A multifunctional ionic iridium complex for field-effect and light-emitting devices†
Jing Li,
Guifang Dong*,
Lian Duan,
Dongxin Ma,
Tao Hu,
Yunge Zhang,
Liduo Wang and
Yong Qiu
Key Laboratory of Organic Optoelectronics and Molecular Engineering of Ministry of Education, Department of Chemistry, Tsinghua University, Beijing, 100084, P. R. China. E-mail: donggf@mail.tsinghua.edu.cn; Fax: +86-010-62795137; Tel: +86-010-62782287
First published on 6th October 2014
Abstract
We synthesized a new ionic iridium complex with interesting multifunctions of both insulativity and light emission. With this material as gate dielectric, we have fabricated a multifunctional device which behaves as a normal transistor at low gate voltage and a light emitting device when the voltage is over 4 V. The emission brightness can be tuned by VGS and VDS separately. This device can potentially be applied in overvoltage alarm systems.
Light-emitting complexes are of great interest with the rapid development of organic electronics.1–5Among these various compounds, ionic iridium complexes have caught much attention due to their high luminous efficiency.6–9 They are generally used in light-emitting electrochemical cells (LECs) which can work effectively at quite low voltage because of their operation mechanism.10–12 Ionic compounds can not only be applied in LECs, but also in transistors where they serve as insulators to induce working voltage.13,14 Therefore, iridium ionic complexes used as emitting materials in LECs have potential to be employed as dielectric materials in transistors as well, leading to realization of the fabrication of multifunctional devices. In this manuscript we synthesized a new ionic iridium complex and successfully fabricated a multifunctional device with it.
The ionic iridium light-emitting complex [Ir(dmfpz)2(dtb-bpy)]PF6, shown in Fig. 1a, contains two kinds of ligands, 1-(9,9-dimethyl-fluorene-2-yl)-1H-pyrazole (dmfpz) and 4,4′-bis(tert-butyl)-2,2-bipyridine (dtb-bpy). These ligands have steric hindrance which enhance luminous efficiency and improve stability.15,16 Synthesis of the material are shown in ESI.† Fig. 1b exhibits the absorption and photo luminescent (PL) spectra of the ionic complex in dilute CH3CN solution and thin film. The absorption spectra are typical for iridium complexes, with the absorption under 350 nm ascribing to 1π–π* transitions from ligands and the absorption above 350 nm corresponding to excitations to MLCT (metal to-ligand charge-transfer) and LLCT (ligand-to-ligand charge-transfer).17 The PL spectra give a wide broad peak without refined structures, which belongs to MLCT and LLCT transitions. The electrochemical behaviour of this complex is investigated by cyclic voltammetry (Fig. 1c). The oxidation potential of complex is 0.83 V, while the reduction potential is −1.81 V, indicating the HOMO and LUMO levels of this complex to be −5.57 eV and −2.93 eV and the level gap is 2.64 eV. The atomic force microscopy (AFM) image (Fig. 1d) demonstrates the thin film of ionic iridium complex is smooth and homogeneous with the RMS of only 0.65 nm.
The dielectric constant of the iridium complex film is measured to be 3.06 (Fig. 2a). The device structure is shown in Fig. 2b. Firstly, a zinc tin oxide (ZTO, a kind of n-type material with high optical transmittance and high mobility18) layer is deposited by spin-coating on a glass substrate with source and drain electrodes (ITO) on it, and then the gate dielectric film of [Ir(dmfpz)2(dtb-bpy)]PF6 is formed also by spin-coating. Finally, the gate electrode film of silver is deposited by thermal evaporation in vacuum. Details of fabrication process are presented in ESI.†
Fig. 2c shows the output characteristics of this top-gate-structured transistor. When the VGS is lower than 4 V, the device exhibits normal n-type characteristics with the source electrode grounded. Fig. 2d displays the transfer curves of the device. From this diagram we estimate on/off current ratio to be 250, electron mobility to be 48.3 cm2 V−1 s−1, while the threshold voltage VT to be only 0.85 V. The low working voltage is attributed to the high capacitance of the electrical double layers (EDLs) formed at the gate–electrolyte and semiconductor–electrolyte interfaces and the large concentration of electrons on the surface of the semiconductor.13,14
When the gate voltage becomes higher (over 4 V), the device acts as a controllable light-emitting electrochemical cell (LEC) rather than a transistor. Fig. 3a shows the electroluminescent (EL) spectra of the device. The EL peak is at 600 nm. The red-shift of EL comparing with PL is because when applying a positive gate voltage to the device, an electric field will be formed, which polarizes the ionic complex and decreases the energy level of the excited states as a result.19,20 The device gives orange light with the CIE coordinates of (0.55, 0.45) (Fig. 3b). Fig. 3c depicts the emission photos of this device. The emission is visible in ambient light through the source and drain ITO electrodes and the glass substrate. It is obvious that the device behaves like a LEC under a high gate voltage. However, different from a traditional LEC, this device has three electrodes rather than two, thus, we investigated functions of these three electrodes (gate, source and drain electrodes).
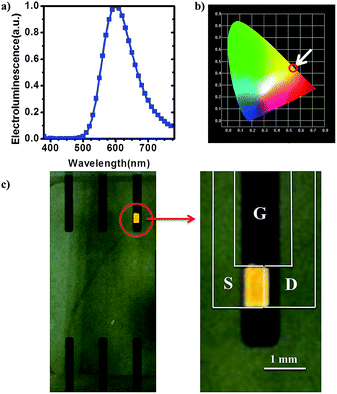 | ||
| Fig. 3 (a) Electroluminescent (EL) spectra of the device; (b)the CIE coordinates of the light emitted; (c) the photo of a working device (VGS = 5 V, VDS = 1 V). | ||
Fig. 4a–c display the emission brightness at different gate voltages. From Fig. 4a, we can find that, under VGS = 6.47 V and VDS = 0.3 V, the highest brightness is 67.7 cd m−2. The changes of brightness along with the drain voltage are obvious in Fig. 4a–c, and this verified that at a settled gate voltage, the emission can be modulated by the drain voltage. The initial increase of brightness can be explained by the response time of the device. Moreover, from the three curves in Fig. 4a–c, it is clear that the gate voltage can also modulate the brightness. As summarized in Fig. 4d, with the increase of the gate voltage, stronger emission can be observed from the device.
 | ||
| Fig. 4 The control of brightness via VGS or VDS: (a–c) the control of brightness via VDS; (d) the control of brightness via VGS. | ||
We suggest the operation mechanism of this multifunctional device as follows (Fig. 5): when a positive voltage is applied to the gate electrode, PF6− will migrate towards the gate electrode, forming an electrochemical double layer. Meanwhile, [Ir(dmfpz)2(dtb-bpy)]+ left near the semiconductor will induce electrons in the semiconductor layer, forming another electrochemical double layer. In this state, the transistor operates as a normal field-effect transistor with low threshold voltage because of the two electrochemical double layers. With the increase of gate voltage, under the help of gate-source field and electrochemical double layer field, holes will be injected from the gate to the iridium complex while electrons from ZTO layer to iridium complex layer. Holes and electrons will drift to the bulk of the complex layer and recombine to give light emission. Because the recombining region is a parallel layer to the transparent substrate, it will achieve an area emission. At this time, the device acts as a controllable LEC. The drain electrode gives an electric field, forming a pinch-off point, which will influence the emission zone and brightness of the device. Such operation mechanism can also be explained and proved by energy level alignment of this light emission system (Fig. 6).
From Fig. 6, it can be found that when gate voltage is lower than 4 V, electrons can accumulate near the interface of ZTO layer and the dielectric layer ([Ir(dmfpz)2(dtb-bpy)]PF6). Under VDS of 6 V and VGS smaller than 4 V, the energy band bending in ZTO is quite large, electron injection to the dielectric layer is forbidden, therefore, the device exhibits field effect rather than light emission characteristic. When gate voltage is higher than 4 V, the energy band bending in the dielectric layer benefits the injection of electrons from ZTO layer and holes from gate electrode to the dielectric layer and results in the light emission in the dielectric layer of the device.
The iridium complex layer plays an important role in the device performance. The film thickness may be one of the influential elements. When the gate voltage is lower than 4 V, the device is a kind of electrolyte-gated transistor. All the applied gate potential is dropped over the EDLs and the capacitance of the electrolyte is essentially independent on its thickness, indicating the transistor performance is insensitive to the thickness of the iridium complex film.14 When the gate voltage is over 4 V, the device behaves as a controllable LEC. Reports have stated that LECs are not quite sensitive to the thickness of light emitting layers.21,22 However, as for LECs based on transition mental complexes, some device characteristics were found to be influenced by the thickness of active layers.23 Therefore, the relationship between the film thickness and the device performance needs to be further investigated in our future research.
Conclusions
In summary, we have synthesized a multifunctional ionic iridium complex. With this complex, we have successfully fabricated a device behaving as a transistor or an emission device at different voltages. The emission brightness can be controlled by both VGS and VDS separately. The versatility of this complex is quite interesting and has potential to be used in overvoltage alarm devices.Acknowledgements
The authors thank the National Natural Science Foundation of China (no. 61177023, 51173096, and 61474069).Notes and references
- C. W. Tang and S. A. Vanslyke, Appl. Phys. Lett., 1987, 51, 913 CrossRef CAS PubMed.
- L. X. Xiao, Z. J. Chen, B. Qu, J. X. Luo, S. Kong, Q. H. Gong and J. J. Kido, Adv. Mater., 2011, 23, 926 CrossRef CAS PubMed.
- H. B. Sun, L. J. Yang, H. R. Yang, S. J. Liu, W. J. Xu, X. M. Liu, Z. Z. Tu, H. Q. Su, Q. Zhao and W. Huang, RSC Adv., 2013, 3, 8766 RSC.
- M. Muccini, Nat. Mater., 2006, 5, 605 CrossRef CAS PubMed.
- F. Cicoira and C. Santato, Adv. Funct. Mater., 2007, 17, 3421 CrossRef CAS.
- J. D. Slinker, A. A. Gorodetsky, M. S. Lowry, J. J. Wang, S. Parker, R. Rohl, S. Bernhard and G. G. Malliaras, J. Am. Chem. Soc., 2004, 126, 2763 CrossRef CAS PubMed.
- C. Ulbricht, B. Beyer, C. Friebe, A. Winter and U. S. Schubert, Adv. Mater., 2009, 21, 4418 CrossRef CAS.
- K. N. Swanick, S. Ladouceur, E. Z. Colman and Z. F. Ding, RSC Adv., 2013, 3, 19961 RSC.
- Y. Zhou, S. Han, G. Zhou, W. Y. Wong and V. A. L. Roy, Appl. Phys. Lett., 2013, 102, 083301 CrossRef PubMed.
- J. D. Slinker, J. A. DeFranco, M. J. Jaquith, W. R. Silveira, Y. W. Zhong, J. M. Mirabal, H. G. Craighead, H. D. Abruña, J. A. Marohn and G. G. Malliaras, Nat. Mater., 2007, 6, 894 CrossRef CAS PubMed.
- T. H. Kwon, Y. H. Oh, I. S. Shin and J. I. Hong, Adv. Funct. Mater., 2009, 19, 711 CrossRef CAS.
- Q. Pei and A. J. Heeger, Nat. Mater., 2008, 7, 167 CrossRef CAS PubMed.
- S. H. Kim, K. Hong, W. Xie, K. H. Lee, S. Zhang, T. P. Lodge and C. D. Frisbie, Adv. Mater., 2013, 25, 1822 CrossRef CAS PubMed.
- L. Herlogsson, X. Crispin, S. Tierney and M. Berggren, Adv. Mater., 2011, 23, 4684 CrossRef CAS PubMed.
- R. D. Costa, E. Ortí, D. Tordera, A. Pertegás, H. J. Bolink, S. Graber, C. E. Housecroft, L. Sachno, M. Neuburger and E. C. Constable, Adv. Energy Mater., 2011, 1, 282 CrossRef CAS.
- H. J. Bolink, E. Coronado, R. D. Costa, N. Lardiés and E. Ortı, Inorg. Chem., 2008, 47, 9149 CrossRef CAS PubMed.
- L. He, L. Duan, J. Qiao, R. Wang, P. Wei, L. D. Wang and Y. Qiu, Adv. Funct. Mater., 2008, 18, 2123 CrossRef CAS.
- Y. Zhao, L. Duan, G. Dong, D. Zhang, J. Qiao, L. Wang and Y. Qiu, Langmuir, 2013, 29, 151 CrossRef CAS PubMed.
- Y. M. Wang, F. Teng, Y. B. Hou, Z. Xu, Y. S. Wang and W. F. Fu, Appl. Phys. Lett., 2005, 87, 233512 CrossRef PubMed.
- J. D. Slinker, A. A. Gorodetsky, M. S. Lowry, J. Wang, S. Parker, R. Rohl, S. Bernhard and G. G. Malliaras, J. Am. Chem. Soc., 2004, 126, 2763 CrossRef CAS PubMed.
- J. Liu, I. Engquist, X. Crispin and M. Berggren, J. Am. Chem. Soc., 2013, 135, 12224 CrossRef CAS PubMed.
- Q. Pei, Y. Yang, G. Yu, C. Zhang and A. J. Heeger, J. Am. Chem. Soc., 1996, 118, 3922 CrossRef CAS.
- J. Slinker, D. Bernards, P. L. Houston, H. D. Abruña, S. Bernhard and G. G. Malliara, Chem. Commun., 2003, 19, 2392 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Synthesis of the ionic iridium light-emitting complex ([Ir(dmfpz)2(dtb-bpy)]PF6) and experimental procedures. See DOI: 10.1039/c4ra09454a |
| This journal is © The Royal Society of Chemistry 2014 |




