol., Biochem., 2002, 66, 532–536 CrossRef CAS PubMed.
- T. Geissman, Pyrrole pigments, isoprenoid compounds and phenolic plant constituents, 1963, vol. 9, p. 265 Search PubMed.
- T. L. Mason and B. P. Wasserman, Phytochemistry, 1987, 26, 2197–2202 CrossRef CAS.
- L. R. Corrêa, N. M. Costa, T. Ishikawa and R. M. L. Lemes, Rev. Eletronica Farm., 2012, 9, 1 Search PubMed.
- M. De, A. K. De and A. B. Banerjee, Phytother. Res., 1999, 13, 616–618 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2014 |