Immobilization of titania nanoparticles on the surface of cellulose fibres by a facile single step hydrothermal method and study of their photocatalytic and antibacterial activities
Abstract
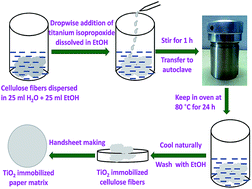
Cellulose fibers immobilized with 2.5 to 21.0 wt% titania (TiO2) nanoparticles of diameter 10–20 nm were prepared by a facile single step hydrothermal method. Paper matrices were prepared by a standard handsheet making procedure using these TiO2 immobilized cellulose fibers. These paper matrices successfully degraded ∼82% of formaldehyde and completely degraded methyl orange in 180 min, when irradiated with sunlight. Moreover, the results improved on increasing either the irradiation time or TiO2 content. Furthermore, these paper matrices showed promising antibacterial activity against Escherichia coli in visible light. The successful immobilization of TiO2 nanoparticles on paper matrices was characterized by field emission scanning electron microscopy (FESEM) and X-ray diffraction studies (XRD).

 Please wait while we load your content...
Please wait while we load your content...